Back to Journals » International Medical Case Reports Journal » Volume 15
Intermammary Hidradenitis Suppurativa: A Case Report
Authors Dharmadji HP, Arianto TR , Sugiri U , Sutedja EK , Achdiat PA , Tsaqilah L , Gunawan H
Received 14 January 2022
Accepted for publication 25 March 2022
Published 11 April 2022 Volume 2022:15 Pages 163—167
DOI https://doi.org/10.2147/IMCRJ.S358514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Hartati Purbo Dharmadji, Tiara Rachmaputeri Arianto, Unwati Sugiri, Eva Krishna Sutedja, Pati Aji Achdiat, Laila Tsaqilah, Hendra Gunawan
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Hartati Purbo Dharmadji, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr Hasan Sadikin General Hospital, Jl. Pasteur No. 38, Bandung, 40161, West Java, Indonesia, Tel +62 222032426 ext. 3449, Fax +62 222032426, Email [email protected]
Abstract: Hidradenitis suppurativa (HS) is a chronic relapsing inflammatory skin disease clinically characterized by recurrent, deep-seated, painful, subcutaneous nodules, sinus tracts, and hypertrophic scarring. This disease affects hair follicles in the apocrine gland-bearing areas such as the axillae, buttocks, as well as the genital and perineal areas. In women, the predilection sites of HS include the anterior part of the body, most commonly on the breasts, inframammary area, and groin. Intermammary location possibly represents a rare phenotype presentation. A case of intermammary HS in a 24-year-old woman was reported. The patient is overweight and is a smoker. Frequent friction and excessive sweating between big breasts were admitted. Chronic painful nodules accompanied by sinuses and scars between the breasts were found. Histopathological examination from a skin lesion on the intermammary area showed follicular occlusion, follicular hyperkeratosis, and destroyed hair follicles. There were also apocrine glands and hair follicles surrounded by massive neutrophils, lymphocytes, and histiocytes, suggestive of HS. The patient was given rifampicin and clindamycin. Clinical improvements were observed on the third week of observation. Typical lesion distribution is one of the criteria for establishing a definite diagnosis of HS. If the lesion is on an atypical site, it must be accompanied by at least one on the typical site. However, the predilection sites of HS often varied. The intermammary area is an intertriginous area, and mechanical friction, follicular occlusion, and rupture of the dilated follicles may contribute to the development of HS in the area. The existence of a typical lesion in an atypical distribution, without any lesion present in typical predilection sites, cannot rule out diagnostic consideration of HS. Therefore, clinicians should be aware and consider the diagnosis of HS even when the required criteria of HS are not fulfilled.
Keywords: hidradenitis suppurativa, intermammary
Introduction
Hidradenitis suppurativa (HS), otherwise known as acne inversa, is a multifactorial, chronic inflammatory disorder of the hair follicles in the apocrine gland-bearing areas.1,2 Robust epidemiologic data on HS is lacking. The reported point prevalence of HS worldwide is between 0.00033% and 4.1%.1 Women are more commonly affected, with a male-to-female ratio ranging from 1:2.7 to 1:3.3.3 To date, the definite diagnosis of HS is based on three clinical criteria. First, one or more typical lesions must be present, including deep-seated painful nodules, abscesses, draining sinuses, double-open comedones, and/or bridged scars. Second, lesions must be located on the typical distribution areas, such as the axillae, groin, buttocks, as well as the perineal or inframammary areas. Atypical or ectopic sites may be affected, but at least one typical area must be involved. Third, there must be a clear history of symptom chronicity and recurrence. Having two recurrences over a six-month period has been suggested as one measure of chronicity.1,4 The primary site of HS involve the intertriginous areas. Although the intermammary area is considered intertriginous, this location is not usually specified within present classifications.5,6 The use of histopathology and a non-invasive imaging assessment such as ultrasound can be beneficial to confirm the possibility of HS.1,7 This case report aimed to present a case of HS occurring only on an atypical distribution area, namely the intermammary area.
Case
A 24-year-old woman presented with a painful lump between the breasts, accompanied by scars and channels that leak pus and blood within 1 year. The lesions first appeared 2 years ago as erythematous macules and papules on both breasts and the intermammary area without pain or itch. Some of the erythematous papules then became painful nodules. The nodules eventually became abscess-like lesions, ruptured, and leaked pus 1 year later. The patient went to a district hospital and was given oral antibiotics. The pus disappeared, and the lesion became less painful. Hypertrophic scars between the breasts with sinuses and purulent discharge were formed 1 year later. Some of the painful nodules between the breasts also reappeared. History of frequent smoking since teenage years, being overweight, and frequent sweating between the breasts were admitted. The patient denied any family history of the same complaints.
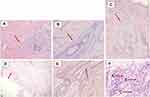
Clinical examination of the lesions revealed painful erythematous nodules, hypertrophic scars, and multiple sinuses with purulent discharge, erosions, hyperpigmented macules, and papules on both breasts as well as between the breasts (Figure 1). The sondage test on the intermammary area was positive (Figure 2), indicating a positive sinus tract formation. Histopathological examination from skin biopsy on the intermammary area revealed follicular occlusion, follicular hyperkeratosis, destroyed hair follicles, as well as apocrine glands and hair follicles surrounded by massive inflammatory cells, including neutrophils, lymphocytes, and histiocytes (Figure 3). This histopathological finding supported the diagnosis of HS. Once-daily 600 mg rifampicin and twice-daily 300 mg clindamycin were given to the patient for 10 weeks. The skin lesion improved on the third week of observation.
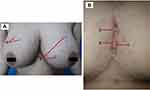 |
Figure 1 Clinical image of skin lesions (A). Macules (A) and papules (B) on the breasts. (B) Hypertrophic scars (A), nodules (B), and sinuses (C) on the intermammary area. |
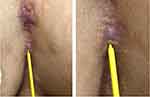 |
Figure 2 Positive sondage test on the intermammary area. |
Discussion
Three criteria are currently used to establish the diagnosis of HS, which are typical lesions, typical distributions, and chronicity, along with recurrence.8 Despite the fact that the three criteria must be included for a definite diagnosis of HS, this disease has an extremely heterogeneous clinical presentation in terms of both lesion appearance and sites of involvement.9 A recent study by Guillem et al stated that since 2006, there had been 89 cases of intermammary HS in their center, but only 6 cases present only on the intermammary area.10 In this case report, chronic recurrent painful nodules with sinus tracts and hypertrophic scars were part of the clinical manifestations. However, the HS lesion was present on a non-predilection area, with no typical areas involved, necessitating further examination to establish the diagnosis.
Histopathological examination can be used to support the diagnosis of HS. Follicular occlusion is a non-specific but universal histopathological finding. Early lesions in HS are characterized by follicular hyperkeratosis of the terminal hair follicles, hyperplasia of the follicular infundibulum, and perifolliculitis.1 There are heavy mixed inflammatory cell infiltrates in the lower half of the dermis, usually extending into the subcutis, consisting of neutrophils, lymphocytes, and histiocytes.2,11 In chronic disease, there may be extensive fibrosis with the destruction of pilosebaceous follicles and sweat glands.11 Although the diagnosis for HS is based on clinical findings, in addition to histopathological examination, adding ultrasound is beneficial to discover the subclinical pathology that can be important for management considerations.7 Combination therapy of oral clindamycin (300 mg twice daily) and rifampicin (600 mg once daily) proved to have excellent efficacy and yielded prolonged remission,4 although relapse often occurs.1 In this patient, follicular occlusion, follicular hyperkeratosis, destroyed hair follicles, and apocrine glands along with hair follicles surrounded by massive neutrophils, lymphocytes, and histiocytes were identified in the histopathological examination. Except for typical distribution, clinical manifestations and histopathological characteristics of HS were all found in this patient. Therefore, ultrasound was not performed. A combination of rifampicin and clindamycin for the treatment of HS was given, and the patient exhibited improvements. The patient has been informed about the possibility of relapse; thus, careful observation is needed.
The etiology of HS is multifactorial and remains to be fully elucidated.4 Individuals with larger skin folds, such as in overweight persons, can cause the occurrence of skin-to-skin contact and enhance mechanical friction. This condition contributes to the development of HS by promoting follicular occlusion and triggering the rupture of dilated follicles in genetically susceptible individuals.12 Follicular clogging, subsequent rupture, and release of follicular content into the surrounding tissue trigger an inflammatory response, resulting in abscess and sinus tract formation.13 The higher-than-usual skin temperature and humid microclimate on the skin fold also favor bacterial growth.12 Bacterial infection has long been implicated in the secondary pathogenesis of HS, as bacterial colonization leads to a series of pathogen-associated molecular pathways, which may contribute to perpetuating and exacerbating local inflammation.12,14 These bacteria also play a role in increasing the severity of the disease; thus, bacterial superinfection of established lesions contributes to maintaining the chronicity of inflammation.15 Big breasts aggravate the occlusion and friction forces already promoted in this area, favoring progression to fistulae and explaining the predominant intermammary disease in women.5 Koebner phenomenon (KP) seems to play a role in HS due to the mechanical stress and may explain the unique distribution of this disease. However, experimental data on the role of KP has not been made available.16 Smoking is also associated with the diagnosis of HS. Nicotine may promote follicular occlusion by increasing sweat gland secretion and inducing hyperplasia of the follicular infundibulum. Smoking cessation may improve symptoms and reduce the recurrence rate after treatment.1
In this case report, the patient complained of repetitive friction and excessive sweat between her breasts. This patient had no distance between her left and right breast, which were considered big breasts. The patient’s body mass index was 26 kilograms per square meter, indicating overweight. A Gram staining examination taken from the pus in the intermammary area was also performed, and revealed Gram-positive cocci bacteria. The patient also had a history of smoking two packs per day since her teenage years. These conditions may induce mechanical friction and favor the development of HS. Although the typical distribution of HS was not found, based on the patient’s history, clinical manifestations, onset, and histopathological examination, this patient was diagnosed with HS.
Conclusion
Typical distribution is one of the criteria for establishing a definite diagnosis of HS. Areas of typical distribution consist of axillae, groin, buttocks, as well as the perineal and inframammary areas. Atypical sites may be affected, but at least one typical area must be involved. The predilection sites of HS varied; it can occur in any intertriginous area on one or more locations, including the intermammary, which is considered an atypical distribution. The existence of a typical lesion in an atypical distribution, without any lesion present in typical predilection sites, apparently cannot rule out the diagnosis of HS, as was seen in this case. However, a careful follow-up is required to identify subsequent lesions in more classical sites. In conclusion, clinicians should be aware and consider HS as a diagnosis, even when the required criteria are not fulfilled, in order to establish an early diagnosis and initiate prompt medical treatment.
Ethic Statement
The publications of images were included in the patient’s consent for publication of the case. The case report has been approved by the institutional ethics committee of Dr. Hasan Sadikin General Hospital, Bandung, Indonesia (Ethical Clearance No.: LB.02.01/x.6.5/28/2022).
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case details and images.
Acknowledgments
The authors would like to thank the staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Okoye GA. Hidradenitis suppurativa. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, editors. Fitzpatrick’s Dermatology.
2. Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60(4):539–561. doi:10.1016/j.jaad.2008.11.911
3. Zouboulis CC, Del MV, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatol. 2015;231(2):184–190. doi:10.1159/000431175
4. Lee EY, Alhusayen R, Lansang P, Shear N, Yeung J. What is hidradenitis suppurativa? Canadian Fam Phys. 2017;63(2):114–120.
5. João AL, Cunha N, Cabete J. Intermammary hidradenitis suppurativa: considerations on a unique presentation. Skin Appendage Disord. 2021;7(3):284–287. doi:10.1159/000514363
6. Ingram JR, Collier F, Brown D, et al. British association of dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019;180(5):1009–1017. doi:10.1111/bjd.17537
7. Wortsman X, Moreno C, Soto R, Arellano J, Pezo C, Wortsman J. Ultrasound in‐depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39(12):1835–1842. doi:10.1111/dsu.12329
8. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11(10):17.
9. Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Investig Dermatol. 2013;133(6):1506–1511. doi:10.1038/jid.2012.472
10. Guillem P, Vlaeminck-Guillem V. Intermammary hidradenitis suppurativa. Skin Appendage Disord. 2022;2022:1–2.
11. Hsiao JL, McMichael AJ, Curtis AR, Guzman-Sanchez D. Folliculitis and other follicular disorders. In: Bolognia JL, Schaffer JV, Cerroni L, editors. Dermatology.
12. Fabbrocini G, De VV, Donnarumma M, Russo G, Monfrecola G. South Italy: a privileged perspective to understand the relationship between hidradenitis suppurativa and overweight/obesity. Skin Appendage Disord. 2016;2(1–2):52–56. doi:10.1159/000447716
13. Zouboulis CC, Benhadou F, Byrd AS, et al. What causes hidradenitis suppurativa?—15 years after. Exp Dermatol. 2020;29(12):1154–1170. doi:10.1111/exd.14214
14. Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000 Med Rep. 2014;6:15.
15. Benzecry V, Grancini A, Guanziroli E, et al. Hidradenitis suppurativa/acne inversa: a prospective bacteriological study of 46 patients and review of the literature. G Ital Dermatol Venereol. 2018;155:459–463.
16. Boer J, Jemec GB. Mechanical forces and hidradenitis suppurativa. Exp Dermatol. 2021;30(2):212–215. doi:10.1111/exd.14234
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.