Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Interrelation Between Increased BDNF Gene Methylation and High Sociotropy, a Personality Vulnerability Factor in Cognitive Model of Depression
Authors Shirata T , Suzuki A, Matsumoto Y, Noto K, Goto K , Otani K
Received 2 March 2020
Accepted for publication 29 April 2020
Published 15 May 2020 Volume 2020:16 Pages 1257—1263
DOI https://doi.org/10.2147/NDT.S252177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Toshinori Shirata,1 Akihito Suzuki,1 Yoshihiko Matsumoto,1 Keisuke Noto,1 Kaoru Goto,2 Koichi Otani1
1Department of Psychiatry, Yamagata University School of Medicine, Yamagata 990-9585, Japan; 2Department of Anatomy and Cell Biology, Yamagata University School of Medicine, Yamagata 990-9585, Japan
Correspondence: Toshinori Shirata Email [email protected]
Purpose: It is suggested that increased methylation of the brain-derived neurotrophic factor (BDNF) gene is involved in the pathogenesis of depression, while sociotropy and autonomy are proposed as personality vulnerability factors in cognitive model of depression. We examined the interrelation between BDNF gene methylation and sociotropy or autonomy, with taking into account the previously reported deleterious effect of parental overprotection on sociotropy.
Materials and Methods: The participants consisted of 90 healthy Japanese volunteers. Methylation levels of the BDNF gene in peripheral blood were quantified by bisulfite pyrosequencing. Sociotropy and autonomy were assessed by the Sociotropy-Autonomy Scale, and perceived parental protection was evaluated by the Parental Bonding Instrument.
Results: In Pearson’s correlation analysis, there was a positive correlation between methylation levels of the BDNF gene and sociotropy scores (p< 0.05) but not autonomy scores, and a positive correlation between maternal protection scores and sociotropy scores (p< 0.05). In structural equation modeling, two models were proposed; the first one is that hypermethylation of the BDNF gene and maternal overprotection independently contribute to high sociotropy, and the second one is that maternal overprotection contributes to high sociotropy which then leads to hypermethylation of the BDNF gene.
Conclusion: The present study suggests an interrelation between increased BDNF gene methylation and high sociotropy.
Keywords: autonomy, BDNF gene, methylation, parental protection, sociotropy
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family which plays an important role in survival of neurons, neural differentiation, and synaptic plasticity.1 Studies using BDNF transgenic mice and human subjects genotyped for the Val66Met BDNF gene polymorphism show that disruption of BDNF activity is related to impaired neurocognitive function, altered sensitivity to stress, and several stress-related psychiatric disorders such as depression, anxiety, and posttraumatic stress disorder.2,3 A meta-analysis shows that blood levels of BDNF are decreased in patients with major depressive disorder.4
DNA methylation is one of the mechanisms causing changes in gene function without modifications of the DNA sequence.5,6 It is demonstrated that an increase in BDNF gene methylation induces decreased synthesis of BDNF in neurons.7 This epigenetic mechanism is influenced by various environmental and life style factors.5,6 A recent study shows increased methylation of the BDNF gene accompanied by decreased BDNF secretion in the brain of suicide subjects.8 An extensive review of cross-sectional studies indicates that methylation of the BDNF gene is higher in depressed patients than in controls.9 Longitudinal studies suggest that high methylation of the BDNF gene is a risk factor for depression after stroke, in breast cancer and in late life.10–12 These studies suggest that increased BDNF gene methylation causing decreased BDNF secretion is involved in the pathogenesis of depression.
In the Beck’s cognitive theory, two personality styles named sociotropy and autonomy, each of which consists of a cluster of negative self-schemas, predispose to depression after experiencing stressful events.13,14 The sociotropic individuals have self-schemas involved in interpersonal closeness, acceptance, and approval from others, and they are sensitive to events perceived as an interpersonal loss such as separation, rejection, and disapproval. The autonomic individuals have self-schemas involved in accomplishment, independence, and self-control, and they are susceptible to events perceived as a failure to accomplish and a loss of independence or self-control. This diathesis-stress model of depression has been confirmed by cross-sectional and prospective studies.15–17
The discussions so far made on BDNF gene methylation and sociotropy/autonomy suggest a relationship between the two factors, which is likely to be involved in the pathogenesis of depression. As BDNF activity modulates various aspects of mental function including neurocognition and stress sensitivity,2,3 it is not surprising if methylation of the BDNF gene causing altered BDNF activity7 affects the formation of these personality vulnerabilities in cognitive theory. In fact, we recently found out that elevated BDNF gene methylation produces high neuroticism,18 another personality vulnerability factor for depression.19 Neuroticism is a dimension in the five-factor personality model derived from factor analysis of personality adjectives in dictionaries and questionnaires, assuming that they cover all meaningful personality descriptions.19,20 Sociotropy/autonomy and neuroticism correlate to each other,21 but they stand on different personality theories as mentioned above, postulate different core features, ie, negative cognition in the former13,14 while negative emotionality in the latter,19,20 also the former refers to coping and interpersonal styles more than the latter does.22 If methylation of the BDNF gene is involved in personality vulnerabilities proposed in different theories and in different aspects of personality construct, it further supports the implication of this epigenetic mechanism in the pathogenesis of depression, especially in the formation of premorbid personalities.
Therefore, the purpose of the present study was to examine the relationship between methylation of the BDNF gene and sociotropy/autonomy. The merits of this study were, firstly, bidirectional relationships between the two factors were examined and, secondly, the previously reported deleterious effect of parental overprotection on sociotropy was taken into account.23
Materials and Methods
Participants
The participants were 90 Japanese volunteers. They were free from severe physical diseases, and from Axis I psychiatric disorders according to the Diagnostic and Statistical Manual of Mental Disorders-IV.24 Their mean±SD of age was 22.4±0.6 years. Forty-four were males and 46 were females. None of them participated in our previous study revealing the harmful effect of maternal overprotection on sociotropy.23 All methods of the present study followed the Declaration of Helsinki, and conformed to the principles of medical ethics. The Ethics Committee of Yamagata University School of Medicine approved the protocol. All participants received a complete explanation of the present study and provided written informed consent.
Analysis of BDNF Gene Methylation
Venous blood was collected from the participants, and genomic DNA was extracted using a QIAamp DNA Blood Kit (Qiagen, Tokyo, Japan). Since DNA methylation levels in peripheral blood are highly correlated with those in brain tissues,25 the present study used BDNF gene methylation in leukocytes as a proxy of that in brain tissue. The human BDNF gene has 11 exons and 9 promoters,26 and previous studies have examined the relationship between methylation levels in several promoter regions and psychiatric disorders.8,27 One of them shows that the promoter IV region of the BDNF gene has the cAMP response element-binding protein (CREB)-binding site and that methylation of this region affects CREB binding and consequent transcription.28 Furthermore, Kundakovic et al. (2015) show that high methylation of the 2 CpG sites in this region reduces BDNF expression.28 Thus, the present study focused on the 2 CpG sites in the promoter IV of the BDNF gene.
The coordinates of the target region are Chr11: 27,723,159–27,723,246 (University of California, Santa Cruz Genome Browser, Human February 2009, GRCh37/hg19) (Figure 1). Methylation levels of the BDNF gene were analyzed through EpigenDx (Worchester, MA, USA), a company specializing in genomic methylation assays, with the assay ID ADS221-FS2re. EpigenDx performed bisulfite conversion using the EZ DNA Methylation Kit (Zymo Research, Inc., CA), PCR amplification and pyrosequencing on the PSQ96 HS System (Pyrosequencing, Qiagen). The methylation status of each CpG site was determined individually as an artificial C/T SNP using QCpG software (Pyrosequencing, Qiagen). A methylation level of each CpG site was calculated as the percentage of the methylated alleles divided by the sum of methylated and unmethylated alleles. The present study used the mean of methylation levels of the 2 CpG sites in statistical analyses.
Assessment of Personality Traits and Parental Protection
Assessment of sociotropy and autonomy was performed by the Japanese version of the Sociotropy-Autonomy Scale,29 which was kindly supplied by Dr Yutaka Ono. The Japanese version is shown to have high reliability and validity.30 The values of Cronbach’s alphas for sociotropy and autonomy subscales in the present data were 0.89 and 0.84, respectively. Perceived protection of parents was evaluated by the Japanese version of the Parental Bonding Instrument.31,32 The Japanese version is demonstrated to have high reliability and validity.31 The values of Cronbach’s alphas for paternal protection and maternal protection in the present data were 0.85 and 0.87, respectively.
Statistical Analyses
The statistical tests used were Pearson’s correlation analysis and structural equation modeling. The statistical software packages used were SPSS 22 and AMOS 22 (IBM Japan, Tokyo, Japan). In structural equation modeling, several models were constructed using BDNF gene methylation levels, sociotropy and autonomy scores, and paternal and maternal protection scores. Goodness-of-Fit-Index (GFI) >0.95, Adjusted GFI (AGFI) >0.90, Comparative Fit Index (CFI) >0.97, and Root Mean Square Error of Approximation (RMSEA) <0.05 were adopted as the criteria for good fit.33 A p value < 0.05 was considered to be statistically significant. In the present study, corrections for multiple testing were not applied because of the exploratory nature of the investigation.
Results
Table 1 shows BDNF gene methylation levels, sociotropy and autonomy scores, paternal and maternal protection scores, and Pearson’s correlation coefficients among them. A positive correlation was found between BDNF gene methylation levels and sociotropy scores (r=0.232, p<0.05) but not autonomy scores (r=0.193, n.s.). Sociotropy scores were positively correlated with maternal protection (r=0.240, p<0.05) but not with paternal protection (r=0.097, n.s.). Autonomy scores were not correlated with any variables.
 |
Table 1 BDNF Gene Methylation Levels, Sociotropy and Autonomy Scores, Paternal and Maternal Protection Scores, and Pearson’s Correlation Coefficients Among Them |
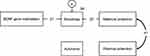
In structural equation modeling, only two out of several models showed good fit. Figure 2 shows the first model with good fit (GFI=0.989, AGFI=0.963, CFI=1.000 and RMSEA=0.000). High methylation levels of the BDNF gene (standardized path coefficient =0.21, p<0.05) and high maternal protection scores (standardized path coefficient =0.22, p<0.05) independently contributed to high sociotropy scores. None of the paths for autonomy was significant.
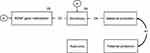
Figure 3 shows the second model with good fit (GFI=0.993, AGFI=0.976, CFI=1.000 and RMSEA=0.000). High maternal protection scores contributed to high sociotropy scores (standardized path coefficient =0.24, p<0.05) which then led to high methylation levels of the BDNF gene (standardized path coefficient =0.23, p<0.05). None of the paths for autonomy was significant.
Discussion
First of all, the present study replicated our previous result that maternal overprotection promotes formation of sociotropy.23 To repeat the former interpretation of this phenomenon,23 a child who grows up to be dependent and immature under maternal overprotection is susceptible to experience failures and feel one’s ineffectiveness in society and to harbor negative self-images such as “being helpless” and “being unworthy” (aka negative core beliefs about the self in cognitive theory of depression), which form the foundation of sociotropic schemas.34
The present study suggests two scenarios regarding interrelations among BDNF gene methylation, sociotropy and maternal protection. The first one is that high methylation of the BDNF gene and maternal overprotection independently contribute to high sociotropy. One of the possible brain structures responsible for the formation of sociotropy is the hippocampus, which plays an important role in memory contextualization and retrieval and evaluation of autobiographical information.35,36 According to Pruessner et al.37 reduced hippocampal volume leads to reduced abilities to recall specific situations and environments when experiencing failures or rejections, resulting in overgeneralized self-perception of being a failure or rejected, ie, negative self-images. It is shown that lower BDNF activity is associated with small hippocampal volume and poor hippocampal-dependent learning.38,39 Meanwhile, BDNF expression is reduced in the hippocampus of suicide victims,40 and increased methylation of the BDNF gene may be the underlying mechanism for this phenomenon.8 Taken together, high methylation of the BDNF gene may contribute to high sociotropy by causing small hippocampal volume accompanied by negative self-images, the foundation of sociotropic schemas.34 Notably, the effect of elevated BDNF gene methylation was independent of that of maternal overprotection in the present study. Also, maternal overprotection did not affect methylation of the BDNF gene. These results suggest that environmental and life style factors other than dysfunctional parenting, eg, nutrition, physical activity, alcohol, pollutants, studying and working habits,5,6 play important roles in increasing BDNF gene methylation. Implication of negative interactions with people other than parents, eg, siblings, classmates, colleagues, partners, cannot be excluded either.
The second scenario is that maternal overprotection contributes to high sociotropy which then contributes to high methylation of the BDNF gene. It is possible that the behaviors according to sociotropic schemas such as “It is important to be liked and approved by others” elicit negative reactions from others.13 In fact, a study shows that sociotropic persons are prone to experience negative social interactions such as criticism, ignorance and betrayal.41 Therefore, a sociotropic person may undergo increases in BDNF gene methylation by experiencing negative interpersonal events.
It is difficult to mention which of the two scenarios is more plausible. However, in either case a vicious cycle between hypermethylation of the BDNF gene and high sociotropy leading to the marked decrease in BDNF secretion observed in depression may be formed.4 In the former scenario once high sociotropy is formed, it may further increase BDNF gene methylation by creating interpersonal stresses,41 while in the latter scenario once hypermethylation of the BDNF gene is formed, it may further increase sociotropy by producing negative self-images through the hippocampal mechanism.37 Recently, we proposed a similar vicious cycle between high methylation of the BDNF gene and high neuroticism,18 another personality vulnerability factor for depression.
In contrast to the result on sociotropy, there was no interrelation between BDNF gene methylation and autonomy. There are two possible explanations for this result. Firstly, epigenetic mechanisms other than DNA methylation regulating BDNF gene function, eg, histone modifications may be related to autonomy.42 Secondly, epigenetics of other genes, eg, glucocorticoid receptor gene may be associated with autonomy.5,6 Apparently, these possibilities are worth testing in future studies.
Our previous study suggests that increased BDNF gene methylation is connected with neuroticism,18 which is most characterized by negative emotionality.19,20 On the other hand, the present study suggests that this epigenetic change is linked with sociotropy, which is characterized by negative cognition and problems in stress coping and interpersonal relationships.13,14,22 Taken together, BDNF gene methylation is likely to be involved in personality vulnerabilities with different theoretical backgrounds and in broad aspects of personality construct. We propose that methylation of the BDNF gene is deeply implicated in the pathogenesis of depression, especially in the formation process of premorbid personalities.
As far as we know, this is the first study to examine the relationship between methylation of the BDNF gene and sociotropy. However, this study has several limitations. Firstly, our subjects were too homogeneous, because they were all young Japanese. Secondly, the sample size was comparatively small, suggesting that a type II error is possible. Consequently, the present results cannot be applied directly to other age groups and other ethnic groups. Thirdly, the present study did not evaluate environmental factors such as current stress, stress history, and trauma, which are shown to alter DNA methylation and BDNF gene polymorphism-dependent affective dysregulation,6,43 suggesting that the relationship between BDNF gene methylation and sociotropy/autonomy might be obscured. Fourthly, the present study could not determine which of the two scenarios presented was more plausible, because of its cross-sectional nature. To clarify this point, a longitudinal study examining changes in sociotropy scores among subjects with different levels of BDNF gene methylation is warranted.
Conclusion
The present study suggests an interrelation between increased BDNF gene methylation and high sociotropy, which is likely to be implicated in the pathogenesis of depression.
Acknowledgments
We thank Dr. Yutaka Ono at the Ono Research Institute for kindly providing the Japanese version of the Sociotropy-Autonomy Scale. This study was supported by funding from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funding had no effect on the present study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen Z-Y, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi:10.1126/science.1129663
2. Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry. 2015;20(8):916–930. doi:10.1038/mp.2015.27
3. Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders [published online ahead of print, 2020 Jan 3]. Mol Psychiatry. 2020. doi:10.1038/s41380-019-0639–2
4. Molendijk ML, Spinhoven P, Polak M, et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014;19:791–800.
5. Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011;3:267–277.
6. Kader F, Ghai M, Maharaj L. The effects of DNA methylation on human psychology. Behav Brain Res. 2018;346:47–65.
7. Martinowich K, Hattori D, Wu H, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–893.
8. Keller S, Sarchiapone M, Zarrilli F, et al. Increased BDNF promoter methylation in the wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–267.
9. Chen D, Meng L, Pei F, et al. A review of DNA methylation in depression. J Clin Neurosci. 2017;43:39–46.
10. Kim J-M, Stewart R, Kang H-J, et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J Affect Disord. 2013;149(1–3):93–99. doi:10.1016/j.jad.2013.01.008
11. Kang H-J, Kim J-M, Kim S-Y, et al. A longitudinal study of BDNF promoter methylation and depression in breast cancer. Psychiatry Investig. 2015;12(4):523–531. doi:10.4306/pi.2015.12.4.523
12. Kang H-J, Kim J-M, Bae K-Y, et al. Longitudinal associations between BDNF promoter methylation and late-life depression. Neurobiol Aging. 2015;36(4):e1–1764.e7. doi:10.1016/j.neurobiolaging.2014.12.035
13. Beck AT. Cognitive therapy of depression: new perspectives. In: Clayton PJ, Barett JE, editors. Treatment of Depression. Old Controversies and New Approaches. New York: Raven Press; 1983:pp 265–284.
14. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York: John Wiley & Sons; 1999.
15. Mazure CM, Maciejewski PK, Jacobs SC, Bruce ML. Stressful life events interacting with cognitive/personality styles to predict late-onset major depression. Am J Geriatr Psychiatry. 2002;10(3):297–304. doi:10.1097/00019442-200205000-00009
16. Hammen C, Ellicott A, Gitlin M, Jamison KR. Sociotropy/autonomy and vulnerability to specific life events in patients with unipolar depression and bipolar disorders. J Abnorm Psychol. 1989;98(2):154–160. doi:10.1037/0021-843X.98.2.154
17. Morse JQ, Robins CJ. Personality–life event congruence effects in late-life depression. J Affect Disord. 2005;84(1):25–31. doi:10.1016/j.jad.2004.09.007
18. Shirata T, Suzuki A, Matsumoto Y, et al. Relation of high neuroticism with increased methylation of the BDNF gene. Neuropsychiatr Dis Treat. 2018;14:1787–1793. doi:10.2147/NDT.S169787
19. Ormel J, Jeronimus BF, Kotov R, et al. Neuroticism and common mental disorders: meaning and utility of a complex relationship. Clin Psychol Rev. 2013;33(5):686–697. doi:10.1016/j.cpr.2013.04.003
20. Cervone DC, Pervin LA. Trait theory: the five-factor model; applications and evaluation of trait approaches to personality. In: Personality: Theory and Research.
21. Zuroff DC. Depressive personality styles and the five-factor model of personality. J Pers Assess. 1994;63(3):453–472. doi:10.1207/s15327752jpa6303_5
22. Zuroff DC, Mongrain M, Santor DA. Conceptualizing and measuring personality vulnerability to depression: comment on coyne and whiffen (1995). Psychol Bull. 2004;130(3):489–522. doi:10.1037/0033-2909.130.3.489
23. Otani K, Suzuki A, Kamata M, et al. Parental overprotection increases sociotropy with gender specificity in parents and recipients. J Affect Disord. 2012;136(3):824–827. doi:10.1016/j.jad.2011.09.033
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
25. Braun PR, Han S, Hing B, et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9(1):47.
26. Notaras M. van den Buuse M. Brain-derived neurotrophic factor (bdnf): novel insights into regulation and genetic variation. Neuroscientist. 2019;25(5):434–454.
27. Zheleznyakova GY, Cao H, Schiöth HB. BDNF DNA methylation changes as a biomarker of psychiatric disorders: literature review and open access database analysis. Behav Brain Funct. 2016;12(1):17.
28. Kundakovic M, Gudsnuk K, Herbstman JB, et al. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Nat Acad Sci U S A. 2015;112:6807–6813.
29. Beck AT, Epstein N, Harrison RP, Emery G. Development of the Sociotropy-Autonomy Scale: A Measure of Personality Factors in Psychopathology. Philadelphia: Unpublished manuscript, University of Pennsylvania; 1983.
30. Izawa R. An examination of two hypotheses for depression: personality style-stress interaction and personality style-life event congruence. Jpn J Pers. 1997;6:1–4.
31. Ogawa M. A study on reliability and validity of Japanese version of PBI (parental bonding instrument). Seishinkachiryogaku. 1981;6:1193–1201.
32. Parker G, Tupling H, Brown LB. A parental bonding instrument. Br J Med Psychol. 1979;52:1–10.
33. Schermelleh-Engel K, Moosbrugger H, Muller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res Online. 2003;8:23–74.
34. Otani K, Suzuki A, Matsumoto Y, Shirata T. Marked differences in core beliefs about self and others, between sociotropy and autonomy: personality vulnerabilities in the cognitive model of depression. Neuropsychiatr Dis Treat. 2018;14:863–866.
35. Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909.
36. Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an FMRI study. J Pers Soc Psychol. 2004;87:421–435.
37. Pruessner JC, Baldwin MW, Dedovic K, et al. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28:815–826.
38. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269.
39. Bueller JA, Aftab M, Sen S, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815.
40. Dwivedi Y, Rizavi HS, Conley RR, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815.
41. Flett GL, Hewitt PL, Garshowitz M, Martin TR. Personality, negative social interactions, and depressive symptoms. Can J Behav Sci. 1997;29:28–37.
42. Chen KW, Chen L. Epigenetic regulation of bdnf gene during development and diseases. Int J Mol Sci. 2017;18:571.
43. Notaras M, Du X, Gogos J, van den Buuse M, Hill RA. The BDNF Val66Met polymorphism regulates glucocorticoid-induced corticohippocampal remodeling and behavioral despair. Transl Psychiatry. 2017;7(9):e1233.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.