Back to Journals » OncoTargets and Therapy » Volume 13
Interfering Human Papillomavirus E6/E7 Oncogenes in Cervical Cancer Cells Inhibits the Angiogenesis of Vascular Endothelial Cells via Increasing miR-377 in Cervical Cancer Cell-Derived Microvesicles
Authors Zhang Y, Liu Y, Guo X, Hu Z, Shi H
Received 25 November 2019
Accepted for publication 11 March 2020
Published 13 May 2020 Volume 2020:13 Pages 4145—4155
DOI https://doi.org/10.2147/OTT.S239979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Ying Zhang,1 Yao Liu,2 Xingrong Guo,2 Zhenhua Hu,1 Huirong Shi1
1Department of Gynaecology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China; 2Department of Gynaecology, Hami Central Hospital, Hami, Xinjiang 839000, People’s Republic of China
Correspondence: Huirong Shi
Department of Gynaecology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Road, 27 District, Zhengzhou City, Henan Province 450052, People’s Republic of China
Email [email protected]
Background: The dysregulation of the human papillomavirus 18 E6 and E7 oncogenes plays a critical role in the angiogenesis of cervical cancer (CC), including the proliferation, migration, and tube formation of vascular endothelial cells. Interfering E6/E7 increases the number of CC cell-derived microvesicles (CC-MVs). Additionally, microRNAs (miRNAs) can modulate CC angiogenesis and can be encapsulated in MVs.
Objective: We aim to investigate whether E6/E7 affects CC angiogenesis via regulating miRNAs in CC-MVs.
Methods: CC-MVs were isolated from a CC cell line (HeLa) which were transfected with small interfering RNAs (siRNAs) against E6/E7 or co-transfected with miR-377 mimics/inhibitors. The expression of several miRNAs in CC-MVs was detected using quantitative real-time PCR. After co-incubating CC-MVs with human umbilical vein endothelial cells (HUVECs), cell proliferation, migration, and tube formation of HUVECs were determined using cell counting kit-8, transwell, and tube formation assays, respectively.
Results: MiR-377 was increased in E6/E7-interfering CC-MVs. Overexpressing miR-377 in CC-MVs suppressed HUVEC proliferation, migration, and tube formation. LPAR2, the cell surface G protein-coupled receptor, was the downstream target of miR-377 in HUVECs. The co-transfection of E6/E7 siRNAs and miR-377 inhibitors in CCs negated the effect of E6/E7 siRNAs on the elevation of miR-377 in CC-MVs. In HUVECs, the co-transfection of E6/E7 siRNAs and miR-377 inhibitors restored the LPAR2 expression which was reduced by the E6/E7 siRNA transfection. Meanwhile, miR-377 mimic reduced LPAR2 expression and inhibited HUVEC proliferation, migration, and tube formation, while such response was negated by LPAR2 overexpression.
Conclusion: Interfering E6/E7 increased miR-377 in CC-MVs, and overexpressing miR-377 in CC-MVs inhibited angiogenesis of HUVECs via reducing LPAR2.
Keywords: human papillomavirus E6/E7, microvesicle, miR-377, LPAR2, angiogenesis
Introduction
Cervical cancer (CC) ranks as one of the major causes of cancer-related death in women and the most frequent malignant tumors in the world.1,2 As reported, the metastasis to lymph nodes and distant organs becomes the main cause of treatment failure for CC.3 The angiogenesis of vascular endothelial cells, which includes cell proliferation, migration and tube formation, is involved in the metastasis of CC by forming new blood vessels to supply tumor cells with oxygen and nutrients.4,5 It is well defined that the metastatic characteristics of CC is closely related to the persistent cervical infection of high-risk human papillomavirus (HPV) genotypes, such as HPV18.6,7 The previous study showed that the aberrant expression of HPV18 E6 and E7 oncogenes is the major cause for the CC angiogenesis, which inhibits the activities of tumor suppressor gene p53 and pRb and leads to the immune escape of cancer cells.8,9 Importantly, researchers have found that microvesicles (MVs) which are derived from CC cells play an important role in E6/E7-mediated carcinogenesis, as silencing E6/E7 oncogenes enhanced the contents and numbers of extracellular MVs which were secreted by HPV-positive CC cells.10 However, the mechanism of E6/E7 oncogenes in the CC angiogenesis remains largely unknown.
Lysophosphatidic acid (LPA), which is a naturally occurring phospholipid, can originate either from cells (cancer cells, fibroblasts, adipocytes, platelets) or non-cells (lipoproteins).11 LPA can regulate cell apoptosis, survival, differentiation, and other processes in a variety of diseases.12,13 It is well known that LPA interacts with LPAR1, LPAR2, and LPAR3, all of which are G protein-coupled receptors in cell surface.14 LPA was suggested to be highly expressed in plasma samples from CC patients than those from healthy controls.15 Meanwhile, the elevated LPAR2 promotes the angiogenesis of vascular endothelial cells, resulting in the increased metastasis of CC.16 These findings indicated that LPAR2 plays a critical role in the CC angiogenesis, whereas whether LPAR2 is correlated with E6/E7 oncogenes is unclear.
Tumor cells can secrete MVs. The genetic materials encapsulated by MVs, such as microRNAs (miRNAs), can be transferred from tumor cells to adjacent cells, such as endothelial cells, thus affecting the tumor microenvironment.17,18 For instance, miR-1246 encapsulated by colorectal cancer cell-derived MVs can activate PML-induced Smad 1/5/8 signaling in endothelial cells, thereby promoting the proliferation, migration and angiogenesis of endothelial cells.19 According to miRNA expression analysis in umbilical cord blood MVs using miRNA PCR arrays, miR-377 was detected in MVs from umbilical cord blood,20 indicating that miR-377 can be carried by MVs. Another research found that miR-377 was expressed in CC cell lines.21 In addition, the downregulation of miR-377 in endothelial cells can promote the angiogenesis.22 These studies indicate that miR-377 may act as a mediator between CC cells and endothelial cells by being encapsulated in CC cell-derived MVs (CC-MVs) to affect the angiogenesis of vascular endothelial cells.
In this study, we determined the expression of several miRNAs in CC-MVs after interfering HPV18 E6/E7 in CC cells, and explored the effect of miR-377 in CC-MVs on regulating the proliferation, migration, and tube formation of vascular endothelial cells.
Methods
Cell Culture
HPV-positive CC cell lines (HeLa cells and Caski cells) and HPV-negative CC cells (C-33A cells and HT-3 cells) were purchased from Shanghai Institute for Biological Sciences (Shanghai, China). Cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma-Aldrich, Saint Louis, MO) at 37°C with 5% CO2.23 Human umbilical vein endothelial cells (HUVECs) were purchased from the cell bank of Wuhan University, and were incubated in a complete medium (EGM BulletKit; Lonza, Walkersville, MD) at 37°C with 5% CO2. The 0.5–1 × 1011 particles/0.5 × 105 cells CC-MVs were added into the medium to be co-cultured with HUVECs.
Cell Transfection
HPV18 E6/E7 lentivirus, small interfering RNA of HPV18 E6/E7 (siHPV18 E6/E7), miR-377 inhibitor and mimic, the siRNA-LPAR2 and its control (siRNA), the LPAR2-overexpressing plasmid (pcDNA-LPAR2) and the control plasmid (pcDNA3.1) were purchased from GenePharma (Shanghai, China). HPV-negative CC cell lines were transfected with HPV18 E6/E7 lentivirus to overexpress E6/E7, and HPV-positive CC cell lines were transfected with siHPV18 E6/E7 to interfere E6/E7 using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA).9 To investigate the impact of miR-377 on CC-MVs, HeLa cells were transfected with miR-377 inhibitor or NC (negative controls) using Lipofectamine 2000 (Thermo Fisher Scientific). Moreover, HUVECs were transfected with miR-377 inhibitor or miR-377 mimic using Lipofectamine 2000 to analysis the influence of miR-377 on HUVECs.
The Collection of CC-MVs
The collection of CC-MVs was performed as described.19 FBS was centrifuged at 3000 rpm for 5 min, followed by flow-through fraction obtained from centrifuged supernatant was ultracentrifuged at 100,000 rpm for 3 h. The supernatant was slightly and carefully removed to act as MV-free FBS, and then was supplemented into DMEM (MV-free DMEM). The transfected CC cell lines were maintained in dishes containing 40 mL MV-free DMEM for 72 h. Centrifugation (2000 rpm, 4°C, and 5 min) was performed to collect the culture medium. The flow-through fraction was obtained from the resulting supernatant which was filtered through 0.45-μm-pore-filter and then 0.22-μm-pore-filter (Millex-GV Filter Unit; Merck Millipore), and was ultracentrifuged at 100,000 rpm for 3 h. Without the disturbance for MV pellet, 1 mL of supernatant was carefully removed to sever as CC-supernatant without MVs (CC-supernatant deprived of MVs). Another 1 mL was taken and used to resuspend the MV pellet (CC-supernatant with collected MVs).
Quantitative Real-Time PCR (qRT-PCR)
The qRT-PCR was done as previously described.9 RNA samples of CC-MVs were prepared via RNA lysis buffer supplied in a NucleoSpin miRNA isolation kit (TaKaRa, Otsu, Japan) added to an MV pellet. NucleoSpin miRNA isolation kit (TaKaRa) was also used to isolate total RNA from cultured CC cells and HUVECs. The prepared RNAs were reverse transcribed to obtain the cDNA using the PrimeScript® RT reagent kit (TaKaRa). The determination of miRNAs levels was conducted by MiScript SYBR®Green PCR Kit (Qiagen, Hilden, Germany), and the detection of the LPAR2 mRNA level was performed with FastStart Universal SYBR Green Master (Roche) in the ABI Prism 7500 (Applied Biosystems). The relative expression levels of RNAs were qualified by the 2−ΔΔCt method.
Western Blotting
Protein isolation from HUVECs and Western blotting experiments were performed according to the previous description.24 Primary antibody (anti-LPAR2, anti-CD63, anti-CD9, anti-HPV18 E6, and anti-HPV18 E7) was purchased from Abcam (Cambridge, UK). Secondary antibody (HRP-conjugated horse anti-mouse and goat antirabbit IgG) were purchased from Cell Signaling. Protein bands were detected with ECL Advance reagent (GE Healthcare Biosciences, Buckinghamshire, UK) using the ECL detection system (Thermo Scientific, Waltham, MA, USA).
Cell Proliferation Assay and Migration Assay
The proliferation of HUVECs was analyzed using a Cell Counting Kit-8 (CCK-8) assay kit (Dojindo, Japan).25 HUVECs were cultured in 96-well plates at a density of 5000 cells/well for 48 h. 10 μL of CCK-8 solution was added to each well for 1 h and absorbance readings at 450 nm were obtained on a spectrophotometric plate reader in triplicate.
HUVECs (1.5 × 105) were placed into Transwell chambers (Corning Incorporated, USA) for migration assay.26 The lower chambers were filled with DMEM containing 10% FBS. After maintaining at 37°C for 24 h, the cells remained on the upper surface of the membrane were removed. HUVECs on the lower surface of the membrane were fixed and stained with crystal violet. The stained cells were visualized and counted under a light microscope.
Tube Formation Assay
The angiogenesis of HUVECs in vitro was measured by tubule formation assay.27 HUVECs were co-cultured with MVs for 24 h. Then, the cell suspension was seeded in 96-well plates that pretreated with Matrigel matrix. Tubes were allowed to form at 37°C in a humidified chamber with 5% CO2 for 9 h. The tube-like network was visualized by a microscope and photographed. The total tube length and the number of tube branch points were measured using Image-Pro Plus software.
Dual-Luciferase Reporter Assay
Dual-Luciferase reporter assay was performed to verify the relationship between miR-377 and LPAR2.9 LPAR2 3ʹ-UTR fragments with wild type (WT) or mutant type (Mut) miR-377 binding sites were cloned into the pGL3-report Luciferase reporter vector. HUVECs were seeded in a 24-well plate. The recombinational luciferase reporter vector and either the miR-377 mimics or the miR-377 inhibitors were co-transfected in the HUVECs using Lipofectamine 2000 (Thermo Fisher Scientific). The luciferase activity was analyzed using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) on a LuminoskanTM Ascent Microplate Luminometer (Thermo Fisher Scientific).
Statistical Analysis
The data were expressed as the mean ± standard deviation. Graphical analysis and statistics were performed with SPSS 20.0 software (SPSS, Chicago, IL, USA). Student’s t-test and One-way ANOVA were used to calculate the significance of differences. A probability value of P less than 0.05 was considered statistically significant.
Results
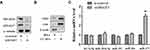
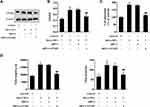
MiR-377 Was Increased in E6/E7-Interfering CC-MVs
The HPV-positive CC cell line (HeLa) was transfected with small interfering RNAs (siRNAs) against HPV18 E6/E7 (si18E6/E7) to downregulate HPV18 E6/E7 (Figure 1A), followed by the isolation of HeLa CC-MVs. The expressions of MV markers (CD63 and CD9) were detected, and the results showed that the isolation was successful (Figure 1B). Meanwhile, miRNAs including let-7a-5p,28 miR-103a-3p,29 miR-191-5p,30 and miR-26a-5p31 have been reported to be related to cell apoptosis, proliferation, migration, and angiogenesis of HUVECs. Therefore, the expressions of let-7a-5p, miR-103a-3p, miR-191-5p, miR-377, and miR-26a-5p were compared in CC-MVs with or without interfering E6/E7. The results showed that compared with the control, the interference of E6/E7 significantly increased the expression of miR-377 in CC-MVs rather than other miRNAs (Figure 1C), suggesting miR-377 may play a role in E6/E7-mediated oncogenesis.
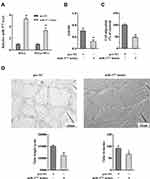
Overexpressing miR-377 in CC-MVs Suppressed Angiogenesis of HUVECs
HeLa cells were transfected with the miR-377 mimic or the negative control (pre-NC). After 72 h, the CC-MVs were collected, and were co-incubated with HUVECs. As shown in Figure 2A, miR-377 was markedly increased in HeLa cells and HeLa-MVs after miR-377 overexpression in comparison with the negative control. The results of CCK-8 assay and Transwell assay revealed that the proliferation and migration of HUVECs were inhibited by the co-incubation with miR-377-overexpressing HeLa-MVs (Figure 2B and C). In addition, the tube formation assay showed that the tube length and tube branches were also reduced by the co-incubation with miR-377-overexpressing HeLa-MVs (Figure 2D). These findings indicated that miR-377 encapsulated in CC-MVs inhibited the proliferation, migration and tube formation of endothelial cells.
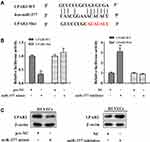
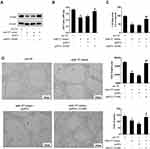
LPAR2 Was a Downstream Target of miR-377 in HUVECs
As shown in Figure 3A, the binding sites of miR-377 on 3ʹUTR of LPAR2 mRNA were predicted by bioinformatics database (TargetScan). The luciferase activity of LPAR2-WT was reduced in the HUVECs co-transfected with miR-377 mimic, while the luciferase activities of LPAR2-Mut were unaffected (Figure 3B). Meanwhile, the luciferase activity of LPAR2-WT was elevated in the HUVECs co-transfected with miR-377 inhibitor, while the luciferase activities of LPAR2-Mut were not obviously changed (Figure 3B). Moreover, overexpression of miR-377 downregulated the protein level of LPAR2 in HUVECs, and the downregulation of miR-377 upregulated LPAR2 protein level (Figure 3C). These data revealed that LPAR2 is a downstream target of miR-377 in HUVECs.
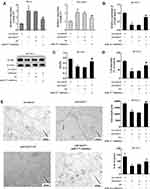
Interfering E6/E7 Inhibited Angiogenesis of HUVECs via Increasing miR-377 in CC-MVs
HeLa cells were transfected with si18 E6/E7 or co-transfected with the miR-377 inhibitor. After 72 h, the CC-MVs were collected and were co-incubated with HUVECs. In both HeLa cells and HeLa-MVs, the co-transfection reduced the enhancement of miR-377 which was raised by interfering E6/E7 (Figure 4A). In HUVECs, the co-transfection restored the LPAR2 expression which was reduced by interfering E6/E7 in HeLa cells (Figure 4B). As shown in Figure 4C and D, the co-transfection restored the cell proliferation and migration which were reduced by interfering E6/E7 in HeLa cells. The results of the tube formation assay showed that the tube length and the tube branches were reduced after interfering E6/E7, while such response was negated after the co-transfection (Figure 4E).
CC-MVs Promoted Angiogenesis of HUVECs via Increasing LPAR2 Expression
HUVECs were transfected with siRNA-LPAR2 or the control (siRNA) followed by the co-incubation with HeLa-MVs. The co-incubation between HeLa-MVs and HUVECs increased LPAR2 expression, and the interference of LPAR2 reduced the LPAR2 protein level (Figure 5A). Cell proliferation and migration were inhibited after interfering LPAR2 (Figure 5B and C). Meanwhile, the tube length and tube branches were also suppressed after interfering LPAR2 (Figure 5D). These data indicated that CC-MVs promoted the proliferation, migration, and tube formation of HUVECs via increasing LPAR2.
Overexpressing miR-377 in CC-MVs Inhibited Angiogenesis of HUVECs via Reducing LPAR2 Expression
HeLa cells were transfected with miR-377 mimics for 72 h, then the CC-MVs were collected and were co-incubated with LPAR2-overexpressing HUVECs. The results showed that overexpressing miR-377 in CC-MVs inhibited LPAR2 expression in HUVECs, while such response was negated by LPAR2 overexpression (Figure 6A). As shown in Figure 6B and C, overexpressing miR-377 in CC-MVs significantly inhibited HUVEC proliferation and migration, while such response was negated by LPAR2 overexpression in HUVECs. In addition, overexpressing miR-377 significantly reduced the tube length and the tube branches, while such response was negated by LPAR2 overexpression (Figure 6D).
Discussion
HPV is a species-specific small double-stranded DNA virus,32 and its infection is highly correlated with the carcinogenesis of CC.33 HPV18 is one of the most common types of HPV families. The oncoproteins coded by E6/E7 genes play key roles in HPV-related induction of transformation and the maintenance of tumorigenic phenotype of HPV-positive cells in CC.34,35 In addition, E6/E7 genes contribute to oncogenesis by altering cell cycles, promoting cell migration, and evading host immune surveillance.36–38 Recently, studies have shown that MVs and exosomes play an important role in E6/E7-mediated carcinogenesis.39 Silencing E6/E7 enhances the contents and numbers of MVs which are secreted by HPV-positive CC cells,10 although the underlying mechanism is still unclear. In the current study, we compared the differential expressions of several miRNAs in CC-MVs, and found that miR-377 was dramatically increased after interfering E6/E7. In addition, our findings indicated that miR-377 encapsulated in CC-MVs inhibited the proliferation, migration, and tube formation of endothelial cells via inhibiting the expression of LPAR2.
MVs are important mediators for signal communications among cells. MVs derived from cancer cells can carry or deliver miRNAs to adjacent cells in the tumor microenvironment, thereby regulating the development of cancers.40,41 Mao et al42 reported that miR-494 secreted by lung cancer cell-derived MVs promoted tumor growth under hypoxic condition by targeting PTEN. Zhang et al40 reported that miR-146b-5p secreted by chronic myelogenous leukemia cell-derived MVs accelerated the leukemic transformation of hematopoietic cells. In this study, we found that HPV18 E6/E7 reduced the expression of miR-377 in CC-MVs, and that overexpressing miR-377 in CC-MVs suppressed the proliferation, migration, and tube formation of endothelial cells. However, the mechanism of HPV18 E6/E7 regulating miR-377 expression in CC-MVs deserves further investigations in the future.
MiRNAs are a class of non-coding RNAs with a length of 18–24 nucleotides. They can regulate the development of cancers via binding to the 3ʹUTR of targeted mRNAs.43,44 According to the prediction of bioinformatics software, there are binding sites between miR-377 and LPAR2 3ʹUTR. LPA is a versatile “phosphoric acid messenger” associated with the occurrence and development of a variety of cancers, including CC,45 and its receptor LPAR2 is reported to promote the migration and proliferation of endothelial cells to accelerate angiogenesis and CC development.16 Therefore, we assumed that miR-377 encapsulated in CC-MVs may regulate endothelial cell proliferation, migration, and tube formation via targeting LPAR2. The results of dual luciferase reporter assay and cell transfection experiments demonstrated that LPAR2 was a downstream target of miR-377, and that LPAR2 expression was negatively regulated by miR-377. In addition, miR-377 inhibitor could reverse the inhibition effect of silencing E6/E7 on LPAR2 mRNA and protein levels. LPAR2 overexpression could reverse the inhibition effect of miR-377 overexpression on endothelial cell proliferation, migration, and tube formation.
In summary, our study demonstrated that interfering HPV18 E6/E7 in CC cells inhibited the proliferation, migration, and tube formation of vascular endothelial cells via increasing miR-377 in CC-MVs. Overexpressing miR-377 in CC-MVs inhibited the angiogenesis of vascular endothelial cells by reducing LPAR2 expression. Our findings provide a novel insight into the mechanisms of CC angiogenesis.
Ethical Statement
This study was approved by the Institute Research Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. Ca a Cancer J Clinicians. 2010;60(5):277. doi:10.3322/caac.20073
2. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Ca a Cancer J Clinicians. 2015;65(2):87–108. doi:10.3322/caac.21262
3. Collaboration., C.f.C.C.M.-A. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–5812. doi:10.1200/JCO.2008.16.4368
4. Park JY, Park DH, Jeon Y, et al. Eupatilin inhibits angiogenesis-mediated human hepatocellular metastasis by reducing MMP-2 and VEGF signaling. Bioorg Med Chem Lett. 2018;28(19):3150–3154. doi:10.1016/j.bmcl.2018.08.034
5. Su C, Zhang P, Liu J, et al. Erianin inhibits indoleamine 2, 3-dioxygenase –induced tumor angiogenesis. Biomed Pharmacother. 2017;88:521–528. doi:10.1016/j.biopha.2017.01.090
6. Hausen HZ. Papillomavirus infections — a major cause of human cancers. Biochim Biophys Acta. 1996;1288(2):F55.
7. Saslow D, Solomon D, Lawson HW, et al. American cancer society, american society for colposcopy and cervical pathology, and american society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;62(3):516. doi:10.1309/AJCPTGD94EVRSJCG
8. Saha SK, Khudabukhsh AR. Berberine alters epigenetic modifications, disrupts microtubule network, and modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical cancer cell HeLa: a mechanistic study including molecular docking. Eur J Pharmacol. 2014;744:132. doi:10.1016/j.ejphar.2014.09.048
9. Jayamohan S, Kannan M, Moorthy RK, et al. Dysregulation of miR-375/AEG-1 axis by human papillomavirus 16/18-E6/E7 promotes cellular proliferation, migration, and invasion in cervical cancer. Front Oncol. 2019;9:847. doi:10.3389/fonc.2019.00847
10. Honegger A, Leitz J, Bulkescher J, et al. Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. Int J Cancer J Int Du Cancer. 2013;133(7):1631–1642. doi:10.1002/ijc.28164
11. Pages C, Simon M-F, Valet P, et al. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64(1–4):1–10. doi:10.1016/S0090-6980(01)00110-1
12. Choi JW, Herr DR, Noguchi K, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50(1):157–186. doi:10.1146/annurev.pharmtox.010909.105753
13. Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116(7):1739–1750. doi:10.1002/cncr.24907
14. Hu YL, Albanese C, Pestell RG, et al. Dual mechanisms for lysophosphatidic acid stimulation of human ovarian carcinoma cells. J Natl Cancer Inst. 2003;95(10):733–740. doi:10.1093/jnci/95.10.733
15. Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280(8):719–723. doi:10.1001/jama.280.8.719
16. Chen RJ, Chen S-U, Chou C-H, et al. Lysophosphatidic acid receptor 2/3-mediated IL-8-dependent angiogenesis in cervical cancer cells. Int J Cancer. 2012;131(4):789–802. doi:10.1002/ijc.26476
17. Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
18. Zhang L-L, Lu Y-M, Zhang L-F. MiR-449b inhibits the migration and invasion of colorectal cancer cells through the negative regulation of MMP2. Clin Surg Res Commun. 2018;2(3):28–34. doi:10.31491/CSRC.2018.9.020
19. Yamada N, Tsujimura N, Kumazaki M, et al. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta. 2014;1839(11):1256. doi:10.1016/j.bbagrm.2014.09.002
20. Wang D, Wang C-M, Wang Y-T, et al. Lactation-related MicroRNA expression in microvesicles of human umbilical cord blood. Med Sci Monit Int Med J Exp Clin Res. 2016;22:4542–4554. doi:10.12659/MSM.901695
21. Liu C, Wang J, Hu Y, et al. Upregulation of kazrin F by miR-186 suppresses apoptosis but promotes epithelial-mesenchymal transition to contribute to malignancy in human cervical cancer cells. Chin J Cancer Res. 2017;29(1):45–56. doi:10.21147/j.issn.1000-9604.2017.01.06
22. Wen Z, Huang W, Feng Y, et al. MicroRNA-377 regulates mesenchymal stem cell-induced angiogenesis in ischemic hearts by targeting VEGF. PLoS One. 2014;9(9):e104666. doi:10.1371/journal.pone.0104666
23. Xia YF, Pei G-H, Wang N, et al. miR-3156-3p is downregulated in HPV-positive cervical cancer and performs as a tumor-suppressive miRNA. Virol J. 2017;14(1):20. doi:10.1186/s12985-017-0695-7
24. Kitange GJ, Carlson BL, Schroeder MA, et al. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-Oncology. 2009;11(3):281–291. doi:10.1215/15228517-2008-090
25. Wu X, Zheng X, Cheng J, Zhang K, Ma C. LncRNA TUG1 regulates proliferation and apoptosis by regulating miR-148b/IGF2 axis in ox-LDL-stimulated VSMC and HUVEC. Life Sci. 2020;243:117287. doi:10.1016/j.lfs.2020.117287
26. Zhao F, Yang X, Xu G, et al. Propranolol suppresses HUVEC viability, migration, VEGF expression, and promotes apoptosis by downregulation of miR-4295. J Cell Biochem. 2019;120(4):6614–6623. doi:10.1002/jcb.27957
27. Anene C, Graham AM, Boyne J, et al. Platelet microparticle delivered microRNA-Let-7a promotes the angiogenic switch. Biochim Biophys Acta Mol Basis Dis. 2018;1864(8):2633–2643. doi:10.1016/j.bbadis.2018.04.013
28. Bammert TD, Hijmans JG, Reiakvam WR, et al. High glucose derived endothelial microparticles increase active caspase-3 and reduce microRNA-Let-7a expression in endothelial cells. Biochem Biophys Res Commun. 2017;493(2):1026–1029. doi:10.1016/j.bbrc.2017.09.098
29. Huang L, Li L, Chen X, et al. MiR-103a targeting Piezo1 is involved in acute myocardial infarction through regulating endothelium function. Cardiol J. 2016;23(5):556–562. doi:10.5603/CJ.a2016.0056
30. Du K, Zhao C, Wang L, et al. MiR-191 inhibit angiogenesis after acute ischemic stroke targeting VEZF1. Aging. 2019;11(9):2762–2786. doi:10.18632/aging.101948
31. Shen H, Li L, Teng Z, et al. Label-free quantitative analysis of protein expression alterations in miR-26a-knockout hela cells using SWATH-MS technology. Sci Rep. 2019;9(1):1399. doi:10.1038/s41598-018-34904-8
32. Karim R, Tummers B, Meyers C, et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte’s innate immune response. PLoS Pathog. 2013;9(5):e1003384. doi:10.1371/journal.ppat.1003384
33. He H, Liu X, Wang D, et al. SAHA inhibits the transcription initiation of HPV18 E6/E7 genes in HeLa cervical cancer cells. Gene. 2014;553(2):98–104. doi:10.1016/j.gene.2014.10.007
34. Von KDM, Oltersdorf T, Schwarz E, Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988;48(13):3780.
35. Mclaughlin-Drubin ME, Mã¼Nger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143(2):195–208. doi:10.1016/j.virusres.2009.06.008
36. Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi:10.1016/0092-8674(90)90409-8
37. Klingelhutz AJ, Foster SA, Mcdougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380(6569):79–82. doi:10.1038/380079a0
38. Mclaughlin-Drubin M, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384(2):335. doi:10.1016/j.virol.2008.10.006
39. Honegger A, Schilling D, Bastian S, et al. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015;11(3):e1004712. doi:10.1371/journal.ppat.1004712
40. Guo AY, Li Q, Zhu X, et al. miR-146b-5p within BCR-ABL1-positive microvesicles promotes leukemic transformation of hematopoietic cells. Cancer Res. 2016;76(10):2901. doi:10.1158/0008-5472.CAN-15-2120
41. Zhang H, Bai M, Deng T, et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016;375(2):331. doi:10.1016/j.canlet.2016.03.026
42. Mao G, Liu Y, Fang X, et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis. 2015;18(3):373–382. doi:10.1007/s10456-015-9474-5
43. Xie F, Hosany S, Zhong S, et al. MicroRNA-193a inhibits breast cancer proliferation and metastasis by downregulating WT1. PLoS One. 2017;12(10):e0185565. doi:10.1371/journal.pone.0185565
44. Zhong C, Liu J, Zhang Y, et al. MicroRNA-139 inhibits the proliferation and migration of osteosarcoma cells via targeting forkhead-box P2. Life Sci. 2017;191:68–73. doi:10.1016/j.lfs.2017.10.010
45. Sui Y, Yang Y, Wang J, et al. Lysophosphatidic acid inhibits apoptosis induced by cisplatin in cervical cancer cells. Biomed Res Int. 2015;2015:598386. doi:10.1155/2015/598386
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.