Back to Journals » Journal of Asthma and Allergy » Volume 16
Interdisciplinary Healthcare Team Experience of Carboplatin and Oxaliplatin Desensitizations in a Tertiary Referral University Hospital
Authors Tunakan Dalgic C , Camyar A, Mete Gokmen N, Kilincer Bozgul SM , Arun MZ , Karaman ZT, Ertuna E
Received 16 May 2023
Accepted for publication 12 July 2023
Published 21 July 2023 Volume 2023:16 Pages 743—753
DOI https://doi.org/10.2147/JAA.S419722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Ceyda Tunakan Dalgic,1 Asuman Camyar,1 Nihal Mete Gokmen,1 Sukriye Miray Kilincer Bozgul,2 Mehmet Zuhuri Arun,3 Zehra Tuba Karaman,2 Elif Ertuna3
1Department of Internal Medicine, Division of Allergy and Immunology, Faculty of Medicine, Ege University, Bornova, Izmir, Turkey; 2Department of Internal Medicine, Faculty of Medicine, Ege University, Bornova, Izmir, Turkey; 3Department of Clinical Pharmacy, Faculty of Pharmacy, Ege University, Bornova, Izmir, Turkey
Correspondence: Elif Ertuna, Department of Clinical Pharmacy, Faculty of Pharmacy, Ege University, Bornova, Izmir, 35040, Turkey, Tel +90 532 672 5988, Fax +90 232 388 5258, Email [email protected]
Purpose: Repeated exposure to platinum compounds increases the risk of immunoglobulin E-mediated immediate hypersensitivity reactions (HSR). To date, many different desensitization protocols with varying success rates have been reported. The presented study is aimed at disseminating the real-world experience of an interdisciplinary healthcare team focusing on platin desensitization.
Patients and Methods: This is a cross-sectional, retrospective study of 7 female patients with carboplatin- or oxaliplatin-induced HSRs. After a discussion with the oncologist and the patient, desensitization protocols were performed by a team consisting of an allergy and immunology specialist, a clinical pharmacist, and a nurse. Clinical data were extracted from the patients’ medical records, and HSRs were reviewed and classified by an allergist according to severity and type.
Results: Twenty-five desensitization protocols were carried out for patients with carboplatin- or oxaliplatin-induced HSRs (N=4 and N=3, respectively; age range: 54– 66). Two of the patients did not experience any HSR during a total of 8 desensitization cycles. The other patients had grade 1– 3 HSRs on 15 cycles, which were successfully managed by oxygen and/or pharmacological interventions and infusions were resumed at a lower rate after stabilization of the patient. Compared to baseline, serum tryptase levels were elevated during HSRs (4.77± 0.21 vs 9.50± 1.71, P=0.028).
Conclusion: All the patients were able to finish the treatment protocol and receive full chemotherapeutic doses. Interdisciplinary teams may facilitate the preparation and administration of platinum-based chemotherapeutics and increase the success rates of desensitization protocols for platin-based chemotherapy, where the concentration and application of drugs differ from standard procedure.
Keywords: platinum compounds, platin hypersensitivity, rapid drug desensitization, immediate reactions, chemotherapy, clinical pharmacist
Introduction
The therapeutic outcome of cancer treatment often relies on the use of first-line chemotherapeutic drugs.1 Platinum compounds such as cisplatin, carboplatin, and oxaliplatin are chemotherapeutic drugs that are commonly used as first-line drugs in the treatment of many solid tumors.2 However, these drugs can trigger an immunoglobulin E (IgE)-mediated allergic reaction, leading to hypersensitivity reactions (HSRs) of varying severity ranging from mild cutaneous eruptions to bronchospasm and anaphylaxis, which can be fatal.2–4
Most of the platinum-induced HSRs have a sudden onset and require repeated exposure, also skin tests are positive in reactive patients. These characteristics support the mast cell/basophil activation and IgE-mediated mechanism of hypersensitivity against platinum compounds.5 It has been reported that multiple exposures to platins increase the risk of sudden HSR, especially in the retreatment of relapsed cancer. An HSR may develop in 25–47% of the cases after the 7th cycle.2,5,6 Pretreatment with corticosteroids (CS) and antihistamines (AH) cannot prevent HSRs against platinum compounds.4 Many patients who are allergic to carboplatin can tolerate cisplatin; however, the true incidence of cross-reactivity is still unknown. It is important to note that fatal cross-reactivity has occurred, and switching to another platinum-based drug may not be an appropriate option for all patients.1,7,8
Drug provocation testing (DPT) is the golden standard to exclude or confirm a true HSR. In DPT, the drug is administered at a standard rate with standard premedication. DPT can prevent the administration of unnecessary desensitization if it is done before a rapid drug desensitization (RDD). However, the appropriateness of performing DPT with platins is controversial. Patients who experienced low to moderate risk HSR (without findings such as chest pain, pressure sensation, change in blood pressure, dyspnea, oxygen desaturation, or laryngeal obstruction) within the first 48 hours after the administration of the drug, and those with negative skin test results are candidates for DPT.9 In a study HSR was excluded in 44% of the patients who underwent DPT.10 Nevertheless, close supervision is recommended during serial subsequent applications, even if the DPT is negative, as there is a possibility of recurrent HSR. DPT is not appropriate in delayed-type drug reactions or severe cutaneous adverse reactions, severe initial HSR history, presence of high-risk comorbidities (presence of uncontrolled asthma, acute infections, and critical diseases or in cases where it is not possible to avoid beta-blocker drugs) or in the presence of pregnancy.
In cases where it is not possible to use an alternative medication or in cases where it is not possible to get an adequate response with the alternative drug, desensitization can be used.4 Desensitization can be defined as the development of temporary tolerance to the offending drug that induces an IgE-mediated immune response by rapid administration of gradually increasing doses of the drug.4,6,11 Rapid drug desensitization protocols inhibit the mast cell responses to the drug and internalization of the antigen/IgE/FceRI complexes, as a result, tryptase, histamine, and other mast cell mediators’ release from the mast cells and basophils is decreased.11 Desensitization is a treatment approach used for the prevention of HSRs caused by platinum compounds.6 Nevertheless, because of the transient nature of the tolerance induced by desensitization, the procedure has to be repeated for each subsequent chemotherapy cycle.2 There is no standard desensitization protocol and diverse protocols are used by different institutions.6 Desensitization can be performed either in an outpatient or inpatient setting and the chosen setting may affect the protocol design. Various desensitization protocols differ in the concentration of the drug, ranging from 1:1000 to 1:1, and the number of steps, which can be 4, 8, 10, 12, or 16. The duration of the desensitization can also vary, with some protocols being completed in a single day, while others may take several days to complete.3,6,8,12,13 Platinum RDD protocols usually start within the range of 1:100–1:1000 dilutions, with subsequently increased concentrations of the drug until 1:1 dilution is reached.8 However, the success rates of these protocols vary. A recent study on a 12-step desensitization protocol that involved several chemotherapeutic drugs, including platinum compounds, showed a complete success rate in 413 procedures, with only a mild or no reaction in 94% of the procedures.14
Due to the potential risks associated with desensitization, it should only be performed at medical centers with experienced medical personnel who can quickly identify and manage any potentially life-threatening breakthrough HSRs. This paper is aimed to clarify the role of an interdisciplinary healthcare team focusing on platin desensitization.
Patients and Methods
A retrospective, cross-sectional review was conducted for patients who experienced immediate HSRs induced by carboplatin or oxaliplatin and received rapid desensitization protocols with the involvement of an interdisciplinary team between November 2018 and November 2021. The patients included in this study were those who were referred to the Allergy and Immunology Clinic of Ege University Hospital due to HSRs from our oncology department or other centers’ outpatient oncology clinics. The patients were identified based on clinical symptoms that were compatible with immediate (within 1 hour) HSRs to carboplatin or oxaliplatin according to the criteria set by the European Network of Drug Allergy and the EAACI Interest Group on Drug Hypersensitivity.15
The clinical data including vitals, desensitization protocols and changes to originally planned protocol, basal and reaction tryptase levels, definitions of reactions that occurred during desensitization, and drugs administered peri-procedurally were extracted from the patients’ hospital records. The HSRs were reviewed and classified by an Allergy and immunology specialist according to severity and type. Grade 1–2 HSRs were considered mild, grade 3 was considered moderate, and finally, grade 4–5 HSRs were categorized as severe according to World Allergy Organization’s 2020 anaphylaxis guidance.16
Standard Operating Procedure of the Interdisciplinary Desensitization Healthcare Team and Desensitization Protocols
Initiation of the standard operating procedure described in Figure 1 was preceded by a meeting attended by all allergy and immunology specialists, nurses, and clinical pharmacists. During the meeting various details related to the robotic chemotherapeutic preparation including instrument sensitivity, margin of error, minimum volume of the solution that can be processed, available serums, and how physician orders should be entered into the hospital electronic order system were discussed. Drug solutions were prepared with the APOTECAchemo robotic chemotherapy drug preparation system (Loccioni humancare, Italy) in negative pressure clean rooms where it is free from microbiological contamination and exposure risks of the worker, under conditions in accordance with international standards (negative pressure indoor air environment in accordance with ISO 5, Class 100 and GMP Class A, double HEPA filtered air cleaning system, safe waste management system, high-capacity laminar flow). In addition, the details about drug administration were also discussed, such as minimum and maximum flow rates of volumetric infusion pumps, amount of medicine remaining in the IV line, procedures for priming and flushing of the IV line, and ensuring stability during drug administration.
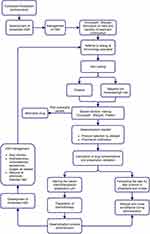 |
Figure 1 Flowchart diagram of the standard operating procedure of the interdisciplinary desensitization healthcare team. Abbreviation: HSR, hypersensitivity reaction. |
An oncologist referred the patient to the allergy clinic and the risks and benefits of continuing therapy were discussed by the oncologist and allergist. An allergist took a detailed history of the patients’ HSR and performed skin tests (prick tests: 10 mg/mL carboplatin or 5 mg/mL oxaliplatin; intradermal test: 0.1 or 1 mg/mL carboplatin or 0.05 or 0.5 mg/mL oxaliplatin) to determine the appropriate desensitization protocol. A time period of 4–6 weeks was chosen after the initial HSR to avoid false negative results when performing the drug skin tests.17,18 Histamine and normal saline were used as positive and negative controls, respectively. Thereafter, a second discussion also involved the patients in the shared decision-making process regarding the administration of drug desensitization.
All patients who had an immediate HSR and could not use an alternative drug were scheduled to receive the 12-step standard RDD protocol, regardless of their skin test results (positive, IgE-mediated, or negative non-IgE-mediated HSRs). After a thorough discussion of potential risks and benefits, patients who provided consent received the desensitization protocol. All desensitization protocols were conducted in an intensive care unit, under the supervision of a physician and a nurse.
Three bags with 10-fold diluted solutions (1:100, 1:10, and 1:1) were used in the 12-step protocol. The protocol involved delivering the drug to the patient with 2x to 2.5x increasing doses and IV flow rates at fixed time intervals (15 min) as previously described.11,14 If a patient had a moderate HSR during prior desensitization, the 12-step protocol was modified to allow, at most, the maximum tolerated infusion rate noted in the previous desensitization in the last step. In one patient a 17-step protocol (1 bag with 1:1 dilution and escalating flow rates)8 was used for 2 desensitizations. The selected protocol and dosage were conveyed to a clinical pharmacist, who calculated the drug concentrations and alerted the robotic chemotherapeutic preparation unit. The pharmacist also conveyed the detailed step-by-step protocol, including drug concentrations and application times, to the physicians and nurses in the intensive care unit where the drug will be administered under the supervision of the specialist. When deemed necessary by the specialist, the desensitization protocol was modified by the pharmacist to allow a lower infusion rate in the last step.
All the patients with severe initial reactions and mild-to-moderate initial reactions were premedicated orally with 0.5–1 mg/kg/day methylprednisolone, ketotifen 2 mg/day (extended-release), famotidine 40 mg, and montelukast 10 mg/day 72 hours or 24 hours before desensitization in patients, respectively. On the day of desensitization pheniramine 45.5 mg, dexamethasone 20 mg, and ondansetron 8 mg were administered intravenously 1 hour before the protocol.
Upon detection of a breakthrough HSR during desensitization, the infusion was stopped, and the patient was managed with oxygen, IV hydration (normal saline), IM epinephrine 0.5 mg, IV pheniramine 45.5 mg, and IV methylprednisolone 40 mg as needed. Once the patient was stabilized; infusion was resumed at the previously tolerated rate and gradually escalated according to the original protocol where possible. Also, in accordance with the literature,5 blood samples for the tryptase test were collected from the patients minimum of 1 and maximum of 2 hours after the breakthrough HSR. The clinical pharmacist was notified if modifications were to be made for the next desensitization cycle.
Skin test results and management and outcome of breakthrough reactions of all patients were presented in the results section.
Ethics
The authors complied with Good Clinical Practice standards throughout the study. This study is approved by the Ethics Committee for Medical Research of the Faculty of Medicine at Ege University (Date: 23.09.2021; No: 21–9.1/4) and is conducted according to the World Medical Association Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Statistical Analysis
The database was constructed using Excel. Continuous variables were expressed as means ± standard error of the mean (SEM). Descriptive analyses were performed, and categorical data were presented in terms of frequencies. Wilcoxon Signed rank test was used for comparisons between two related samples of continuous variables. All statistical tests were performed using SPSS version 25.0 (IBM SPSS Statistics for Windows, Version 25.0; IBM Corp., Armonk, NY, USA). P ≤ 0.05 was considered statistically significant.
Results
A total of 25 desensitization cycles were administered with the contribution of the interdisciplinary team in the study period; 15 of which were carboplatin (4 patients), and 10 of which were oxaliplatin (3 patients) desensitization. All the patients were female, and their ages ranged from 54 to 66 (Table 1). Fifteen carboplatin desensitization cycles in 4 patients and ten oxaliplatin desensitization cycles in 3 patients were successfully completed. None of the patients experienced laryngeal edema. One patient receiving carboplatin and one receiving oxaliplatin did not experience any HSR during 8 desensitization cycles. One patient had an atypical immediate reaction (back pain) to oxaliplatin. Half of the remaining 6 patients had a severe initial reaction, while the other half had a moderate initial reaction. All desensitization protocols were completed, allowing the patients to receive their full intended dose. Any allergic symptoms that occurred during the desensitization were milder than the initial HSR reaction. Five patients had grade 1–3 HSRs which were successfully managed by oxygen and/or pharmacological interventions (Table 2).
 |
Table 1 Patient and Initial HSR Characteristics |
Rapid 12-step desensitization (3 bags with 3x10-fold dilutions) was used in the majority of the protocols (88.5%). Among these, all the HSRs occurred in the last and most concentrated bag of the infusion protocol, 92.3% of which was in the 12th step (Table 1). During 4 of the RDDs, the patient had a moderate HSR. Therefore, they received a modified protocol tailored to their individual needs for their next scheduled desensitization. In 3 of the 4 modified protocols, the infusion rate of concentrated (1:1) solution was decreased in the last step to 40, 50, or 60 mL/h. In the other modification, four additional steps were added to the last bag (1:1 solution) and the solution was planned to be infused at 10, 20, 30, 40, 50, 60, 70, and 80 mL/h rate. However, she had a HSR at 40 mL/h which required interruption of infusion and treatment, but the patient was able to tolerate 40 mL/h when infusion was resumed.
Mast cell tryptase is considered a specific marker of mast cell degranulation. Although 15 HSRs occurred during desensitization, because of the retrospective design of our study, we were able to obtain 6 results for serum tryptase levels that were taken when the patient had an HSR. Compared to baseline, serum tryptase levels were elevated during HSRs (N=6, 4.77±0.21 vs 9.50±1.71, P=0.028).
Forty-five HSR symptoms occurred during desensitization. The patients had 8 constitutional, 9 skin-related, 12 respiratory, 5 cardiovascular, and 1 gastrointestinal symptoms. These symptoms are presented in Figure 2 together with the drug used, and HSR grade.
Individual case histories for initial HSRs and their definitions, and skin test results are presented in Table 1. Desensitization protocols, and management of breakthrough HSRs are presented in Table 2.
 |
Table 2 Desensitization Protocols, Breakthrough HSRs, and Their Management |
Discussion
Hereby we presented 15 carboplatin RDDs in 4 patients and 10 oxaliplatin RDDs in 3 patients, which were performed using our standard operating procedure for interdisciplinary desensitization teams. Preparation of the complex desensitization solutions was facilitated, and prescription errors and stability issues regarding light sensitivity or storage were prevented by validation of each process step with the constant interaction between team members and the robotic preparation unit. Furthermore, the interdisciplinary approach facilitated the management of problems during the administration of chemotherapeutics in the intensive care unit.
Carboplatin and oxaliplatin are widely used in the first-line treatment of numerous malignancies including ovarian, breast, colorectal, lung, and gastrointestinal cancers.2 The incidence of HSRs increases with repeated exposure to platins. The overall HSR risk was reported to be less than 1% for the first 5 cycles of carboplatin therapy, while the incidence rises dramatically to 27% in patients receiving more than 7 cycles of treatment.1,2 The incidence of HSRs with oxaliplatin is as high as 19% and the reactions generally develop after six cycles.7 Patients can become temporarily desensitized to platinum compounds when the target dose is administered by divided incremental steps in a short period.8,14 While the HSRs caused by platinums are mostly IgE-mediated immediate-type reactions, and occur with repetitive administration of the drug, the reactions caused by taxanes and other monoclonal antibodies are often not mediated by IgE and occur in the first or second infusion. Hypertension or hypotension, back- and abdominal pain, presyncope, rigors, fever, flushing, and pruritus can be seen in these non-IgE-mediated reactions with direct mast cell/basophil stimulation.19 In addition, paclitaxel and docetaxel preparations can degranulate basophils and mast cells owing to their cremophor and polysorbate 80 content, respectively.20,21 A different clinical converter phenotype was also defined with taxanes. In this case, the HSR that starts as a non-immediate reaction turns into immediate (Type 1) HSR during RDD.22 Monoclonal antibodies, on the other hand, can cause anti-drug antibody-mediated (IgE/IgG) HSR in addition to CRS in the first infusion. While the severity of CRS decreases, the severity of IgE-mediated reactions increases as the number of drug infusions increases. In the management of mild to moderate non-IgE-mediated HSRs related to taxanes and monoclonal antibodies, infusion rate reduction and/or increased premedication is recommended, while RDD is recommended for severe initial reactions.23
Skin tests are performed for patients with initial HSRs, however, the sensitivity and specificity of the skin tests performed with platinums are variable. In previous studies, while carboplatin skin test sensitivity was 85%, test results were false negative in 8% of cases. In addition, the positive predictive value of platinum skin tests was high (about >90%), while the negative predictive value was low (about 50%).9,24 All of our patients had severe initial HSRs (grade 3 or above). Since the negative predictive value of skin tests are low and DPT is a high-risk procedure in severe immediate HSRs, RDD was preferred instead of DPT in all of the cases in our study.
In the present study, 15 cycles of carboplatin desensitization were performed in 4 patients, and 10 cycles of oxaliplatin desensitization were administered in 3 patients. Overall, 10 cycles were completed without HSRs (40%). Patients did not experience any breakthrough HSRs in 5 carboplatin cycles (33.25%) and 5 oxaliplatin cycles (50%). The overall safety of carboplatin desensitization was reported to be good and 68% of the patients with low to moderate initial reactions tolerated carboplatin with no HSRs during the desensitization protocol.25 On the contrary, in pediatric patients with low-grade glioma, only 20% of the patients had no reaction during a rapid carboplatin desensitization protocol.26 The rate of patients suffering from breakthrough reactions was higher in our case series than previously reported,14,25 which might be due to the difference in the severity of the initial HSRs. Indeed, Vetter et al have reported nearly 65% breakthrough reactions in moderate-to-high-risk patients, and Caiado et al reported that the severity of the initial HSR increased the odds of breakthrough HSRs.3,27 In our study 3 of the patients had grade 4 or 5 initial reactions, and an additional patient had an atypical reaction to oxaliplatin.
In cases with breakthrough HSRs during RDD, premedication was added before the step with HSR, and the last bag infusion time was extended for subsequent RDDs. In cases with cytokine release syndrome (CRS)-like findings (flushing, fever, chills, rigor, pain), normal saline infusion was preferred alongside paracetamol and opioid. For cutaneous symptoms, corticosteroids were used in combination with antihistamines (H1 and H2 antagonists). In the literature, it is recommended to use aspirin for cutaneous reactions that cannot be overcome by antihistamines and corticosteroids.28 In our study HSR was treated in all cases with the interventions described above, therefore aspirin use was unnecessary. In addition, considering that thrombocytopenia may develop as a side effect of chemotherapeutics and the use of ASA may increase the risk of bleeding the use of aspirin was not preferred.
None of the patients experienced laryngeal edema during desensitization. Forty percent of the overall reactions were mild, and 33% were moderate. These rates are comparable to those of previous reports of carboplatin and oxaliplatin desensitizations.3,29 Compared to their initial reaction most of the patients experienced milder or no symptoms during desensitization. All reactions occurred during the infusion of the last bag (1:1 dilution) in the 12-step protocol. Additionally, all reactions were resolved when infusion was paused, and appropriate treatment was administered. These findings are in line with the previous observations of Castells et al.14
Some patients with oxaliplatin hypersensitivity experience atypical symptoms, which are thought to occur because of cytokine release. In addition to IgE-mediated reactions, the atypical reactions can also be prevented by desensitization.30 IL-1 and IL-6 originating from macrophages and monocytes, which are the main cell group of myeloid cells, increase in CRS. Fever, chills, rigors, back or abdominal pain, hypotension, and hypoxia may be seen. It may progress to acute respiratory distress syndrome. Cytokines reach their maximum level in the blood around 100 minutes and remain for 10 hours.31,32 IL-6 blockade can prevent CRS. Tocilizumab, an IL-6 receptor antibody, is used as first-line therapy in CRS. In addition, anakinra, an IL-1 receptor antibody, is also effective in the prophylaxis and treatment of CRS symptoms, including neurological symptoms.33
In our study, one patient who suffered from initial back pain, which is an atypical reaction to oxaliplatin, experienced less severe back pain in 4 desensitization cycles. Another patient’s (Case 2) skin test was negative, but she experienced chest tightness and flushing during her initial HSR and did not experience breakthrough HSRs during RDD. This case might have had CRS or mixed HSR (CRS + IgE mediated HSR). Albeit the HSR is mediated by CRS or IgE, RDD is recommended for the continuation of the culprit drug.34–36 Although anaphylaxis could not be proven by skin test or tryptase increase, the patient had grade 3 index HSR with severe symptoms such as flushing, face edema, tightness of chest, and dyspnea. Due to the severity of the initial HSR DPT could not be performed and because of the variability of skin test sensitivity and specificity, HSR could not be excluded. Thus, drug administration was preferred to be carried out by RDD based on the history.
Despite the 100% success rate in our study, desensitization protocols can be error-prone as there is a need for multiple calculations, dilutions, and dose preparations.8 In addition, the special circumstances of drug administration like the need to change infusion rates every 15 minutes, longer than usual infusion times, and administration of the drug in the ICU by a relatively untrained nurse on chemotherapeutics can compromise patient safety. While planning RDDs for chemotherapeutics, oncologists/hematologists, internal medicine specialists, allergists, nurses, and pharmacists should work together. Chemotherapeutic agent and premedication to be used during RDD should be decided by the collaboration of oncology/hematology specialists and allergy specialists.37 Therefore, before implementing platinum desensitization protocols in our hospital, an interdisciplinary team was established to increase patient safety and procedural success. With the attendance of all team members, details of desensitization protocols and pitfalls regarding all aspects of preparation and administration were discussed. Upon review of the literature, a workflow for rituximab desensitization for a multidisciplinary team by Amoros-Reboredo et al was identified,38 and modifications were made to form a standard operating procedure suitable for our setting (Figure 1). The standard operating procedure aimed to reduce medication and administration errors by cross-checking before, during, and after the procedure and by ensuring the continuity of communication between team members. As a result, no medication errors were reported and a success rate of 100% was achieved for platinum desensitization cycles of all patients referred to our hospital during the study period.
Recently, a study in patients with immediate HSRs reported that the overall survival was higher in patients who continued to receive carboplatin via desensitization compared to platin discontinuation.39 In a large European series of RDD, desensitization was found to be highly effective and safe.27 In another study, RDD was safely administered with no effect on overall health costs. Furthermore, patients’ life expectancy was slightly increased in carboplatin RDD group compared to controls.40 Therefore interventions to improve the success rates for desensitization procedures should be encouraged. We strongly believe that a strong collaboration between physicians, nurses, and clinical pharmacists is crucial to attain therapeutic success.
Limitations
There were some limitations of our study. First of all, the number of patients was low, and previously defined protocols for RDDs were used. Because of the retrospective design of the study not all patients had tryptase levels recorded in their patient records, and in some cases, negative skin tests and lack of HSR tryptase values did not allow us to clearly determine whether the observed reaction was allergic or not. The decision for using RDD was made considering patient history in these cases. The lack of a control group or a historical cohort made it impossible to compare RDD outcomes.
Conclusion
This study highlights the importance of an interdisciplinary approach to desensitization protocols for patients with a history of hypersensitivity reactions to platinum compounds. Administering chemotherapy to patients with a history of severe hypersensitivity reactions and anaphylaxis can be challenging. Using a standard operating procedure and close collaboration between physicians (oncology, allergy, and intensive care specialists), nurses, and clinical pharmacists, we had been able to achieve a 100% success rate in desensitization cycles for carboplatin and oxaliplatin. This approach not only increases the likelihood of treatment success for patients but also reduces the risk of medication errors and enhances patient safety. We hope that our findings will encourage the implementation of similar interdisciplinary approaches to desensitization protocols in other healthcare settings.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Otani IM, Wong J, Banerji A. Platinum chemotherapy hypersensitivity: prevalence and management. Immunol Allergy Clin North Am. 2017;37(4):663–677. doi:10.1016/j.iac.2017.06.003
2. Giavina-Bianchi P, Patil SU, Banerji A. Immediate hypersensitivity reaction to chemotherapeutic agents. J Allergy Clin Immunol Pract. 2017;5(3):593–599. doi:10.1016/j.jaip.2017.03.015
3. Vetter MH, Khan A, Backes FJ, et al. Outpatient desensitization of patients with moderate (high-risk) to severe platinum hypersensitivity reactions. Gynecol Oncol. 2019;152(2):316–321. doi:10.1016/j.ygyno.2018.10.037
4. Weiss ME, Bernstein DI, Blessing-moore J, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273.e78. doi:10.1016/j.anai.2010.08.002
5. Caiado J, Picard M. Diagnostic tools for hypersensitivity to platinum drugs and taxanes: skin testing, specific IgE, and mast cell/basophil mediators. Curr Allergy Asthma Rep. 2014;14(8). doi:10.1007/s11882-014-0451-7
6. Miyamoto S, Okada R, Ando K. Platinum hypersensitivity and desensitization. Jpn J Clin Oncol. 2015;45(9):795–804. doi:10.1093/jjco/hyv081
7. Castells Guitart MC. Rapid drug desensitization for hypersensitivity reactions to chemotherapy and monoclonal antibodies in the 21st century. J Investig Allergol Clin Immunol. 2014;24(2):72–79.
8. O’Malley DM, Vetter MH, Cohn DE, Khan A, Hays JL. Outpatient desensitization in selected patients with platinum hypersensitivity reactions. Gynecol Oncol. 2017;145(3):603–610. doi:10.1016/j.ygyno.2017.03.015
9. Martí-Garrido J, Vázquez-Revuelta P, Lleonart-Bellfill R, Molina-Mata K, Muñoz-Sánchez C, Madrigal-Burgaleta R. Pilot experience using drug provocation testing for the study of hypersensitivity to chemotherapy and biological agents. J Investig Allergol Clin Immunol. 2021;31(2):166–168. doi:10.18176/JIACI.0552
10. Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019;7(2):618–632. doi:10.1016/J.JAIP.2018.07.031
11. Tunakan Dalgiç C, Mete Gökmen EN, Özişik M, Alkaya Baklan M, Yüceyar N. Successful rapid drug desensitization with a modified protocol to alemtuzumab in a multiple sclerosis patient with severe immediate-type hypersensitivity reaction. Arch Neuropsychiatr. 2022;59(3):237. doi:10.29399/NPA.27371
12. Choi J, Harnett P, Fulcher DA. Carboplatin desensitization. Ann Allergy Asthma Immunol. 2004;93(2):137–141. doi:10.1016/S1081-1206(10)61465-2
13. Toyohara Y, Sone K, Nishida H, et al. Desensitization strategy for hypersensitivity reactions to carboplatin in five patients with gynecological cancer. J Obstet Gynaecol Res. 2020;46(11):2298–2304. doi:10.1111/jog.14443
14. Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122(3):574–580. doi:10.1016/j.jaci.2008.02.044
15. Cernadas JR, Brockow K, Romano A, et al. General considerations on rapid desensitization for drug hypersensitivity - A consensus statement. Allergy. 2010;65(11):1357–1366. doi:10.1111/J.1398-9995.2010.02441.X
16. Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10):100472. doi:10.1016/j.waojou.2020.100472
17. Picard M, Pur L, Caiado J, et al. Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J Allergy Clin Immunol. 2016;137(4):1154–1164.e12. doi:10.1016/J.JACI.2015.10.039
18. Hong DI, Madrigal-Burgaleta R, Banerji A, Castells M, Alvarez-Cuesta E. Controversies in allergy: chemotherapy reactions, desensitize, or delabel? J Allergy Clin Immunol Pract. 2020;8(9):2907–2915.e1. doi:10.1016/J.JAIP.2020.08.005
19. Picard M. Management of hypersensitivity reactions to taxanes. Immunol Allergy Clin North Am. 2017;37(4):679–693. doi:10.1016/J.IAC.2017.07.004
20. Szebeni J, Muggia FM, Alving CR. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst. 1998;90(4):300–306. doi:10.1093/JNCI/90.4.300
21. de Leon MCB, Bolla S, Greene B, Hutchinson L, Del Priore G. Successful treatment with nab-paclitaxel after hypersensitivity reaction to paclitaxel and docetaxel. Gynecol Oncol Case Rep. 2013;5:70–71. doi:10.1016/J.GYNOR.2013.05.003
22. Jimenez-Rodriguez TW, Manuel Marco de la Calle F, Lozano-Cubo I, et al. Converter phenotype: a new profile that is not exclusive to taxanes. Front Allergy. 2022;2. doi:10.3389/FALGY.2021.785259
23. Bonamichi-Santos R, Castells M. Diagnoses and management of drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: reactions to taxanes and monoclonal antibodies. Clin Rev Allergy Immunol. 2018;54(3):375–385. doi:10.1007/S12016-016-8556-5
24. Pradelli J, Verdoire P, Boutros J, et al. Allergy evaluation of hypersensitivity to platinum salts and taxanes: a six-year experience. J Allergy Clin Immunol Pract. 2020;8(5):1658–1664. doi:10.1016/J.JAIP.2019.12.032
25. Li Q, Cohn D, Waller A, et al. Outpatient rapid 4-step desensitization for gynecologic oncology patients with mild to low-risk, moderate hypersensitivity reactions to carboplatin/cisplatin. Gynecol Oncol. 2014;135(1):90–94. doi:10.1016/j.ygyno.2014.07.104
26. Dodgshun AJ, Hansford JR, Cole T, Choo S, Sullivan MJ. Carboplatin hypersensitivity reactions in pediatric low grade glioma are protocol specific and desensitization shows poor efficacy. Pediatr Blood Cancer. 2016;63(1):17–20. doi:10.1002/PBC.25686
27. Caiado J, Brás R, Paulino M, Costa L, Castells M. Rapid desensitization to antineoplastic drugs in an outpatient immunoallergology clinic: outcomes and risk factors. Ann Allergy Asthma Immunol. 2020;125(3):325–333.e1. doi:10.1016/J.ANAI.2020.04.017
28. Breslow RG, Caiado J, Castells MC. Acetylsalicylic acid and montelukast block mast cell mediator-related symptoms during rapid desensitization. Ann Allergy Asthma Immunol. 2009;102(2):155–160. doi:10.1016/S1081-1206(10)60247-5
29. Park HJ, Lee JH, Kim SR, et al. A new practical desensitization protocol for oxaliplatin-induced immediate hypersensitivity reactions: a necessary and useful approach. J Investig Allergol Clin Immunol. 2016;26(3):168–176. doi:10.18176/jiaci.0038
30. Madrigal-Burgaleta R, Berges-Gimeno M, Angel-Pereira D, Guillen-Ponce C, Sanz M, Alvarez-Cuesta E. Desensitizing oxaliplatin-induced fever: a case report. J Investig Allergol Clin Immunol. 2013;20(6):435–436.
31. Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SGA. Elevated serum cytokines during human anaphylaxis: identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol. 2009;124(4):786–792.e4. doi:10.1016/J.JACI.2009.07.055
32. Jakubovic BD, Sanchez-Sanchez S, Hamadi S, Lynch DM, Castells M. Interleukin-6: a novel biomarker for monoclonal antibody and chemotherapy-associated hypersensitivity confirms a cytokine release syndrome phenotype-endotype association. Allergy. 2021;76(5):1571–1573. doi:10.1111/ALL.14644
33. Liu D, Zhao J. Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol. 2018;11(1). doi:10.1186/S13045-018-0653-X
34. Silver J, Garcia-Neuer M, Lynch DM, Pasaoglu G, Sloane DE, Castells M. Endophenotyping oxaliplatin hypersensitivity: personalizing desensitization to the atypical platin. J Allergy Clin Immunol Pract. 2020;8(5):1668–1680.e2. doi:10.1016/J.JAIP.2020.02.013
35. Caiado J, Venemalm L, Pereira-Santos MC, Costa L, Barbosa MP, Castells M. Carboplatin-, oxaliplatin-, and cisplatin-specific IgE: cross-reactivity and value in the diagnosis of carboplatin and oxaliplatin allergy. J Allergy Clin Immunol Pract. 2013;1(5):494–500. doi:10.1016/J.JAIP.2013.06.002
36. Caiado J, Castells M. Presentation and diagnosis of hypersensitivity to platinum drugs. Curr Allergy Asthma Rep. 2015;15(4). doi:10.1007/S11882-015-0515-3
37. McKenzie MG, Bissell BD, Disselkamp MA, Hildebrandt GC, Cox JN. Sensitizing the interdisciplinary team to desensitizations: an alemtuzumab case report. J Oncol Pharm Pract. 2020;26(3):742–746. doi:10.1177/1078155219865313
38. Amorós-Reboredo P, Sánchez-López J, Bastida-Fernández C, et al. Desensitization to rituximab in a multidisciplinary setting. Int J Clin Pharm. 2015;37(5):744–748. doi:10.1007/s11096-015-0136-x
39. Narui C, Tanabe H, Shapiro JS, et al. Readministration of platinum agents in recurrent ovarian cancer patients who developed hypersensitivity reactions to carboplatin. In vivo. 2019;33(6):2045–2050. doi:10.21873/invivo.11702
40. Sloane D, Govindarajulu U, Harrow-Mortelliti J, et al. Safety, costs, and efficacy of rapid drug desensitizations to chemotherapy and monoclonal antibodies. J Allergy Clin Immunol Pract. 2016;4(3):497–504. doi:10.1016/J.JAIP.2015.12.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

