Back to Journals » Cancer Management and Research » Volume 10
Intermittent androgen deprivation therapy: recommendations to improve the management of patients with prostate cancer following the GRADE approach
Authors Bonfill X , Arevalo-Rodriguez I, Martínez García L , Quintana MJ , Buitrago-García D, Lobos Urbina D, Cordero JA
Received 7 February 2018
Accepted for publication 2 April 2018
Published 2 August 2018 Volume 2018:10 Pages 2357—2367
DOI https://doi.org/10.2147/CMAR.S164856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Xavier Bonfill,1–3 Ingrid Arevalo-Rodriguez,4,5 Laura Martínez García,3 Maria Jesús Quintana,1,2 Diana Buitrago-Garcia,4 Diego Lobos Urbina,6 José Antonio Cordero7
On behalf of the IADT Spanish Study Group
1Department of Clinical Epidemiology and Public Health, Hospital de la Santa Creu i Sant Pau, Institut de Recerca Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; 2CIBER of Epidemiology and Public Health, Barcelona, Spain; 3Iberoamerican Cochrane Centre, Barcelona, Spain; 4Centro de Investigación en Salud Pública y Epidemiología Clínica, Facultad de Ciencias de la Salud Eugenio Espejo, Universidad Tecnológica Equinoccial, Quito, Ecuador; 5Hospital Ramon y Cajal (IRYCIS), Clinical Biostatistics Unit, CIBER of Epidemiology and Public Health, Madrid, Spain; 6Pontificia Universidad Católica de Chile, Santiago, Chile; 7School of Health Sciences Blanquerna – Ramon Llull University, Barcelona, Spain
Purpose: The purpose of this study was to provide evidence-based recommendations of intermittent androgen deprivation therapy (IADT) compared with continuous androgen deprivation therapy (CADT) for men with prostate cancer (PCA).
Methods: We conducted a comprehensive search in MEDLINE, EMBASE, The Cochrane Library, CINAHL, and ECONLIT, from the database inception to December 2017. We adhered to the Grading of Recommendations, Assessment, Development and Evaluation framework to assess the quality of the evidence and to formulate recommendations.
Results: We included one systematic review with 15 trials as well as three additional studies that assessed IADT versus CADT, all of them focused on PCA patients in advanced stages. The findings did not show differences for critical and important outcomes, including adverse events. Trials reported the benefits of IADT in terms of selected domains of health-related quality of life, although with high heterogeneity. Evidence quality was considered moderate or low for most of the assessed outcomes. We identified a patient preference study reporting a high preference for IADT, due to issues related to quality of life, general well-being, and side effects, among others. We did not identify economic studies comparing these regimes. We formulate four recommendations: one no-recommendation, one conditional recommendation, and two good practice points.
Conclusion: For men in early stages of PCA, it is not possible to make any recommendation about the preferable use of IADT or CADT due to the lack of available evidence. For men in advanced stages of the disease, an IADT should be considered as soon as clinically reasonable (weak recommendation and low certainty of the evidence). Clinicians should discuss the risks and benefits of IADT and CADT with their patients, taking into account their values and preferences.
Keywords: hormone deprivation therapy, prostate cancer, prostate neoplasm, evidence-based medicine, GRADE approach
Introduction
Androgen deprivation therapy (ADT) has been used to treat patients with prostate cancer (PCA) since the 1940s. Several options to block testosterone action have been proposed, including chemical castration, achieved using antiandrogens or luteinizing hormone-releasing hormone (LHRH) analogs and antagonists, and surgical castration. Most patients prefer the treatment with LHRH analogs due to the advantages of organ preservation, despite being associated with a wide range of adverse effects related to the duration of treatments.1–10
Intermittent ADT (IADT) has been proposed as a rational strategy to overcome deleterious adverse effects related to the management of these patients while maintaining its benefits. IADT consists in temporarily interrupting the continuous ADT (CADT) when the patient shows no clinical progression of the neoplasm. Continuous monitoring based on prostate-specific antigen (PSA) values and testosterone levels is recommended in order to consider restarting a new IADT cycle. IADT strategy claims other potential advantages such as an improvement in the quality of life (QoL),11 a reduction in the high cost associated with LHRH analogs,12 and a potential delayed onset of drug resistance,13 among others.
Despite the potential role that IADT might have in the hormonal management of PCA and the existing recommendations favoring its use,1,14,15 it continues to be controversial or not well accepted in some settings. One of the reasons may be that recent publication of new and updated evidence on IADT could warrant some changes in previous knowledge. The aim of this study was to update and provide evidence-based recommendations on IADT compared with CADT for patients with PCA by adopting the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework approach.
Materials and methods
This report assessed the following clinical question:
Is intermittent hormone therapy as effective and safe as continuous hormone therapy in men receiving long-term hormonal therapy for PCA? (Supplementary materials).
Information sources and search strategy
For clinical information about the effectiveness and safety of the assessed interventions, we searched MEDLINE (PubMed, from 1966 to October 2017) and EMBASE (OVID, from 1980 to October 2017). We established language restrictions (only publications in English). For information about values and preferences, as well as economic studies, we searched MEDLINE, EMBASE, The Cochrane Library, CINAHL, and ECONLIT, from the database inception to October 2017. The search strategies are available in the Supplementary materials. In addition, we hand-searched the reference lists of the included studies and consulted experts.
Eligibility criteria
Types of studies
We included systematic reviews published during the last 5 years to assess the effectiveness and safety of ADT regimens, and we used these as a source of original studies. We prioritized the inclusion of randomized controlled trials followed by observational studies in order to inform about the risks and benefits of IADT and CADT, as well as to update potential systematic reviews about the effectiveness and safety of IADT versus CADT. In addition, we included studies to inform about patients’ preferences and economic evaluations to inform the economic aspects, if available.
Types of patient profiles
We defined two clinical profiles: 1) patients in early stages and 2) patients in advanced stages (locally advanced, metastatic or recurrent disease).
Types of interventions
We included studies that compared IADT versus CADT.
Types of outcome measures
To assess the effectiveness and safety of ADT regimens, we included studies reporting data for at least one of the following types of outcomes: 1) benefits (overall/specific survival, progression-free survival, and health-related QoL [HRQoL]) and 2) risks (adverse events including hot flashes, gynecomastia, and sexual activity within the previous month or impotence). In addition, we included studies about patients’ preferences (treatment preferences and determinants to choose a treatment) and cost-effectiveness of ADT regimens. We present our results and the quality of evidence per outcome.
Study selection and methodological quality assessment
Two reviewers independently screened titles and abstracts to identify references potentially eligible for inclusion. They obtained full-text copies of potentially eligible references for further assessment. Disagreements were solved by consensus. We used the Epistemonikos database (www.epistemonikos.org/en) to illustrate, in a matrix of evidence, the identified systematic reviews, as well as the trials included in each systematic review.
One reviewer assessed the risk of bias. This process was subjected to quality control by a second reviewer, who checked a random sample of 20% of the included studies. We used the Cochrane risk of bias tool for assessing clinical trials included in this report,16 and the AMSTAR tool for assessing included systematic reviews.17 Quality of evidence was not evaluated for evidence related to values and preferences of patients.
Quality assessment and formulating recommendations
We adhered to the GRADE framework to assess and synthesize available evidence and to formulate recommendations.18 We rated the quality of the evidence per outcome from high to very low, considering the standard GRADE domains (risk of bias, imprecision, inconsistency, indirectness, and publication bias). Finally, we formulated evidence-based recommendations in favor of IADT or CADT according to the clinical characteristics of the patients with PCA.
Updating process
We implemented a continuous surveillance process of new evidence to keep recommendations up to date. We conducted monthly pragmatic searches (Supplementary materials), screened the references, assessed their impact on the recommendations to identify new relevant studies, and modified the recommendations, if necessary.19 We defined relevant references as topic-related references that met the study design criteria but not enough to trigger an immediate update, and potential-key references as references that could potentially trigger an update in the short-term.20
Target users of the recommendations
These recommendations are intended to be considered by PCA specialists, urologists, oncologists, radiation oncologists, and other clinicians involved in the management of these patients.
Results
Study selection
In regard to the risks and benefits of assessed intervention, we identified 38 nonduplicated references related to systematic reviews of randomized controlled trials (RCTs) and excluded 24 of these 38 references after examining their titles and abstracts. We reviewed 14 full texts and excluded 13 references (Supplementary materials). The screening process is summarized in a flow diagram (Figure 1A and B). We included a systematic review with information until March 2014 (Magnan et al).21 This systematic review included information from 15 trials about IADT versus CADT in the management of patients with PCA at any stage. Figure 2 shows the amount of evidence incorporated in Magnan et al in comparison with other systematic reviews about IADT versus CADT (Figure 2).
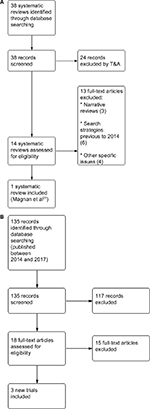  | Figure 1 (A) Study flow diagram: selection of systematic reviews; (B) selection of additional trials (published between 2014 and 2017). Abbreviation: T&A, title and abstract. |
  | Figure 2 Matrix of evidence. Note: Figure created with Epistemonikos (www.Epistemonikos.com). |
In order to update the information provided by Magnan et al,21 we searched for new RCTs published until September 2017 (Figure 1B). We identified 135 nonduplicated references about new RCTs and excluded 117 of them after examining their titles and abstracts. We reviewed 18 full texts and excluded 15 references (Supplementary materials). We included information from three new trials in addition to those identified by Magnan et al.22–24 The justified reference list of included and excluded studies is presented in Supplementary materials.
Likewise, regarding patients’ values and preferences, we identified one study assessing preferences across ADT regimens in PCA patients.25 The flow diagrams for these results are included in Supplementary materials. We did not find any economic studies comparing IADT versus CADT for PCA patients. We present additional information related to general ADT costs in the corresponding section.
Characteristics of included studies
We identified a systematic review of RCTs,21 which provided information from 15 trials that recruited 6856 patients and were published from 2000 to 2013. Included studies had a median of 201 patients per trial; patients had a median age of 70 years, and the most common PCA stage in included trials was metastatic hormone sensitive (six trials).21 The most common ADT regimen was combined androgen blockade (seven trials). Eight of 15 trials had variable duration of treatment periods, and all but one had variable off-treatment periods. Duration of follow-up ranged from 23.2 to 117.6 months.21
Furthermore, we identified three additional trials published from 2014 to 2017.22–24 These additional studies included from 74 to 701 patients; the median age of patients ranged from 72 to 74 years. One study focused on locally advanced PCA patients.22 Median follow-up in these studies ranged from 14 to 48 months.
In regard to values and preferences in PCA patients, one study was identified and included in this report.25 This study included 36 PCA patients (locally advanced, recurrent or metastatic) from a cancer center in Canada; the mean age ranged from 71 to 72 years; patients received a questionnaire focused on a list of factors that they could consider when choosing between IADT and CADT.
Risk of bias assessment
Magnan et al assessed the risk of bias for each primary outcome (overall survival, QoL and the primary outcome of each included trial).21 The authors considered that all trials but one had an unclear or high risk of bias for overall survival and QoL, respectively. Blinding was unclear or not performed in all included trials. Considerable withdrawals (from 21% to 61%) and important loss to follow-up (from 1% to 15%) were reported in some included trials.21 AMSTAR score for Magnan et al was eight of 11 items (Supplementary materials).
In addition, we evaluated the risk of bias of the three additional identified trials (Supplementary materials). These trials were affected by unclear or lack of blinding of participants, personnel, and outcome assessor. Besides, they suffered losses at follow-up or premature ending of the trial due to low accrual.
Risks and benefits of IADT versus CADT
Patients with PCA in an early stage
We did not find RCTs on patients with PCA in early/nonmetastatic stages.
Patients with PCA in locally advanced/metastatic stage
Overall survival
Magnan et al did not find differences between intermittent and continuous therapy in terms of overall survival (eight trials, 5352 patients; hazard ratio (HR) for death =1.02, 95% CI =0.93 to 1.11; I2=23%).21 An additional trial published in 2016 focused on patients with nonmetastatic PCA and reported that 86 men died within 5 years of study entry: 42 in the IADT arm and 44 in the CADT arm, but the difference between these groups was not statistically significant (P=0.969).22 Quality of evidence was downgraded from high to moderate due to issues related to the risk of bias (Table 1).
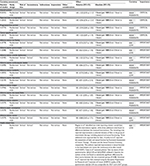  | Table 1 Evidence profile: IADT versus CADT in patients with prostate cancer: subgroup advanced stages Notes: aMultiple trials in Magnan et al21 have unclear or high summary risk of bias. bAn additional trial published in 2016 focusing on patients with nonmetastatic prostate cancer reported that 86 men died within 5 years of study entry: 42 in the IADT arm and 44 in the CADT arm, but the difference between these groups was not statistically significant (P=0.969). cI2=75%. dSchulman et al22 reported no differences between IADT and CADT groups in relation to time to PSA progression (P=0.718) in patients with relapsing M0 or locally advanced prostate cancer. eAn additional trial published in 2016 found no statistically significant differences between CADT and IADT groups in patients with relapsing M0 or locally advanced prostate cancer (43 vs 41 events; P=0.865). An additional trial published in 2017 reported the number of patients with disease progression (defined as PSA≥ 4 ng/mL biochemical failure [BF] and/or metastases); the authors found three patients with disease progression in the intermittent group versus zero patients in the continuous arm after follow-up, and they concluded that there were no significant differences between the assessed groups related to this outcome. fI2=93%; gI2=91%; hI2=80%; iI2=78%; jI2=73%; kThere was a considerable range of instruments and high variability in the report of quantitative data for this outcome, as well as different schedules for patient assessment. Abbreviations: CADT, continuous androgen deprivation therapy; CI, confidence interval; HR, hazard ratio; IDAT, intermittent androgen deprivation therapy; PSA, prostate-specific antigen; QLQ, quality of life questionnaire; QoL, quality of life; RR, risk ratio. |
Progression-free survival
Magnan et al found 12 trials reporting disease progression in two ways. First, five trials reported time to progression, the analyses of which did not show differences between intermittent and continuous ADT regimens (five trials, 3523 patients; HR for time to progression =0.96, 95% CI =0.76 to 1.21; I2=75%).21 Likewise, Schulman et al reported no differences between IADT versus CADT groups related to time to PSA progression (P=0.718) in patients with relapsing M0 or locally advanced PCA.22 Quality of evidence was downgraded from high to low due to issues related to risk of bias and inconsistency (Table 1).
In addition, four trials reported progression-free survival, and their respective analyses did not show differences between assessed regimens either (four trials, 1774 patients; HR for time to progression-free survival =0.94, 95% CI =0.84 to 1.05; I2=0%).21 An additional trial published in 2016 did not find statistically significant differences between CADT and IADT groups in patients with relapsing M0 or locally advanced PCA (43 versus 41 events; P=0.865).22 Another trial published in 2017 reported the number of patients with disease progression (defined as PSA ≥4 ng/mL and/or metastases);24 the authors found three patients with disease progression in the intermittent group versus zero patients in the continuous arm after follow-up, but they concluded that there were no significant differences between assessed groups related to this outcome.24 Quality of evidence was downgraded from high to moderate due to issues related to the risk of bias (Table 1).
Cancer-specific survival
Magnan et al did not find differences between intermittent and continuous therapy in terms of cancer-specific survival (five trials, 3613 patients; HR for cancer-specific survival =1.02, 95% CI =0.87 to 1.19; I2=4%).21 Quality of evidence was downgraded from high to moderate due to issues related to the risk of bias (Table 1).
Adverse events
Magnan et al found 12 of 15 trials reporting data about drug-related adverse effects in terms of number of patients who experienced adverse events at least once during the follow-up period. Patients receiving IADT experienced less adverse fewer in comparison to those receiving CADT, although the differences were not statistically significant. The adverse effects included: hot flashes (six studies, 3778 participants; risk ratio (RR) =0.76, 95% CI =0.57 to 1.00; I2=93%), gynecomastia (five studies, 3588 participants; RR =0.63, 95% CI=0.36 to 1.10; I2=91%), erectile dysfunction (four studies, 2182 participants; RR =1.03, 95% CI =0.74 to 1.43; I2=80%), cardiovascular deaths (four studies, 3490 participants; RR =0.86, 95% CI =0.73 to 1.02; I2=0%), headache (four studies, 3025 participants; RR =0.70, 95% CI =0.48 to 1.02; I2=78%), depression (three studies, 2139 participants; RR =0.91, 95% CI =0.39 to 2.13; I2=56%), fatigue (two studies, 1946 participants; RR =0.94, 95% CI =0.60 to 1.48; I2=24%), decreased libido (two studies, 1946 participants; RR =1.01, 95% CI =0.95 to 1.07; I2=0%), dyspnea (two studies, 1579 participants; RR =0.82, 95% CI =0.44 to 1.54; I2=53%), constipation (two studies, 1579 participants; RR =0.71, 95% CI =0.35 to 1.42; I2=65%), and nausea (two studies, 1579 participants; RR =0.88, 95% CI =0.45 to 1.71; I2=73%).21 Two additional trials reported information about selected adverse events.22,23 This information is shown in Table 2. Quality of evidence was downgraded from high to moderate or low due to issues related to risk of bias and inconsistency (Table 1).
HRQoL
Magnan et al detected high heterogeneity in the assessment of QoL provided in 12 of the 15 trials included in this review.21 They found a considerable range of instruments and high variability in the reporting of quantitative data for this outcome, as well as different schedules for patient assessment. Nine trials used a version of the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ)-C30, while the remaining studies used other assessment tools. The authors identified two trials reporting a better overall QoL with intermittent regimen, while three additional trials found no differences between the assessed interventions.21 The remaining trials reported improvement in selected domains of QoL in the group of intermittent therapy, including physical and sexual functioning.
Three additional trials reported data for QoL. Crawford et al reported the assessment of QoL and sexual function by Functional Assessment of Cancer Therapy – Prostate and the Sexual Function Inventory, respectively.26 The authors reported improvements in sexual function in the intermittent arm versus the continuous arms after month 14 (P=0.027). Casas et al assessed patients’ QoL by means of two questionnaires: QLQ-C30 and QLQ-PR25 validated in Spanish;24 the authors of this trial found no statistically significant differences for these scores between the two treatment groups (P=0.08). Schulman et al reported that QoL assessed using the EORTC QLQ-C30 was comparable for IADT and CADT groups.22 Quality of evidence was downgraded from high to low due to issues related to risk of bias and inconsistency (Table 1).
Patients’ preferences
We identified one study regarding values and preferences of PCA patients receiving ADT. Chun-Leung Chau et al assessed the preferences about ADT regimens and reasons for their choice in 36 PCA patients with locally advanced, recurrent and metastatic stages.25 The authors developed a questionnaire including a trade-off table between IADT and CADT to help patients reflect the reasons taken into consideration when choosing the ADT regimen. Thirty-six patients were enrolled in this pilot study in 2014, and 32 patients chose IADT as their preferred ADT regimen. The most important reasons for deciding on intermittent ADT were QoL, chances of the cancer returning, general well-being, overall life span, and side effects (including hot flashes and physical function).25 Patients preferring CADT prioritized overall life span and cancer recurrence as well as risk of death. The study had some limitations due to patient age (>65 years) as well as due to a high prevalence of patients sexually inactive (69%).25
Cost-effectiveness
No economic evaluations comparing IADT versus CADT in patients with PCA were identified.
Formulation of recommendations
Based on the previous information, we have elaborated and propose the following recommendations to guide the administration of ADT in PCA patients:
- No recommendation is provided against or in favor of a specific ADT regimen for PCA patients in early stages of their condition.
Summary and interpretation of the evidence: We did not identify controlled clinical trials in the subgroup of patients with PCA in early stages. Specific evidence-based information is needed to provide recommendations for these patients.
- In men with PCA in advanced stages (including locally advanced and metastatic), we suggest considering an intermittent regimen in the provision of long-term ADT as soon as clinically reasonable (weak recommendation and low certainty of the evidence). When considering the prescription of long-term deprivation therapy, clinicians should discuss the alternative regimens with their patients, taking into account individual factors such as:
- Trade-off balance between risks and benefits of ADT regimens;
- Expectations about QoL and general well-being;
- Values and preferences about death and cancer returning;
- Values and preferences about the frequency of treatment and hospital visits; and
- Availability and access to hospital resources and indirect costs related to ADT.
Summary and interpretation of the evidence: We identified a significant number of trials comparing IADT versus CADT in PCA patients in advanced stages. There were no differences in most of the critical and important outcomes assessed, including overall survival, progression-free survival, and adverse events, among others. Narrative, but contradictory, reports about the benefits of IADT were found for selected scales evaluating HRQoL. Evidence was classified as low or moderate in most cases due to issues related to risk of bias as well as to inconsistency of results. We identified no cost-effectiveness studies comparing IADT versus CADT in these patients. In addition, we identified a survey study assessing values and preferences for selecting ADT regimens in PCA patients; findings suggest that a considerable number of patients can choose IADT as the preferred ADT regimen due to issues related to QoL, general well-being, and side effects, among others.
The authors consider that the decision about the selection of a specific regimen has to include other criteria, such as the use of resources and values and preferences of patients. Despite finding no economic studies comparing these ADT alternatives, we believe that the reduction of the doses and the frequency of hospital visits could have an important economic impact for patients, especially in those whose health insurances or social security systems do not include these therapies. We consider that clinicians should discuss the available alternatives with each patient in order to consider all individual-related factors, values, beliefs, and preferences.
Updating process
We have conducted three cycles of the updating process (from September 2017 to January 2018). During the surveillance period, we reviewed 71 additional references, we identified cero-relevant references, and we identified no key reference (those that could potentially trigger an update).
Discussion
Uncertainties remain regarding the appropriate use of IADT in the management of PCA and its potential role in relation to CADT. In order to generate recommendations on IADT versus CADT in patients with PCA, we analyzed and used information about risks and benefits of these regimens from one systematic review containing 15 trials and three additional trials published after 2014. In addition, we searched for studies focused on values and preferences, and economic studies. In general, we found no differences in most of the critical and important assessed outcomes, including overall survival, progression-free survival, and adverse events, among others. Narrative but contradictory reports about benefits of IADT were found for selected scales evaluating HRQoL. Evidence was classified as low or moderate in most cases, due to issues related to risk of bias as well as inconsistency of results. We identified a survey assessing values and preferences for selecting ADT regimens in PCA patients; findings suggest that a considerable number of patients may consider IADT as the preferred ADT regimen, due to QoL-related issues, general well-being, and side effects, among others. In addition, we identified no cost-effectiveness studies comparing IADT versus CADT in these patients.
Administration and prescription of ADT should be guided by a decision-making process based on evidence of the best possible quality. Bultijnck et al assessed the implementation of evidence-based recommendations for the management of ADT in daily practice of clinicians in Europe.27 The authors included information of 489 clinicians working in a multidisciplinary oncologic team; over 70% of clinicians administered LHRH agonist with or without an antiandrogen, especially in the palliative metastatic settings. Likewise, over 70% of physicians reported to apply at least one evidence-based strategy for preventing and managing ADT-related side effects. The authors evaluated recommendations related to the management of erectile and sexual dysfunction, and they found that only 25% of clinicians provided an evidence-based strategy for this issue as a first-line management.27 Regarding the administration of ADT, Liede et al assessed which factors were related to physician’s ADT prescription for patients with nonmetastatic PCA.28 The authors surveyed 441 urologists/oncologists from 19 countries, with at least 10 nonmetastatic PCA patients managed per month, during 2012. Physicians reported that around 38% of nonmetastatic patients received ADT, with 36% of them receiving gonadotropin-releasing hormone agents; CADT was prescribed to 54% of PCA patients. The decision for prescribing CADT was related to PSA levels, Gleason score, and treatment guidelines, whereas the administration of IADT was guided by PSA levels, patient request, desire to maintain sexual function, the presence of comorbidities, and patient age.28 In addition, Hurwitz et al assessed the factors involved in the treatment decision-making for PCA in 925 newly diagnosed PCA patients between 2006 and 2014.29 The authors found that >60% of patients preferred an active role in the decision about their PCA treatment.
Our review has several strengths. First, we exhaustively searched, identified, and assessed published systematic reviews about ADT regimens in PCA, which gathered up-to-date evidence until 2017. Second, we adhered to the GRADE framework to formulate the recommendations according to patient profiles,18 which has allowed us to consider all the important elements needed to guide clinical decisions. In addition, we used Epistemonikos, a new database that provides a comprehensive overview of the evidence, in order to illustrate the amount of evidence about IADT versus CADT in terms of systematic reviews and controlled clinical trials. Finally, we implemented a continuous surveillance process for new evidence to keep recommendations up-to-date.19 As potential limitations, we identified no clinical trials for patients in early stages of PCA. Furthermore, we found no cost-effectiveness studies comparing ADT regimens and testing the hypothesis that IADT could save direct and indirect costs. Finally, we had to formulate weak recommendations due to the low quality of the available evidence, as well as the lack of studies on patients’ values and preferences when choosing between IADT and CADT. We will consider updating the recommendations in light of new evidence.
Conclusion
In conclusion, for men in early stages of PCA, it is not possible to make any recommendation about the preferable use of IADT or CADT due to the lack of available evidence. For men in advanced stages of the disease, we suggest considering an intermittent regimen of ADT as soon as clinically reasonable (weak recommendation and low certainty of the evidence). Clinicians should always discuss risks and benefits of IADT and CADT with their patients, taking into account their values and preferences in relation to therapeutic regimens, QoL issues, the frequency of visits, and monitoring, as well as costs.
Acknowledgments
This study has been funded by Instituto de Salud Carlos III through the project “PI15/00886” (cofunded by European Regional Development Fund/European Social Fund, “Investing in your future”). The authors would like to acknowledge the IADT Spanish Study Group: Marcos Agustín Acha (Hospital Universitario Cruces, Barakaldo, Spain); Xavier Bonfill (Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Spain, and CIBER of Epidemiology and Public Health [CIBERESP], Spain); Josep Anton Cordero (Blanquerna School of Health Science, Universitat Ramon Llull, Barcelona, Spain); José Manuel Cozar (Hospital Universitario Virgen de las Nieves, Granada, Spain); José Ignacio Emparanza (Hospital Universitario Donostia, BioDonostia, San Sebastian, Spain, and CIBERESP, Spain); Albert Frances (Hospital del Mar, Parc de Salut Mar, Barcelona, Spain); Josep M Gaya (Fundació Puigvert, Barcelona, Spain); David Hernández (Hospital Regional de Málaga, Málaga, Spain); Bernardo Herrera-Imbroda (Hospital Universitario Virgen de la Victoria, Málaga, Spain); Asunción del Carmen Hervas (Hospital Ramón y Cajal, Madrid, Spain); Emilio Julve (Hospital Universitario Virgen de la Victoria, Málaga, Spain); Emilio Marqués (Hospital General Universitario de Valencia, Valencia, Spain); María Manuela Morales-Suárez-Varela (University of Valencia, Valencia, Spain, CIBERESP, Spain, and Hospital Universitario Virgen de la Victoria, Málaga, Spain); Dimelza Osorio (Hospital Universitari Vall d’Hebron, Institut de Recerca [VHIR], Barcelona, Spain); Joan Palou (Fundació Puigvert, Barcelona, Spain); M Jesús Quintana (Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Spain, and CIBERESP, Spain); Elena Rodríguez (Hospital Universitario Cruces, Barakaldo, Spain); Begoña San José Ruiz (Hospital Universitario Cruces, Barakaldo, Spain); Rita Sainz de Rosas (Hospital Universitario Cruces, Barakaldo, Spain); Javier Sánchez (Hospital Regional de Málaga, Málaga, Spain); Gemma Sancho (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain); Juan Pablo Sanz (Hospital Universitario Donostia, San Sebastian, Spain); Daysi Yoe-Ling Chang (Escuela Andaluza de Salud Pública, Granada, Spain).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Carroll PR, Parsons JK, Andriole G, et al. NCCN Clinical Practice Guidelines Prostate Cancer Early Detection, Version 2.2015. J Natl Compr Canc Netw. 2015;13(12):1534–1561. | ||
Pagliarulo V, Bracarda S, Eisenberger MA, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012;61(1):11–25. | ||
Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol. 2005;7(Suppl 5):S37–S43. | ||
Giacalone A, Quitadamo D, Zanet E, Berretta M, Spina M, Tirelli U. Cancer-related fatigue in the elderly. Support Care Cancer. 2013;21(10):2899–2911. | ||
Bourke L, Kirkbride P, Hooper R, Rosario AJ, Chico TJ, Rosario DJ. Endocrine therapy in prostate cancer: time for reappraisal of risks, benefits and cost-effectiveness? Br J Cancer. 2013;108(1):9–13. | ||
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166(4):465–471. | ||
Grossmann M, Zajac JD. Hematological changes during androgen deprivation therapy. Asian J Androl. 2012;14(2):187–192. | ||
Jespersen CG, Norgaard M, Borre M. Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population-based cohort study. Eur Urol. 2014;65(4):704–709. | ||
Langley RE, Cafferty FH, Alhasso AA, et al. Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09). Lancet Oncol. 2013;14(4):306–316. | ||
Krahn MD, Bremner KE, Luo J, Alibhai SM. Health care costs for prostate cancer patients receiving androgen deprivation therapy: treatment and adverse events. Curr Oncol. 2014;21(3):e457–e465. | ||
Tunn UW, Canepa G, Kochanowsky A, Kienle E. Testosterone recovery in the off-treatment time in prostate cancer patients undergoing intermittent androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2012;15(3):296–302. | ||
Rosario DJ, Bourke L. Reply: Endocrine therapy in prostate cancer: time for reappraisal of risks, benefits and cost-effectiveness? Br J Cancer. 2013;108(10):2194. | ||
Bruchovsky N, Rennie PS, Coldman AJ, Goldenberg SL, To M, Lawson D. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50(8):2275–2282. | ||
Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–757. | ||
Horwich A, Hugosson J, de Reijke T, et al. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24(5):1141–1162. | ||
Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. Accessed October 1, 2017. | ||
Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. | ||
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. | ||
Martinez Garcia L, Sanabria AJ, Araya I, et al. Efficiency of pragmatic search strategies to update clinical guidelines recommendations. BMC Med Res Methodol. 2015;15:57. | ||
Martinez Garcia L, Sanabria AJ, Garcia Alvarez E, et al. The validity of recommendations from clinical guidelines: a survival analysis. CMAJ. 2014;186(16):1211–1219. | ||
Magnan S, Zarychanski R, Pilote L, et al. Intermittent vs continuous androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. JAMA Oncol. 2015;1(9):1261–1269. | ||
Schulman C, Cornel E, Matveev V, et al. Intermittent versus continuous androgen deprivation therapy in patients with relapsing or locally advanced prostate cancer: a phase 3b randomised study (Iceland). Eur Urol. 2016;69(4):720–727. | ||
Crawford D, Shore N, Higano CS, Neijber A, Yankov V. Intermittent androgen deprivation with the gonadotrophin releasing hormone antagonist degarelix. J Urol. 2014;191(4 Suppl. 1):e766. | ||
Casas F, Henriquez I, Bejar A, et al. Intermittent versus continuous androgen deprivation therapy to biochemical recurrence after external beam radiotherapy: a phase 3 GICOR study. Clin Transl Oncol. 2017;19(3):373–378. | ||
Chun-Leung Chau D, Wang D, Tedesco A, et al. Prostate cancer patients’ preferences for intermittent vs. continuous androgen deprivation—a pilot institutional study. J Med Imaging Radiat Sci. 2016;47(1):108–112.e102. | ||
Crawford D, Shore N, Higano CS, Neijber A, Yankov V. Intermittent androgen deprivation with the GnRH antagonist degarelix in men with biochemical relapse of prostate cancer. Am J Hematol Oncol. 2015;11(12):6–13. | ||
Bultijnck R, Surcel C, Ploussard G, et al. Practice patterns compared with evidence-based strategies for the management of androgen deprivation therapy–induced side effects in prostate cancer patients: results of a European web-based survey. Eur Urol Focus. 2016;2(5):514–521. | ||
Liede A, Hallett DC, Hope K, Graham A, Arellano J, Shahinian VB. International survey of androgen deprivation therapy (ADT) for non-metastatic prostate cancer in 19 countries. ESMO Open. 2016;1(2):e000040. | ||
Hurwitz LM, Cullen J, Elsamanoudi S, et al. A prospective cohort study of treatment decision-making for prostate cancer following participation in a multidisciplinary clinic. Urol Oncol. 2016;34(5):233.e17–25. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

