Back to Journals » Journal of Pain Research » Volume 7
Interstitial Cystitis – Elucidation of Psychophysiologic and Autonomic Characteristics (the ICEPAC Study): design and methods
Authors Chelimsky T, Chelimsky G, McCabe NP, Louttit M, Hijaz A, Mahajan S, Sanses T, Buffington CT, Fenton B, Janicki T, Ialacci S, Veizi E, Zhang D, Daneshgari F, Elston R, Janata J
Received 7 December 2013
Accepted for publication 14 February 2014
Published 8 May 2014 Volume 2014:7 Pages 243—253
DOI https://doi.org/10.2147/JPR.S58853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Thomas Chelimsky,1 Gisela Chelimsky,1 N Patrick McCabe,2 Megan Louttit,3 Adonis Hijaz,3 Sangeeta Mahajan,3 Tatiana Sanses,3 CA Tony Buffington,4 Bradford Fenton,5 Thomas Janicki,3 Sarah Ialacci,2 Elias Veizi,3 Di Zhang,2 Firouz Daneshgari,2,3 Robert Elston,2 Jeffrey Janata2,3
1The Medical College of Wisconsin, Departments of Neurology and Gastroenterology, Milwaukee, WI, 2Case Western Reserve University, Department of Neurology, Cleveland, OH, 3University Hospitals Case Medical Center, School of Medicine, Cleveland, OH, 4The Ohio State University, Department of Veterinary Clinical Sciences, Columbus, OH, 5Summa Health System, Department of Gynecology, Akron, OH, USA
Background and purpose: Interstitial cystitis/bladder pain syndrome (IC/BPS) is relatively common and associated with severe pain, yet effective treatment remains elusive. Research typically emphasized the bladder's role, but given the high presence of systemic comorbidities, the authors hypothesized a pathophysiologic nervous system role. This paper reports the methodology and approach to study the nervous system in women with IC/BPS. The study compares neurologic, urologic, gynecologic, autonomic, gastrointestinal, and psychological features of women with IC/BPS, their female relatives, women with myofascial pelvic pain (MPP), and healthy controls to elucidate the role of central and peripheral processing.
Methods and results: In total, 228 women (76 IC/BPS, 76 MPP, 38 family members, and 38 healthy controls) will be recruited. Subjects undergo detailed screening, structured neurologic examination of limbs and pelvis, tender point examination, autonomic testing, electrogastrography, and assessment of comorbid functional dysautonomias. Interpreters are blinded to subject classification. Psychological and stress response characteristics are examined with assessments of stress, trauma history, general psychological function, and stress response quantification. As of December 2012, data collection is completed for 25 healthy controls, 33 IC/BPS ± MPP, eight MPP, and three family members. Recruitment rate is accelerating and strategies emphasize maintaining and encouraging investigator participation in study science, internet advertising, and presentations to pelvic pain support groups.
Conclusion: The study represents a comprehensive, interdisciplinary approach to sampling autonomic and psychophysiologic characteristics of women with IC/BPS. Despite divergent opinions on study methodologies based on specialty experiences, the study has proven feasible to date and different perspectives have proved to be one of the greatest study strengths.
Keywords: interstitial cystitis, bladder pain syndrome, autonomic nervous system, psychophysiology, pelvic pain, myofascial pain
Introduction
This report describes the design, implementation, and feasibility of the Interstitial Cystitis: Elucidation of Psychophysiologic and Autonomic Characteristics (ICEPAC) Study. Interstitial cystitis/bladder pain syndrome (IC/BPS) causes severe bladder pain and afflicts about 2.5% of the population.1 The authors hypothesized abnormality of bladder perception at the central and/or peripheral nervous system levels. Typical symptoms and findings support this hypothesis (Table 1), and suggest IC/BPS is better conceptualized as either a chronic pain disorder, one involving autonomic nervous system dysfunction, or a combination of both. Classification as a chronic pain disorder may imply aberrant afferent (sensory) processing, whereas autonomic nervous system disorders are more often conceptualized as reflecting a primary efferent disturbance (Table 1). If IC/BPS reflects a nervous system disorder, the large number of comorbid diagnoses associated with IC/BPS2 suggests a widespread abnormality, either in a large portion of peripheral nerves or in a single location of the central nervous system responsible for end-organ input, control, or both.
  | Table 1 Neural mechanisms related to specific urologic symptoms |
A number of IC/BPS comorbid psychiatric, chronic pain, and autonomic disorders have been described previously.3,4 Examination of the interstitial cystitis database suggests significant overlap of IC/BPS with other pain areas, including lower back, pelvic, and abdominal pain in 60%–80% of subjects.5 The presence of headache, abdominal pain, tachycardia, panic attacks, dry eyes and mouth, heat intolerance, and cold-sensitive fingers and toes suggests increased sympathetic activity in IC/BPS.3,6,7 Studies investigating autonomic function in IC/BPS identified the presence of abnormal vasomotor tone,8–10 increased bladder sympathetic neuron density,6,11 and increased urine norepinephrine excretion.12
Stress response is partially explored in chronic pain disorders like IC/BPS. Prior provocative testing was restricted to physical stressors, though the stress response system behaves differently when activated by physical or psychosocial stressors.13 Moreover, disease activity at testing is generally not reported, further complicating interpretation. In feline IC/BPS, sympathetic outflow increases14–16 while the hypothalamic–pituitary–adrenal axis does not respond to external threat.17 Sympathetic nervous system outflow may become exaggerated since adrenocortical hormone activity normally restrains sympathetic nervous system outflow.3,18 Adrenocorticotropic hormone concentrations appear to decline during the stress of an IC/BPS flare in women3 and in men with chronic pelvic pain syndrome.19 Lutgendorf et al15 found that although mean urinary or salivary cortisol did not differ between women with IC/BPS and controls, subjects with higher morning cortisol had significantly less pain and urgency and those with higher urinary free cortisol reported less overall symptomatology (P<0.05). Thus, information to date suggests correlation between lower circulating cortisol concentration, higher sympathetic nervous system outflow, and higher pain levels.
The study aims to define autonomic, psychological, and other IC/BPS comorbid disorders, evaluate associated changes in autonomic and sensory nervous system structure and function, and characterize stress response physiology. The broad scientific questions raised are outlined in Table 2. This paper describes the study design and feasibility in a population that is difficult to research in adequate number.20
Material and methods
Developed initially by a core group of investigators, the protocols utilized in ICEPAC evolved in the first year of the study through the guidance of an advisory board with expertise in each of the fields encompassed in this complex interdisciplinary effort. The board meets annually to review study progress, advise the investigators, and serves as the Data Safety and Monitoring Board.
Subjects
ICEPAC plans to enroll 228 women aged 18–80 years: 76 IC/BPS subjects, 76 MPP subjects, 38 family member subjects (female siblings/parents/children of IC/BPS patients), and 38 healthy controls. The MPP group was selected to determine if findings are specific to IC/BPS or occur with other pelvic pain syndromes. IC/BPS was defined by at least 6 months of urgency, frequency, and bladder pain clearly linked to bladder filling and emptying. Specialist evaluation excluded other competing diagnoses. MPP was defined by at least 3 months of chronic pelvic pain unrelated to bladder state and a minimum of two of five pelvic floor tender points scoring >4/10 on a numeric rating scale (NRS) when examined applying 2 kg pressure with the index finger. The differing pain durations for subjects to be classified as IC/BPS or MPP, though not optimally comparable, are required for two reasons: 1) these durations reflect the current definitions found in the literature, and 2) subjects with MPP often become diagnosed and treated, effectively reducing pain burden, before 6 months have elapsed leading to recruitment difficulties if MPP inclusion requires 6 months of recent pain. Family members of IC/BPS subjects are excluded if they have any history of IC/BPS, MPP, or chronic pelvic pain as they would then be indistinguishable from subjects with pelvic pain. Healthy controls must have no history, symptoms or signs of fibromyalgia, chronic fatigue syndrome, IC/BPS, MPP, chronic pelvic pain, migraine headache, or any other putative IC/BPS comorbid disorders and be age matched to within ±3 years of an IC/BPS subject.
General exclusion criteria include: attempted, recent, or existing pregnancy, recurrent urinary tract infections in the last 12 months, pelvic or bladder neoplasm, major organ impairment, uncontrolled illness or unstable medical disorder, central or peripheral nervous system disorder associated with its own confounding autonomic and neurologic findings, drug/medical device or noninvasive treatment initiation (eg, bladder instillation, pelvic floor therapy) within 30 days, major surgical intervention (eg, cystoplasty, sacral neuromodulation therapy) within 120 days, use of hormones (except insulin, thyroid replacement, or oral contraceptives), opioid medications or allergy to bupivacaine, inability to stop all autonomic and gastrointestinal motility modifying agents prior to testing, any condition impairing ability to consent and comply with study procedures, or math/speech/needle phobia. All subjects sign an informed consent prior to undergoing any study procedures in accordance with the Institutional Review Board of University Hospitals Case Medical Center in Cleveland, OH, USA.
Study structure and exam standardization
The study administration is handled by the principal investigator (PI), co-PI, and project manager. Five satellite locations are used to screen and refer patients. To further increase recruitment, subcontracts were developed with Summa Health Systems (Akron, OH, USA) and the University of Toledo Medical Center (Toledo, OH, USA) to allow recruitment from other hospital systems located in the region.
With a diverse group of investigators performing the screening visit, it was necessary to standardize the examination to eliminate procedural bias. A total of four independent screening examinations were performed with each investigator; the first two conducted by the ICEPAC Study PI while the trained investigator observed. After observing two examinations, the investigator in training then performed two examinations under supervision. This process took place on four different days and with four different patients to establish examiner consistency. Additional rigor is brought to subject selection by requiring a structured screening examination, even if the referral came from a study investigator trained to perform the visit.
Data collection and storage
A customized FileMaker® Pro (FileMaker, Inc., Santa Clara, CA, USA), password protected database (Figure 1) resides on an encrypted network at the medical center. Research assistants (RA) perform real-time entry of all registration, visit, and testing data that is internally validated and stored in the database. Investigators are blinded to all disease and demographic information.
The ICEPAC Online Survey System, developed specifically for this study, allows subjects to enter questionnaire responses into the database. The RA monitors responses in real time at a different terminal to validate subject response patterns, speed, and subject attention. Study subjects access the instruments online with a user account and password. Data are stored in the ICEPAC research database and the ICEPAC Online Survey System is backed up daily. Data stored within the two systems can be exported and analyzed using the desired analytic software package.
Recruitment strategy
Patient recruitment began in February 2011. Completion is anticipated in early 2015 with enrollment through December 2012 depicted in Figure 2. Investigators in their individual clinics identify most potential subjects. Subjects may contact ICEPAC Study staff or give permission for study staff to contact them and schedule a screening visit (Visit 0). In the first year, a steady referral volume originated from a bimonthly interdisciplinary pelvic pain clinic, structured so that appropriate interested subjects could be consented, enrolled, and have their screening visit performed that day. Family members are recruited through nomination by the IC/BPS subjects. Mothers, sisters, and daughters accompanying study subjects to their visits are directly solicited. In addition to flyers at satellite clinics and postings in internal university/hospital communications, the study is listed on ResearchMatch.com and ClinicalTrials.gov. The study is advertised through the Interstitial Cystitis Association and Interstitial Cystitis Network. Investigators also guest lecture at regional IC support group meetings throughout Northeast and Central Ohio. Meeting attendees are invited to learn more about the study by contacting study coordinators.
The advisory board was critical in pointing out potential bottlenecks in recruitment strategies. For example, initial inclusion criteria excluded MPP from the IC/BPS group and excluded IC/BPS from the MPP group to enable clean comparisons. The advisory board was concerned about recruitment and how realistic it might be to find patients with IC/BPS who did not have MPP and recommended that the IC/BPS group be allowed to have comorbid MPP, while keeping the MPP group clean (without IC/BPS). This change proved critical. Enrollment (not completed subjects) of the IC/BPS group through December 2012 numbers 37, of whom 25 also have MPP. Had this change not been implemented, only twelve subjects would be enrolled while 25 would have been excluded.
Study visits
Enrollment and screening (Visit 0)
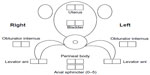
After informed consent, the initial standard medical evaluation includes a medical history, physical exam, pregnancy, and urine dipstick tests. Physical evaluation includes height, weight, lying and standing blood pressure and heart rate, body temperature, and body mass index. An RA leads each visit to ensure direct, in-person contact between clinical and study personnel.21 Once the above information is obtained, a study investigator reviews subject information and performs a routine physical examination of the head, eyes, ears, nose, throat, heart, lungs, extremities, and abdomen and assesses neurologic function. Particular attention is paid to any neurologic findings (eg, neuropathy, Parkinsonian features) that would require exclusion. Next, the RA and study investigator begin the first of several scripted examinations. Figures 3 and 4 show the locations of points examined for tenderness over the abdominal musculature, the inguinal ligaments, adductor muscles, and the pelvic floor musculature. The abdomen, inguinal region, and adductors are examined by applying 3 kg of force using an algometer (Model FDN-50; Wagner Instruments, Greenwich, CT, USA). Both the force associated with the first pain experience and a pain score (NRS: 0–10) at 3 kg (or the maximum tolerated force) are recorded. Pelvic floor muscle examination is done using 2 kg finger pressure where the study investigator standardizes the pressure applied by the digit with the algometer prior to starting the examination. The vulvodynia examination involves moving a lubricated cotton-tipped applicator back and forth inside the vagina (reported as a pressure or pain sensation) followed by a standard six-point assessment of the vulvar mucosa. The subject is oriented to the sensation of pin and cotton on the thigh and asked to determine whether similar contact on the vulva is experienced as pin or cotton and to rank any pain on an NRS of 0–10. A diagnosis of vulvodynia requires three or more points ranked at a pain NRS of four or more. The fibromyalgia examination is conducted per American College of Rheumatology criteria22 using 4 kg of pressure applied by the thumb standardized against the pressure algometer at standard sites. The fibromyalgia examination is conducted at Visit 0 only for healthy controls – as fibromyalgia is exclusionary for healthy controls – and at Visit 2 for IC/BPS, MPP, and family members. The RA records the subject pain ratings and study investigator comments pertaining to the examination. Based on history, examination, and referring physician records, subjects are grouped as IC/BPS ± MPP, MPP alone, or healthy subject. If the subject qualifies for the study, the RA schedules additional study visits and the subject is sent home with a urine hat and 24-hour voiding diary to be completed before her next visit.
Study ineligibility may lead to one of two actions: 1) the subject may qualify at a later date (ie, if subject had a recent procedure that requires a specific delay before enrollment), and will be contacted to return at the appropriate time; or 2) subject will be excluded from the study indefinitely.
Questionnaires and instillation (Visit 1)
Subjects arrive with their completed 24-hour voiding diary having not voided in the prior hour. Subjects use a portable computer to complete the psychological and Ohio Dysautonomia (ODYSA) questionnaires40 on the ICEPAC Online Survey System while the RA monitors progress and validates responses. The questionnaires listed in Table 2 are administered in random order to neutralize any questionnaire order effect with the exception of the ODYSA, which is given last due to its length. The ODYSA questionnaire was developed over the last several years to query for the presence of symptoms suggestive of comorbid disorders such as chronic fatigue syndrome, fibromyalgia, Raynaud’s syndrome, complex regional pain syndrome, migraine headache, irritable bowel syndrome, cyclic vomiting syndrome, functional dyspepsia, syncope, and orthostatic intolerance. Subjects are informed that they are not required to answer all questions and may skip questions should they feel uncomfortable for any reason. The RA will verify missing questionnaire data with the subject to determine whether omission was intentional before proceeding forward with the study visit.
Visit 1 also includes a 20 mL bladder instillation of a 0.75% bupivacaine solution performed on IC/BPS and MPP subjects only. The local anesthetic solution is prepared by the University Hospitals Research Pharmacy (University Hospitals, Cleveland, OH, USA) and dropped off at the procedure location. Since the intent of this procedure is to relate the change in bladder function to local anesthetic induced change in afferent processing, subjects undergo a baseline bladder ultrasound and uroflow (FloPoint® Elite; Verathon, Bothell, WA, USA) measurement so that pre- and post-procedure volumes can be matched as closely as possible for an “apples to apples” comparison of detrusor and sphincter functions. The bladder must contain at least 100 mL before uroflow measurement.
Prior to receiving the bladder instillation, the investigator cleans the subject’s urethra with three benzalkonium chloride swabsticks (Aplicare, Inc., Meriden, CT, USA), and then inserts a lubricated 8F pediatric catheter (C.R. Bard, Inc., Murray Hill, NJ, USA). The subject is asked to hold the solution in the bladder for a minimum of 60 minutes if possible. Subjects are asked to give pain ratings pre- and post-instillation. While waiting to void, subjects are given water to drink and the ODYSA questionnaire to complete. After 60 minutes of holding the solution, bladder volume is determined via ultrasound for comparison to the baseline volume reading. If the volume is reduced compared to baseline, more water is given and the bladder is subjected to ultrasound again after a suitable period of time has elapsed. Once the volume is approximately equal to that of the baseline recording (±10%), a final uroflow is performed. Subjects who received an instillation are given a 3-day voiding diary to return at the final study visit and reminded which medications must be stopped prior to their next visit. At this visit, the investigator reviews medications the subject will need to temporarily discontinue/halt prior to the autonomic testing study visit (Visit 2).
Autonomic and neurologic examination (Visit 2)
Autonomic testing includes the cardiac responses to deep breathing and to the Valsalva maneuver, the post-ganglionic quantitative sudomotor axon reflex test, and a tilt table test. RAs complete all autonomic nervous system testing with a trained investigator required only during the tilt table test. The methods of the autonomic nervous system laboratory have been previously described23 and will be reiterated here in brief. The cardiac response to deep breathing is a fairly pure measure of pulmonary afferent stretch receptor and cardiac parasympathetic efferent activities.24 The subject takes six full breaths per minute for 1 minute while an electrocardiogram records heart rate. This procedure is repeated three times. The difference between inspiration (higher heart rate) and expiration (lower heart rate) is averaged for the five best breaths and compared to age-based norms. The Valsalva maneuver is performed by asking the subject to blow against a pressure gauge holding 40 mmHg for 15 seconds with an open glottis (insured by a small leak in the connecting tubing) while continuous blood pressure, measured by digital plethysmography (Nexfin Monitor Model 1; BMEYE B.V., Amsterdam, the Netherlands), and heart rate are recorded. Measurements include the highest heart rate at the end of the pressure holding period (Phase II) – reflecting cardiac sympathetic function, the lowest heart rate after release (Phase IV) – reflecting cardiac parasympathetic function, and maintenance of diastolic pressure throughout Phase II – reflecting vasomotor sympathetic function. Age-based norms are expressed as a ratio of the highest to lowest heart rates (Valsalva ratio). The tilt-table test requires the subject to remain inclined at 70 degrees for 30 minutes preceded and followed by baseline blood pressure and heart rate readings taken each minute for 5–10 minutes. The subject is asked every few minutes for any standard orthostatic symptoms such as lightheadedness, nausea, diaphoresis, blurred or darkening vision, and fatigue, ranked on a NRS from 0–10. Tilt results are interpreted as either normal, showing orthostatic hypotension (>20 mmHg systolic or >10 mmHg diastolic blood pressure drop in the first 3 minutes of tilt), postural tachycardia syndrome (>30 beats per minute (bpm) rise in heart rate in the first 10 minutes of tilt in the presence of orthostatic symptoms and in the absence of a drop in blood pressure), or reflex syncope (an abrupt drop in pressure usually accompanied by a drop in heart rate that would have led to loss of consciousness had the subject not been reclined). The heart rate record is also analyzed for heart rate variability. During the tilt test, the gastric electrical activity (Polygram Net™ analysis package; Medtronic, Minneapolis, MN, USA) is recorded for 10 minutes with the patient in the supine position and then during the 30 minutes of head-up tilt. The quantitative sudomotor axon reflex test provides a specific measure of cholinergic sympathetic efferent small fiber function. In brief, a battery sourced current is driven for 5 minutes across a 10% solution of acetylcholine located in the outer chamber of a dual-chambered capsule applied to the skin. Sweat rate is detected through the change in humidity in the inner chamber of the same capsule. Since each sudomotor nerve innervates multiple sweat glands, the axon stimulated by the acetylcholine iontophoresis signals the other sweat glands and results in sweat output into the inner chamber.25
The remainder of Visit 2 is performed in a standard examination room. The 3-day voiding diary is collected from the subject and reviewed. A water load test26 is administered, which consists of recording the maximal volume of water a subject can consume in 5 minutes with comparison to normal values. Subjects classified as IC/BPS, MPP, or family members then undergo a fibromyalgia examination based on the 1990 American College of Rheumatology22 criteria, checking the 18 standard tender points applying 4 kg of pressure with the thumb, calibrated using an algometer every fourth point. An examination of the extremities (Small Fiber Score) looking for generalized small (and large) fiber peripheral nerve dysfunction is performed assessing pin, temperature, vibration sensation, superficial and deep allodynia, distal muscle strength, and reflexes (Figure 5). A pelvic neurologic examination27 is performed to evaluate dermatomes T12, L1, L2, S1, S2, S3, S4, and S5 for pin prick and vibration sensations. Subjects are compensated for their time and offered participation in other ICEPAC substudies such as the Trier Social Stress Test study28 or an ICEPAC sleep study investigating sleep disorders in women with IC/BPS.
Results and discussion
Recruitment
As of December 2012, data collection has been completed for 25 healthy controls, 33 IC/BPS ± MPP, eight MPP, and three family members. Of 194 potential subjects contacted, 83 subjects or 43% have consented. Rate of recruitment has shown a pattern of slow but accelerating referrals. Initially, the PI and co-PI were the main source of referrals, but this pattern was not sustainable once clinic populations were exhausted. Efforts at maintaining contact with investigators to encourage referrals have led to gradual referral growth across a broader group of referrers. Study investigators are kept up-to-date on study progress through an ICEPAC Investigator Newsletter, which also serves as a reminder to refer.
Adverse advents
To date, the only adverse events have been associated with the bupivacaine instillation procedure. As a result, the investigators, guided by the ICEPAC Data Safety Monitoring Board adopted a smaller, pediatric hydrophilic catheter and, since then, no further adverse events of any type have been reported.
Challenges
Studies involving chronic pain disorders such as IC/BPS pose two very specific challenges. First, in the current era, most successful clinical–translational studies on disorders involving chronic pain are interdisciplinary in design, requiring significant education, cross-training, and front-end work for the team to be successful. In fact, the National Institutes of Health (Bethesda, MD, USA) is currently funding much of the work on IC/BPS through the Multidisciplinary Approach to the study of chronic Pelvic Pain (MAPP) multisite network. Second, these populations have been difficult to recruit in large numbers.20 The purpose here is to present an approach to both of these challenges for the benefit of others who are performing related studies and spell out which strategies were and were not successful. The original proposal arose from a collaboration funded by the Interstitial Cystitis Association of America between Dr Buffington and Dr Chelimsky on the familial characteristics of IC/BPS and an interdisciplinary team seeing pelvic pain patients together in a clinic setting, with a set of questions regarding these patients. The team included a psychologist, urologist, gynecologist, anesthesiologist, and neurologist. Disagreements on methods (eg, the best way to examine the pelvic floor musculature) were incorporated as questions to be answered by the grant. Thus, this study is noteworthy for its true interdisciplinary nature, from conception to implementation, and there was strong investment in the grant by the research team. Although such a process takes longer, the authors believe the design quality was higher, and the scientific questions posed were more clinically relevant.
An independent advisory board of nationally recognized investigators monitors project progress. The board reflects the interdisciplinary composition nature of the research team and is comprised of a psychophysiologist with expertise in heart rate variability and stress responses, a pelvic pain neurologist, an urologist, a gynecologist specializing in pelvic pain and detailed vulvar examinations, a psychiatrist, and a sleep specialist. The board provided refinements to the study and helped validate that the project was capable of addressing important questions with an adequately sophisticated design.
A simple issue pertained to the practicality of scheduling meetings for 20 busy surgeons and clinicians. This was initially addressed by scheduling monthly conference calls at an optimal time with topical discussions that were highly relevant and intellectually stimulating. Once major issues were resolved, attendance levels dropped and never recovered. An investigator newsletter was implemented as a means of maintaining investigator engagement and transparent communication.
The collaboration of disciplines that usually do not understand each other’s technical terms required significant education, with an initially steep learning curve. Cross-disciplinary training for standardization of pelvic floor, neurologic, and urologic examinations conducted during study visits was completed by each investigator. This was a highly rewarding process for investigators from varied disciplines, resulting in novel questions on each area driven by an entirely fresh perspective.
Another set of challenges arose from sheer study size. A protocol comprised of multiple investigators from different departments and medical centers required multiple revisions in order to obtain Institutional Review Board approval. An area of contention surrounded the development of the pelvic floor examinations. Urologists and gynecologists have different views on methodology and the relative importance of different portions of the examinations. It took several months of re-evaluations and standardization to obtain a final protocol, a feat not possible without the guidance and expertise of the ICEPAC Study advisory board members. The number of investigators, of whom consensus was required, slowed attempts to modify the study. Exploration of new ideas or protocol modifications, such as assessment of sleep pattern and sleep disorder causality or measurement of stress and tight junction related cytokines in serum in subjects undergoing the Trier Social Stress Test, require more discussion and take longer to implement. As a result, however, the design is more likely to be embraced by specialists in diverse areas of medicine (eg, urologists, gynecologists).
The number of physicians trained to perform study visits (which was the only way to recruit the large number of subjects proposed) required particular attention to investigator training (described in Methods) and protocol standardization. To this end, attention was paid to protocol scripts, and most of these were designed to be read and controlled by the RA rather than the investigator, which minimizes protocol variability. Measurement precision was improved early in the study for some of the assessments. For example, semiquantitative tuning forks29 (US Neurologicals LLC, Poulsbo, WA, USA) for the assessment of vibration sensation were introduced, the temperature of the hot water stimulus was standardized to 115°F, and a digital algometer (Model FDIX 10; Wagner Instruments) to standardize the force used to assess pin sensation to 0.55 N (about 2 oz) were implemented as part of standard operating procedure.
Selection of the psychological questionnaires required great care, ensuring that appropriate psychological constructs were evaluated while simultaneously limiting the time requirement burden on subjects and reducing the impact on statistical power of the number of variables employed. After extensive discussion and consideration of alternatives, the advisory board ultimately supported the selection of psychological proposed instruments. The psychological questionnaire set includes 313 questions and the ODYSA either 158 or 330 questions, depending on the subject’s symptomatic history. Experiences with the first group of subjects enrolled have been mixed. Though all subjects have completed the question set to date, a small number of subjects have expressed concern about questionnaire time burden. Breaks are offered in the process and no time constraints are placed on subjects to complete the questionnaires.
The database presented some informatics challenges. With current privacy regulations and concerns, the database had to be accessible by subjects for entry but not for look-up, and specific layouts needed to remain inaccessible to some team members while accessible to others (eg, demographic and diagnostic information needed to be blinded to the investigator to eliminate bias). Using the FileMaker Server and Database platform in cooperation with University Hospitals Information Technology and Solutions allowed the authors to design a database with all of these features, which may well be useful for other similar studies in the future. This level of sophistication though required the fulltime effort of a data manager for 3 years.
As with all studies on pelvic pain, recruitment presents a challenge. In an effort to maintain study awareness, the PI, co-PI, and project manager visited each of the investigators to encourage active participation. Investigators are invited to annual advisory board meetings to encourage a shared sense of academic participation and monthly phone conferences were conducted. Advertisements were placed on several research subject recruitment websites and patient support organizations (as outlined in the Methods section). Recruitment returns from the effort at the subject level far exceeded those from efforts at the investigator and provider level. Recurrent communication with eight interested providers and potential collaborators from neighboring institutions resulted in few referrals. In contrast, promoting ICEPAC Study information to patient support groups, both regional and national, resulted in higher enrollment than referrals alone.
A final challenge relates to many clinical studies that use hospital resources. The study utilizes the autonomic laboratory and clinic rooms for subject examinations that are also employed by patients for neurology and urology clinics. Coordinating open times requires negotiation and reminders to hospital administration of the importance of the study in fostering clinical referrals. Clinical calendars frequently change leading to double bookings of resources and require conflict management. However, every effort is made to maintain an excellent working relationship with clinic staff and faculty, which results in excellent cooperation. When hospital staff turnover occurs, renewed educational efforts help replacement staff get up to speed on the study.
ICEPAC has been designed to allow careful phenotyping of subjects, which affords the novel identification of potentially important patient subgroups. The study is exploratory in nature, collecting a broad and comprehensive array of data, and its conclusions should generate specific, testable hypotheses that form the basis for future studies. Moreover, ICEPAC can be seen as a template for the study of other complex pain phenomena, such as gastrointestinal and other visceral and nonvisceral referred pain.
Conclusion
The ICEPAC Study represents a comprehensive, interdisciplinary approach to understanding the autonomic and psychophysiologic characteristics of IC/BPS. The study has proven feasible to date, although it is too early to report findings. The major obstacles have been by-products of the different perspectives and areas of expertise that a diverse group of investigators has brought, which, interestingly has also been one of the study’s greatest strengths. That is, as perspectives have been shared and a common approach has been forged, each specialty has benefited by the input of the others. The investigators are hopeful that the findings of the ICEPAC Study will reflect the strength of this collaborative process.
Acknowledgments
The ICEPAC Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (5R01DK083538). The following individuals are members of the ICEPAC Study Advisory Board and have helped shape the design and methodologies describe herein: Debra Erickson (Department of Surgery, University of Kentucky College of Medicine, Lexington, KY, USA), Kathleen Pajer (IWK Health Centre, Dalhousie University, Halifax, NS, Canada), Julian Thayer (Department of Psychology, The Ohio State University, Columbus, OH, USA), Ursula Wesselmann (Department of Anesthesiology, UAB School of Medicine, Birmingham, AL, USA), Phyllis Zee (Center for Sleep and Circadian Biology, Northwestern University, Evanston, IL, USA), and Denniz Zolnoun (Department of Obstetrics and Gynecology, UNC School of Medicine, Chapel Hill, NC, USA).
Disclosure
The authors report no conflicts of interest in this work.
References
Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544. | |
Warren JW, Howard FM, Cross RK, et al. Antecedent nonbladder syndromes in case–control study of interstitial cystitis/painful bladder syndrome. Urology. 2009;73(1):52–57. | |
Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172(4 Pt 1):1242–1248. | |
Peters KM, Carrico DJ, Diokno AC. Characterization of a clinical cohort of 87 women with interstitial cystitis/painful bladder syndrome. Urology. 2008;71(4):634–640. | |
FitzGerald MP, Brensinger C, Brubaker L, Propert K; ICDB Study Group. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):69–72. | |
Hohenfellner M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147(3):587–591. | |
Pontari MA, Hanno PM, Ruggieri MR. Comparison of bladder blood flow in patients with and without interstitial cystitis. J Urol. 1999;162(2):330–334. | |
Galloway NT, Gabale DR, Irwin PP. Interstitial cystitis or reflex sympathetic dystrophy of the bladder? Semin Urol. 1991;9(2):148–153. | |
Irwin PP, James S, Watts L, Fleming LL, Galloway NT. Abnormal pedal thermoregulation in interstitial cystitis. Neurourol Urodyn. 1993;12(2):139–144. | |
Irwin PP, Galloway NTM. Urinary cystitis: the neurovascular perspective. In: Sant GR, editor. Interstitial Cystitis. Philadelphia, PA: Lippencott-Raven; 1997:129–135. | |
Peeker R, Aldenborg F, Dahlstrom A, Johansson SL, Li JY, Fall M. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J Urol. 2000;163(4):1112–1115. | |
Stein PC, Torri A, Parsons CL. Elevated urinary norepinephrine in interstitial cystitis. Urology. 1999;53(6):1140–1143. | |
Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. | |
Buffington CA, Pacak K. Increased plasma norepinephrine concentration in cats with interstitial cystitis. J Urol. 2001;165(6 Pt 1):2051–2054. | |
Lutgendorf SK, Kreder KJ, Rothrock NE, et al. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. J Urol. 2002;167(3):1338–1343. | |
Lutgendorf SK, Latini JM, Rothrock N, Zimmerman MB, Kreder KJ Jr. Autonomic response to stress in interstitial cystitis. J Urol. 2004;172(1):227–231. | |
Westropp JL, Kass PH, Buffington CA. Evaluation of the effects of stress in cats with idiopathic cystitis. Am J Vet Res. 2006;67(4):731–736. | |
Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. | |
Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CA, Nickel JC. Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008;71(2):261–266. | |
Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg L Jr. The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161(2):553–557. | |
Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012;13(9):910–920. | |
Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. | |
Safder S, Chelimsky TC, O’Riordan MA, Chelimsky G. Gastric electrical activity becomes abnormal in the upright position in patients with postural tachycardia syndrome. J Pediatr Gastroenterol Nutr. 2010;51(3):314–318. | |
Low PA, Benarroch EE. Laboratory Evaluation of Autonomic Failure. In: Clinical Autonomic Disorders. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:130–163. | |
Low PA. Autonomic nervous system function. J Clin Neurophysiol. 1993;10(1):14–27. | |
Jones MP, Hoffman S, Shah D, Patel K, Ebert CC. The water load test: observations from healthy controls and patients with functional dyspepsia. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G896–G904. | |
Howard FM, Perry CP, Carter JE, El-Minawi AM. Pelvic Pain: Diagnosis and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. | |
Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test” – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. | |
Merkies IS, Schmitz PI, van der Meche FG, van Doorn PA. Reliability and responsiveness of a graduated tuning fork in immune mediated polyneuropathies. The Inflammatory Neuropathy Cause and Treatment (INCAT) Group. J Neurol Neurosurg Psychiatry. 2000;68(5):669–671. | |
Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26(3):327–332. | |
Kubany ES, Haynes SN, Leisen MB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12(2):210–224. | |
Goodman LA, Corcoran C, Turner K, Yuan N, Green BL. Assessing traumatic event exposure: general issues and preliminary findings for the Stressful Life Events Screening Questionnaire. J Trauma Stress. 1998;11(3):521–542. | |
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. | |
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. | |
Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. | |
The Pennebaker Inventory of limbic languidness. In: Pennebaker JW. The Psychology of Physical Symptoms. New York, NY: Springer-Verlag; 1982:169–170. | |
Kerns RD, Turk DC, Rudy TE. The West Haven–Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23(4):345–356. | |
Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain. 2001;91(1–2):147–154. | |
Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. | |
Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. 2011;158(1):20–23. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.