Back to Journals » Clinical Ophthalmology » Volume 18
Interleukin-8 Promoter Polymorphism −251 A/T and Treatment Response in Neovascular Age-related Macular Degeneration
Authors Thomsen AK , Krogh Nielsen M, Liisborg C, Sørensen TL
Received 9 November 2023
Accepted for publication 11 January 2024
Published 21 February 2024 Volume 2024:18 Pages 537—543
DOI https://doi.org/10.2147/OPTH.S448794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alexander Kai Thomsen,1,2,* Marie Krogh Nielsen,1,* Charlotte Liisborg,1 Torben Lykke Sørensen1,2
1Clinical Eye Research Division, Department of Ophthalmology, Zealand University Hospital, Roskilde, Denmark; 2Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
*These authors contributed equally to this work
Correspondence: Alexander Kai Thomsen, Department of Ophthalmology, Zealand University Hospital, Sygehusvej 10, Roskilde, 4000, Denmark, Tel +45 26369327, Fax +45 46321055, Email [email protected]
Purpose: Interleukin-8 (IL-8) is a potent pro-angiogenic and pro-inflammatory chemokine, suggested to hold a role in neovascular age-related macular degeneration (nAMD). Our aim is to study the association of the single-nucleotide polymorphism − 251 A/T (rs4073) in the IL-8 promoter region with the treatment response to intravitreal anti-vascular endothelial growth factor (VEGF) injections in nAMD.
Patients and Methods: This is a prospective study of treatment-naïve patients with nAMD. Treatment response after a loading dose of three intravitreal anti-VEGF injections was defined as functional response based on change in visual acuity, and morphological response based on change in central retinal thickness (CRT) and intraretinal fluid on optical coherence tomography. Morphological response was categorized in good, partial, and poor responders. Blood DNA was analyzed for − 251 A/T genotype.
Results: The IL-8 promoter polymorphism − 251 A/T was not significantly associated to functional treatment response (P=0.09). No significant association was found between genotype and morphological treatment response (P=0.799). Older age was significantly associated to good morphological responders compared to partial and poor responders (P=0.014).
Conclusion: The IL-8 polymorphism − 251 A/T is not associated to morphological nor functional treatment response to intravitreal anti-VEGF injections in patients with nAMD.
Keywords: anti-VEGF, IL-8, rs4073, single-nucleotide polymorphism, treatment response, age-related macular degeneration
Introduction
The pathogenesis of age-related macular degeneration (AMD) is largely unknown. This retinal disease is the leading cause of visual impairment in the Western world,1 with the late stage neovascular AMD (nAMD) accounting for the majority of cases of severe visual loss.2
Vascular endothelial growth factor (VEGF) is a key driver of neovascularization in nAMD and treatment with intravitreal anti-VEGF injections has proven to inhibit the retinal pro-angiogenic environment.3 Treatment with anti-VEGF has significantly reduced the rates of disability related to nAMD,4 but although anti-VEGF is an efficient treatment option, many patients respond insufficiently to treatment.3,5
Chronic low-grade inflammation plays a critical role in the development of AMD, evident by elevated levels of systemic biomarkers for chronic inflammation such as interleukin-6,6,7 C-reactive protein,8 chemokines,6,9 CD11b and CD200 on monocytes.10
Interleukin-8 (IL-8) is a pro-inflammatory and pro-angiogenic chemokine expressed by vascular endothelium and retinal pigment epithelial cells.11,12 The single-nucleotide polymorphism (SNP) −251 A/T (rs4073) is located in the promotor region of the IL-8 gene. The −251 A allele is associated to higher levels of systemic IL-8.13,14 Previous studies have shown conflicting results regarding the association between IL-8 −251 AA genotype and the development of nAMD,15,16 while others have suggested an association to the onset of nAMD.17 Furthermore IL-8 −251 A/T has been suggested to affect the response to anti-VEGF treatment in nAMD. Patients harboring the risk-associated allele A have shown a diminished morphological treatment response after loading dose,12 while the homozygous AA genotype was found to be associated with a diminished response on retinal sensitivity and morphology after three months, as well as visual acuity after 12 months.18
The aim of this study is to investigate the role of the SNP IL-8 −251 A/T in treatment response to intravitreal anti-VEGF in patients with nAMD. Both functional and morphological outcomes of treatment response is studied.
Methods
We prospectively included patients newly diagnosed and subsequently undergoing treatment for nAMD at the Department of Ophthalmology, Zealand University Hospital, Denmark. The study has been approved by the Regional Committee of Ethics in Research of the Region of Zealand, Denmark (journal no: SJ-385) and was performed in adherence to the Declaration of Helsinki. All participants were informed as to the purpose of the study before signing informed consent, which was obtained from all participants prior to inclusion. Inclusion criteria included being treatment naïve to intravitreal anti-VEGF and age 60 years or older. Patients were also included in a study regarding immunological alterations in AMD,10 and therefore, individuals with any immunological or infectious diseases were excluded.
Participants and Retinal Imaging
All participants were diagnosed by a retinal specialist, after thorough examination including best-corrected visual acuity (BCVA) according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol,19 slit-lamp biomicroscopy, spectral domain optical coherence tomography (OCT), and fluorescein and indocyanine-green angiography. Participants were treated with a loading dose of three intravitreal anti-VEGF injections at monthly intervals and were reexamined a month later including visual acuity and follow-up OCT. The anti-VEGF agent consisted of either aflibercept or ranibizumab, as this study was conducted in a transitional period of treatment agent. The median follow-up period, time from the date of diagnosis to the fourth visit when visual acuity and OCT were reevaluated, was 4.7 months (IQR 4.0–5.2).
Only one eye was included for each participant. If participants started bilateral treatment for nAMD, the right eye was chosen.
Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) was used to obtain OCT scans. Scan area was 20° × 15°, with a distance of 236 µm between each B scan. All scans were evaluated for macular morphology at baseline (time of diagnosis) and follow-up (examination after loading dose). The analysis was an assessment of presence of intraretinal fluid and central retinal thickness (CRT) defined as average thickness of a 1 mm circle centered on the fovea.
The functional response was determined by BCVA by evaluating the change in letters at follow-up compared to baseline as a continuous variable.
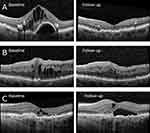
The morphological response was graded from the OCT scans and categorized in groups (Figure 1) inspired by a classification system proposed by Amoaku et al20 according to presence of intraretinal fluid and CRT: good responders having total regression of intraretinal fluid or a reduction of CRT >75% at follow-up, partial responders showing persistence of intraretinal fluid and a reduction of CRT >0–75%, and nonresponders having persistence of intraretinal fluid and unchanged or increased CRT.
Single-nucleotide Polymorphism Genotyping
Venous blood was sampled from the antecubital vein in a 5 mL tube coated with ethylenediaminetetraacetic acid from all participants the day of diagnosis. Samples were genotyped using the Kompetitive Allele Specific Polymerase Chain Reaction (KASP) genotyping assay. The KASP assay for single SNP analysis includes two allele specific forward primers and one common reverse primer. Both were designed and validated by LGC Genomics. The assay preparation and polymerase chain reaction (PCR) amplifications were performed according to the user’s guide and manual (LGC Genomics, Herts, UK).
Statistics
Statistical analysis was performed using R software version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Categorical demographic data is presented in absolute number and percentage (gender, treatment agent, smoking status). Normally distributed continuous demographic data is presented as mean and standard deviation (SD) (age, visual acuity change, CRT). Nonnormally distributed demographic data is presented as median and interquartile range (IQR) (visual acuity at baseline and after treatment). The demographic characteristic’s effects on treatment response were analyzed with a generalized linear model (GLM) correcting for confounders. Comparisons between distributions was performed with the nonparametric tests Kruskal–Wallis and paired Wilcoxon, with Bonferroni adjustments in statistically significant results. SNP and treatment response analysis was performed using Fisher’s exact test. A P-value <0.05 is interpreted as statistically significant.
Sample Size
A power calculation was performed with Robin Ristl’s Sample Size Calculator21 with an alpha level of 0.05 and power level of 80%. Based on previous a study18 investigating the correlation between the IL-8 −251 A/T genotype and treatment response we found a minimum sample size of 67 participants, but we allowed further recruitment.
Results
Clinical Outcome and Demographic Data
A total of 86 participants were enrolled in this study and all completed the follow-up examination. Three participants were excluded due to a missed amplification in the SNP genotyping, resulting in 83 included participants. Participants were categorized according to their morphological treatment response based on OCT findings. Based on morphological response 44 participants were good responders, 23 partial responders, and 16 nonresponders. The clinical and demographic data is shown in Table 1. No significant difference in gender, smoking status, treatment agent or length of follow-up period was found between the groups of morphological responders. However, older age was significantly associated to good morphological response (P=0.014, GLM). When comparing each morphological response group to each other, a statistically significant association was found in older age in good responders compared to partial responders, as well as old age in good compared to poor responders (P=0.045 and P=0.045, paired Wilcoxon test with Bonferroni adjustment) of 2.97 years (95%CI: 1.67–8.55) and 3.33 years (2.33–9.51), respectively. No significant association was found between age and partial responders to poor responders (P=0.647, paired Wilcoxon test).
 |
Table 1 Demographic Data of Patients and Morphological Response |
Single-nucleotide Polymorphism and Treatment Response
The total sample of participants showed an increase in BCVA of 5±10 ETDRS letters (P=0.003) from baseline to follow-up. The functional treatment response defined as change in number of ETDRS letters did not correlate with the IL-8 −251 A/T genotype (P=0.090, Kruskal–Wallis test) or allele frequency (P=0.603).
The total sample of participants showed a decrease in CRT of −80±133 µm (P<0.001) from baseline to follow-up. The assumed risk genotype, homozygous AA, was carried by 25.0% of morphological nonresponders, 26.1% of partial responders and 18.2% of good responders. There was no significant correlation between IL-8 −251 A/T genotype or allele frequency and morphological treatment response (P=0.799, Fisher’s exact test and P=0.364, respectively) (Table 2). No correlation was found between genotype and CRT change either (P=0.127).
 |
Table 2 Distribution of Genotype and Allele Frequency of IL-8 −251 A/T and Treatment Response |
Morphological and Functional Treatment Response
There was a significant association between CRT and visual acuity at baseline (P=0.002, Kruskal–Wallis test with Bonferroni adjustment) and follow-up (P=0.002). The CRT was also significantly associated to presence of intraretinal fluid at baseline (P=0.006) and follow-up (P=0.002). Presence of intraretinal fluid too was significantly associated to visual acuity at baseline (P=0.002) and trending at follow-up (P=0.062).
Discussion
Identifying biomarkers and genetic factors associated with the treatment response of nAMD may lead to insights necessary to pave the way for new and efficient treatment options for the large patient population, who suffers from this life-altering disease. In this study, we found no association between the SNP IL-8 −251 A/T (rs4073) and treatment response to anti-VEGF in nAMD. Neither morphological nor functional response showed any significant correlation with the genotype or allele frequency. The presumed risk genotype homozygous AA and risk allele A did not correlate with poorer response.
Two previous studies have investigated the relationship of the polymorphism IL-8 −251 A/T and treatment response to anti-VEGF in nAMD. Similar to our study, Hautamäki et al did not find a significant association between the genotype and functional treatment response. They did, however, demonstrate the homozygous AA genotype was associated with morphological nonresponders after loading dose. Hautamäki et al defined nonresponders as patients with persisting neuroepithelial detachments after treatment or the reduction of the combined area of intraretinal cysts being <70% in this retrospective study.12 Lazzeri et al showed a significant association between the homozygous AA genotype and reduced treatment response of morphology and mean retinal sensitivity after three months of treatment. Morphological treatment response was defined according to change of CRT, and retinal sensitivity treatment response as 12 degrees central retinal sensitivity evaluation with microperimetry. Lazzeri et al also found patients harboring the IL-8 −251 AA genotype had significantly lower visual acuity after 12 months of treatment, but did not find any association after three months.18 The two aforementioned studies investigated a slightly older population which may be a factor of morphological treatment response. Hautamäki et al defined morphological treatment response differently by not including CRT, which might be a factor in our conflicting results, but we were not able to replicate the results of Lazzeri et al either even with the same definition of CRT change after treatment.12,18
Inflammation plays a pivotal role in the pathogenesis of AMD and multiple pro-inflammatory cytokines and chemokines are increased in patients with AMD.6 Moreover, the immunological profiles differ between the late stages of AMD, geographic atrophy and nAMD, as well as polypoidal choroidal vasculopathy.6,10,22,23 The polymorphism IL-8 −251 A/T is associated with several inflammatory diseases,13,14,24 but has shown differing results in AMD.15,16 A previous study suggests IL-8 −251 A/T is associated to earlier onset of nAMD, while not finding this polymorphism to be associated to AMD in general.17 A previous meta-analysis has shown the level of IL-8 in aqueous humor is significantly higher in patients with nAMD compared to a control group of healthy eyes, but no significant association was found in serum IL-8 compared to healthy controls, suggesting the systemic level of IL-8 might not influence the development of nAMD. Haplotypes involving both −251 A/T (rs4073) and +781C/T (rs2227306) have been found to correlate with AMD, and the relationship between the polymorphisms in the IL-8 regulatory region might be interlinked.25 The polymorphism IL-8 −251 A/T has not been shown to be associated with branch or central retinal vein occlusion,26,27 but has been found associated to diabetic retinopathy and high-risk proliferative diabetic retinopathy.28
In this study, we found age to be significantly associated to good morphological response, thus suggesting late onset as a predictor for better morphological treatment response. Previous studies have found the same association,29,30 while others find no such association.12
Our study was limited by the relatively small population size, which might have masked an association between IL-8 −251 A/T and treatment response. Furthermore, a limitation was lack of previous comparable studies examining IL-8 −251 A/T which defined morphological response similarly to this current study, thus a sample size calculation for this metric was undefinable. However, a sufficient sample size was included for the continuous variables of BCVA and CRT. The differences in study population regarding age and exclusion of participants with immune and infectious diseases might have caused our nonsignificant results. The prospective study design, high completion of follow-up, single SNP analysis and statistical adjustment for multiple comparisons are strengths.
Conclusion
We did not find an association between treatment response in nAMD and IL-8 −251 A/T. There is still an unmet need for personalized medicine and new therapeutic targets in treatment for nAMD, as a large proportion of patients have an inadequate response to current treatment.
Abbreviations
BCVA, best-corrected visual acuity; CRT, central retinal thickness; ETDRS, Early Treatment of Diabetic Retinopathy Study; IL-8, interleukin-8; nAMD, neovascular age-related macular degeneration; OCT, optical coherence tomography; SNP, single-nucleotide polymorphism.
Acknowledgments
This study was supported by the Velux Foundation (00024226, Søborg, Denmark) and Region Zealand PhD Grant (R29-A1337, Sorø, Denmark). The funding organizations had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi:10.1016/S2214-109X(13)70145-1
2. Morris B, Imrie F, Armbrecht AM, Dhillon B. Age-related macular degeneration and recent developments: new hope for old eyes? Postgrad Med J. 2007;83(979):301–307. doi:10.1136/pgmj.2006.052944
3. Martin DF. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2011;364(20):1897–1908. doi:10.1056/NEJMoa1102673
4. Bloch SB, Larsen M, Munch IC. Incidence of Legal Blindness From Age-Related Macular Degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153(2):209–213.e2. doi:10.1016/j.ajo.2011.10.016
5. Gillies MC, Campain A, Barthelmes D, et al. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2015;122(9):1837–1845. doi:10.1016/j.ophtha.2015.05.010
6. Rozing MP, Durhuus JA, Krogh Nielsen M, et al. Age-related macular degeneration: a two-level model hypothesis. Prog Retin Eye Res. 2020;76:100825. doi:10.1016/j.preteyeres.2019.100825
7. Subhi Y, Krogh Nielsen M, Molbech CR, et al. Plasma markers of chronic low-grade inflammation in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Acta Ophthalmol (Copenh). 2019;97(1):99–106. doi:10.1111/aos.13886
8. Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291(6):704–710. doi:10.1001/jama.291.6.704
9. Falk MK, Singh A, Faber C, Nissen MH, Hviid T, Sørensen TL. CX3CL1/CX3CR1 and CCL2/CCR2 Chemokine/Chemokine Receptor Complex in Patients with AMD. PLoS One. 2014;9(12):e112473. doi:10.1371/journal.pone.0112473
10. Subhi Y, Krogh Nielsen M, Molbech CR, et al. CD11b and CD200 on Circulating Monocytes Differentiate Two Angiographic Subtypes of Polypoidal Choroidal Vasculopathy. Invest Opthalmol Vis Sci. 2017;58(12):5242. doi:10.1167/iovs.17-22479
11. Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res off J Am Assoc Cancer Res. 2008;14(21):6735–6741. doi:10.1158/1078-0432.CCR-07-4843
12. Hautamäki A, Kivioja J, Vavuli S, et al. INTERLEUKIN 8 PROMOTER POLYMORPHISM PREDICTS THE INITIAL RESPONSE TO BEVACIZUMAB TREATMENT FOR EXUDATIVE AGE-RELATED MACULAR DEGENERATION. Retina. 2013;33(9):1815–1827. doi:10.1097/IAE.0b013e318285cf92
13. Taguchi A, Ohmiya N, Shirai K, et al. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2005;14(11 Pt 1):2487–2493. doi:10.1158/1055-9965.EPI-05-0326
14. Hildebrand F, Stuhrmann M, van Griensven M, et al. Association of IL-8-251A/T polymorphism with incidence of Acute Respiratory Distress Syndrome (ARDS) and IL-8 synthesis after multiple trauma. Cytokine. 2007;37(3):192–199. doi:10.1016/j.cyto.2007.03.008
15. Goverdhan SV, Ennis S, Hannan SR, et al. Interleukin-8 promoter polymorphism −251A/T is a risk factor for age-related macular degeneration. Br J Ophthalmol. 2008;92(4):537–540. doi:10.1136/bjo.2007.123190
16. Tsai YY, Lin JM, Wan L, et al. Interleukin Gene Polymorphisms in Age-Related Macular Degeneration. Invest Opthalmol Vis Sci. 2008;49(2):693. doi:10.1167/iovs.07-0125
17. Hautamäki A, Seitsonen S, Holopainen JM, et al. The genetic variant rs4073 A→T of the Interleukin‐8 promoter region is associated with the earlier onset of exudative age‐related macular degeneration. Acta Ophthalmol (Copenh). 2015;93(8):726–733. doi:10.1111/aos.12799
18. Lazzeri S, Orlandi P, Piaggi P, et al. IL-8 and VEGFR-2 polymorphisms modulate long-term functional response to intravitreal ranibizumab in exudative age-related macular degeneration. Pharmacogenomics. 2016;17(1):35–39. doi:10.2217/pgs.15.153
19. Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of Snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans Am Ophthalmol Soc. 2009;107:311–324.
20. Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye. 2015;29(6):721–731. doi:10.1038/eye.2015.48
21. Ristl R. Sample Size Calculator at the University of Vienna. Available from: https://homepage.univie.ac.at/robin.ristl/samplesize.php;.
22. Krogh Nielsen M, Subhi Y, Molbech CR, Falk MK, Nissen MH, Sørensen TL. Systemic Levels of Interleukin-6 Correlate With Progression Rate of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2019;60(1):202–208. doi:10.1167/iovs.18-25878
23. Krogh Nielsen M, Subhi Y, Molbech CR, Falk MK, Nissen MH, Sørensen TL. Chemokine Profile and the Alterations in CCR5-CCL5 Axis in Geographic Atrophy Secondary to Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2020;61(4):28. doi:10.1167/iovs.61.4.28
24. Andia DC, de Oliveira NFP, Letra AM, Nociti FH, Line SRP, de Souza AP. Interleukin-8 gene promoter polymorphism (rs4073) may contribute to chronic periodontitis. J Periodontol. 2011;82(6):893–899. doi:10.1902/jop.2010.100513
25. Ricci F, Staurenghi G, Lepre T, et al. Haplotypes in IL-8 Gene Are Associated to Age-Related Macular Degeneration: a Case-Control Study. PLoS One. 2013;8(6):e66978. doi:10.1371/journal.pone.0066978
26. Steinbrugger I, Haas A, Maier R, et al. Analysis of inflammation- and atherosclerosis-related gene polymorphisms in branch retinal vein occlusion. Mol Vis. 2009;15:609–618.
27. Maier R, Steinbrugger I, Haas A, et al. Role of inflammation-related gene polymorphisms in patients with central retinal vein occlusion. Ophthalmology. 2011;118(6):1125–1129. doi:10.1016/j.ophtha.2010.10.014
28. Dong L, Bai J, Jiang X, et al. The gene polymorphisms of IL-8(−251T/A) and IP-10(−1596C/T) are associated with susceptibility and progression of type 2 diabetic retinopathy in northern Chinese population. Eye Lond Engl. 2017;31(4):601–607. doi:10.1038/eye.2016.287
29. Bek T, Klug SE. Age, sex, and type of medication predict the effect of anti-VEGF treatment on central retinal thickness in wet age-related macular degeneration. Clin Ophthalmol Auckl NZ. 2018;12:473–479. doi:10.2147/OPTH.S158760
30. Bjerregaard T, Krogh Nielsen M, Molbech CR, Subhi Y, Sørensen TL. Treatment failure in neovascular age-related macular degeneration is associated with a complex chemokine receptor profile. BMJ Open Ophthalmol. 2019;4(1):e000307. doi:10.1136/bmjophth-2019-000307
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.