Back to Journals » Clinical Ophthalmology » Volume 14
Inter-Eye Vault Differences of Implantable Collamer Lens Measured Using Anterior Segment Optical Coherence Tomography
Authors Cerpa Manito S, Sánchez Trancón A, Torrado Sierra O, Baptista AMG , Serra PM
Received 24 April 2020
Accepted for publication 12 August 2020
Published 29 October 2020 Volume 2020:14 Pages 3563—3573
DOI https://doi.org/10.2147/OPTH.S258817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Santiago Cerpa Manito,1 Angel Sánchez Trancón,1 Oscar Torrado Sierra,1 António MG Baptista,2 Pedro Miguel Serra1,3
1Ophthalmology Clinic Vista Sánchez Trancón, Badajoz, Spain; 2Centre of Physics, University of Minho, Braga, Portugal; 3Optics and Optometry Department, Instituto Superior de Educação e Ciências, Lisbon, Portugal
Correspondence: Pedro Miguel Serra Email [email protected]
Purpose: The distance between an implantable collamer lens (ICL) and the crystalline lens, namely vault, is a space regulated by the interaction of the ICL and the anatomical structures of the eye. This study analysed the differences in vault size between fellow eyes with similar anterior segment biometry.
Patients and Methods: A retrospective case series analysed 109 cases of patients bilaterally implanted with EVO-V4c. Patients were analysed pre- and postoperatively using anterior segment optical coherence tomography. The range of vault inter-eye differences was defined as the 95% confidence interval of the differences. Bivariate correlation was applied to seek for associations between vault inter-eye differences with biometric and lens parameters (angle-to-angle, anterior chamber depth, crystalline lens rise, central corneal thickness, central keratometry, ICL spherical equivalent, horizontal compression, postoperative pupil diameter and vault).
Results: Mean vault inter-eye differences were similar between fellow eyes (26.0 ± 122.5 μm). The 95% confidence interval range of the differences was ± 240.1 μm, nearly 50% of the cases presented vault inter-eye differences higher than 100 μm. The vault of the first operated eye explained 81% of the variance in the second eye vault. Vault inter-eye differences were positively correlated with the level of horizontal compression and with vault magnitude.
Conclusion: Vaults measured in fellow eyes may present considerable differences, which can reach 25% of the common vault range. This reflects some degree of baseline variability in the vault. Clinically, these differences assume special relevance in cases where low or high vaults are expected.
Keywords: implantable collamer lenses, vault, inter-eye differences, variability
Introduction
Refractive surgery using Implantable Collamer Lenses (ICL) is a safe and efficient technique for the correction of ametropias, in particular myopia1 and astigmatism.2 The ICL is implanted in the posterior chamber between the iris and the crystalline lens, with the lens’ haptics ideally resting on the ciliary muscle-sulcus complex.3 The distance from the ICL posterior surface to the crystalline lens anterior surface is an important safety postoperative parameter called vault.4
The recommended range of vaults, i.e., a vault between 100 and 1000 μm,4 avoids the contact between the ICL and the crystalline lens (low vaulting), minimizing the risk of anterior subcapsular cataract development.5 Also, it reduces the chance of iridocorneal angle closure (high vaulting), which could put the patient at risk of developing ocular hypertension.6
The need for predicting the vault has led to the development of predictive models using biometric and lens features.7–11 Despite the variety of associations put forward, the ability of explaining the vault preoperatively is limited to approximately 40%9,10 and decreases to 14% if the prediction is restricted to an optimal vault range.12 Several arguments have been presented to justify the limited predictability of the models. Lee et al have argued that the vault may depend on the dampening ability of the ciliary-sulcus complex.9 Later, Lee et al suggested that pupil myosis produces an anterior-posterior compression induced by the iris influencing the vault.13 The influence of the pupil size on the vault has driven the concept that the vault is a dynamic entity.14 Recently, imaging studies using ultrasound biomicroscopy (UBM) showed that the ICL haptics adopt different positions in the posterior chamber which has a major influence in the vault magnitude, thus affecting the ability of predicting the vault preoperatively.15,16 All together, these facts point out for the presence of a degree of variability in the vault which is intrinsic to the anatomical properties, physiological behaviour of the eye and surgical procedure. This leads to the question: what are the common differences in the vault between eyes expected to present the same vault?
A study from Kamiya et al found similar vault sizes in fellow eyes implanted with an ICL with and without central port.17 The authors, however did not report the range of differences between eyes (inter-eye difference), which would have been informative of the clinical variability regarding the vault in fellow eyes. Schmidinger et al using an earlier ICL model (V4 version) reported an absolute vault difference between fellow eyes of 74 μm (range: 0–280 µm).18
This study retrospectively analysed cases implanted bilaterally with the same type of ICL, to measure the common range of differences in the vault. The findings have several clinical implications, first, they may be indicative of what should be regarded as a clinical significant difference in vault between fellow eyes; second the range of differences could be used as a safety boundary when performing the second eye surgery allowing the surgeon to consider a change in the lens size or a vertical implantation of the lens; and third the variability in vault measurements found between fellow eyes could assist in interpreting the levels of performance achieved by mathematical models for vault prediction.
Patients and Methods
Study Design
This retrospective case series comprised patients that had undergone bilateral ICL implantation for the correction of myopia and astigmatism (EVO-V4c, STAAR Surgical AG, Nidau, Switzerland). The patients were operated in the Ophthalmology Clinic Vista Sánchez Trancón between January 2012 and December 2017. The surgeries were performed on two different days, with the right eye (RE) being the first operated eye. The patients were considered for the analysis if they presented a manifest myopia between −3.00 and −20.00 DS, had a refractive astigmatism lower than −5.00 DC, internal anterior chamber depth (ACD) ≥2.8 mm and an endothelial cell density ≥2000 cells/mm2. From these patients, those implanted with the same ICL type (spherical and toric) and same ICL size in both eyes were included. Patients with previous corneal refractive surgery or presence of corneal ectasia were excluded alongside patients with ICL implanted vertically, and patients with missing data or non-suitable exams. Upon application of the criteria, the number of eligible cases was 118 and 62 for the spherical and toric group, respectively, Figure 1. This research followed the tenets of the Declaration of Helsinki. Ethical approval was granted by the local ethics committee (Comité Ético de Investigación Clínica de Badajoz), on the basis of the retrospective nature of the study, the impossibility of obtaining individual patient consent for research purposes and ensuring that patient confidentiality was maintained throughout the research.
Preoperative and Postoperative Protocol
Preoperatively, all patients had a complete ophthalmologic exploration which included presenting visual acuity (VA), manifest refraction, objective cycloplegic refraction and best corrected distance VA. Anterior segment anatomy was assessed using a slit-lamp, intraocular pressure measured with a Goldman tonometer and retina observed by indirect ophthalmoscopy. Optical tomography (Pentacam HR, OCULUS Optikgeräte GmbH, Wetzlar, Germany) was used for measuring the horizontal visible iris diameter, i.e., white-to-white (WTW), central keratometry (KC) and central corneal thickness (CCT). Regarding WTW measurements, the Pentacam shows a marginal overestimation of 0.1 mm compared to the standard WTW for ICL sizing (using Orbscan).19 Anterior segment optical coherence tomography (AS-OCT) (Visante, Zeiss Meditec AG, Jena, Germany) was used for measuring the horizontal anterior angle distance (ATA), crystalline lens rise (CLR) and ACD. The AS-OCT Visante scans have a transverse and axial resolution of 20 and 60 µm, respectively.20 The repeatability for the ACD, CLR, ATA and vault measurements was reported to be 99.00 µm, 79.96 µm, 0.14 mm and 58.80 µm, respectively.21,22 Due to the retrospective nature of this study, and since the AS-OCT Visante does not provide a quality centration index, all cases included had the scans reanalysed by one operator where the scan centration was rechecked and the measurements redone. The scans were performed using the single-scan protocol along the horizontal meridian (0–180 degrees) with the scan centred on the pupil, Figure 2. The ATA, CLR and ACD were measured using the software inbuilt calliper “chamber”, Figure 2A. The ATA (mm) represents the distance connecting the nasal and temporal iridocorneal angle recess, the CLR (µm) was defined by the line perpendicular to the ATA line connecting the crystalline lens apex and the ATA line.23 The ACD (mm) was measured along the line perpendicular to the ATA connecting the corneal endothelium and crystalline lens apex. The CLR was defined as positive if the crystalline lens apex was anterior to the ATA line and negative if the opposite.24 Endothelial cell count was performed using a noncontact specular microscope (Topcon SP-2000P, Topcon Corporation, Tokyo, Japan), using a sample of 12 points in the central part of the cornea. On average three months after surgery, the central vault and the horizontal pupil diameter were measured using AS-OCT. The postoperative scans followed the same protocol as the one followed preoperatively. The vault (µm) was measured using the inbuilt “vault” calliper and was defined as the distance from the crystalline lens apex and the central most anterior point of the ICL posterior surface, Figure 2B. The horizontal pupil diameter was measured with a “line” calliper as the distance between pupil margins. The ICL size and power was ordered in accordance to the manufacturer’s Online Calculation and Ordering System (OCOS™) using preoperative data. The measurements were performed in a room with dim light conditions and the patients were instructed to look at the fixation systems of each instrument.
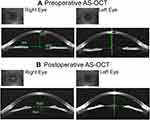 |
Figure 2 (A) Preoperative measurements for the right and left eye using the AS-OCT chamber calliper. (B) Postoperative measurements Vault and Pupil diameter using linear callipers. |
Surgical Protocol
The surgery was performed under local anaesthesia using 2% intracameral preservative free Lidocaine (B.Braun® 20mg/mL) 50% diluted with saline solution. The anterior chamber was filled with 2% Methylcellulose (Medicontur, Zsámbék, Hungary) as viscoelastic and introduced through an anterior chamber paracentesis. The ICL was introduced in the anterior chamber through a 3.2 mm clear temporal corneal incision using the manufacturer injector cartridge (STAAR Surgical Co. Monrovia, CA, USA) and moved to the posterior chamber through the pupil. Upon positioning the lens, the viscoelastic was removed using a balanced saline solution and aspirated from the anterior chamber. Finally, a diluted antibiotic solution (Ceftazidime 50mg/mL and Vancomycin 50mg/mL) was injected in the anterior chamber via the paracentesis. After surgery, antibiotic (Oftalcilox®, Ciprofloxacin 3mg/mL), corticoid (Predforte®, Prednisolone acetate 10 mg/mL) and anti-inflammatory (Voltaren® Diclofenac sodium 1mg/mL)25 drugs were prescribed four times a day during the following three weeks.
Statistical Analysis
Primarily, the inter-eye differences (ΔE) for the preoperative (ATA, ACD, CLR, KC and CCT), postoperative parameters (vault and pupil diameter) and ICL Spherical Equivalent (ICL SE) were calculated as the difference between the RE and left eye (LE). This was done independently for the spherical and toric ICL group. The ATA was chosen at the expense of WTW to represent the transversal size of the eye since, in previous studies, ATA showed stronger correlation with the sulcus-sulcus considered as the gold-standard measure for the ICL size determination.26,27 Secondly, for each parameter the ΔE were compared between the spherical and toric ICL groups. This was done by comparing the shapes of the distributions using the Kolmogorov–Smirnov test and the central tendency using the Independent samples T-test or Mann–Whitney test. In neither parameter did the shape of the distribution and central tendency differ between groups, thus merging both groups. Thirdly, in order to rule out eyes that were structurally different, the cases presenting preoperative ΔE outside the 95% confidence interval (CI) for a specific parameter (ATA, CLR ACD, CCT and KC) were excluded from the analysis. Similarly, the influence of the refractive power on the ICL sagittal depth9 was controlled by including only the cases with ICL SE ΔE within ±1.00 DS, Figure 1.
The absolute agreement between RE and LE parameters was determined using intraclass correlation coefficient (ICC)28 and ΔE were compared to zero (reference level) by applying One-sample T-test or One Sample Wilcoxon Test, as appropriate. The significance level for the multiple comparisons was adjusted using the Bonferroni correction; thus, dealing with the problem that as the number of tests increase, so does the likelihood of a type I error i.e. concluding that significant differences were present when they was not.29 Bland-Altman analysis was used for determining the mean vault ΔE and the limits of agreement were defined as the mean difference ± 1.96 the standard deviation of the vault ΔE. Bivariate correlation analysis was applied for determining the influence of the parameter’s magnitude (RE and LE average: ATA, horizontal compression (HC), ACD, CLR, CCT, KC, ICL SE, pupil diameter and vault) on the absolute vault ΔE, to understand whether parameters’ magnitude influenced the vault ΔE. Horizontal compression was computed as the difference between ICL size and ATA. Also, absolute pupil ΔE were correlated with absolute vault ΔE, to investigate whether vault differences could be related to inter-eye differences in postoperative pupil sizes. The absolute vault ΔE were compared to three vault groups (average RE and LE), low vault <250 µm, intermediate/optimal [250; 750] µm and high vault >750 µm12 and for the three lens sizes (12.6, 13.2 and 13.7) mm using the Kruskal–Wallis test. Statistical analysis was performed using IBM SPSS Statistics V23.0.
Results
From the 180 eligible cases, upon excluding those with preoperative anatomical ΔE outside the 95% CI and ICL SE differences higher than ±1.0DS, the number of cases was reduced to 109. The group mean age was (mean ± SD) 31.5 ± 7.2 years old (range: 18 to 50 y/o) and the sample comprised 75 (69%) women. The number of cases implanted with a 12.6, 13.2 and 13.7 mm ICL was 23, 70 and 16, respectively. Table 1 summarizes the demography, refraction and endothelial cell count for the selected cases.
 |
Table 1 Sample’s Demographic Summary After Similarity Control Between Fellow Eyes (n=109). The Values are Represented by the Mean, Standard Deviation and Range |
Table 2 shows the biometric preoperative and postoperative parameters and the ICL SE for the sample. Right and left eye parameters showed strong intraclass correlation coefficients (R range: 0.79 to 0.98) and the differences between eyes did not differ from zero for any of the parameters (Bonferroni adjustment p=0.05/8). For the specific case of the vault, the two eyes had a Pearson-R2 of 0.81, indicating that 81% of the LE vault could be explained by the RE vault.
The Bland-Altman analysis showed a mean vault ΔE equal to 26.0±122.5 µm and a 95% CI range of the differences equal to ±240.1 µm, informing that although the mean difference is close to zero, fellow eyes can exhibit large differences in the vault, Figure 3A and B. No significant correlation was found between vault ΔE and the average vault of both eyes (R=−0.07 p=0.455).
The bivariate correlation analysis aiming to determine the influence of parameters’ magnitude (average RE and LE) on the absolute vault ΔE (amount of the difference) showed a weak but significant statistical difference for the amount of HC (R=0.27 p=0.004), Figure 4, and vault magnitude (R=0.31 p=0.001). This indicates that the stronger the compression and the size of the vault, the larger the vault differences between eyes. The remaining correlations studied, failed to reach statistical significance (for all, R≤0.16 p>0.05).
 |
Figure 4 Association between average horizontal compression (HC= ICL Size – ATA) and absolute inter-eye vault differences. |
Analysing the patients in three vault ranges, low (<250 µm), intermediate/optimal (250 to 750 µm) and high vault (>750 µm) the mean absolute vault ΔE were 50.0 ± 50.2, 85.1 ± 75.5 and 137.4 ± 71.4 µm, respectively; and for all patients 97.7 ±77 µm. The differences between vault groups were statistically different Kruskall-Wallis χ2=14.4, p=0.001, with the high vault group showing larger vault ΔE compared to the low (Mann–Whitney: U=36.5, p=0.001) and intermediate vault groups (U=638.5, p=0.007), Figure 5. The percentages of vault ΔE adjusted for vault interval (Adjusted vault ΔE =100x absolute vault ΔE/average vault RE and LE) were: Median =31.6% (range: 0.0–200.0%), M= 11.4% (range: 0.0–73.0%), M=12.5% (range: 0.0 −24.0%), M=12.3% (range: 0.0 −200.0%) respectively for the low, intermediate, high vault groups and all cases. The amount of vault ΔE was analysed for the type of lens implanted but these were not statistically significant (ΔE 12.6mm= 75.7 ± 75.5µm; ΔE 13.2mm =98.6 ± 78.1 µm; ΔE 13.7mm =105.3 ± 70.4 µm), Kruskall-Wallis χ2=1.32, p=0.519.
Table 3 shows the cumulative percentage of cases. When all patients are included, only 14% of the cases presented vault ΔE lower than 20 µm and nearly half of the cases had differences below 100 µm. In the low vault group, approximately 43% of the patients had vault ΔE below 20 µm and all patients showed differences smaller than 160 µm. In the intermediate vault group, approximately 13% of the cases had differences below 20 µm and 82% had differences lower than 160 µm. In the high vault group, the percentage of cases showing differences lower than 20 µm and 160 µm decreased to 6.5% and 58%, respectively.
 |
Table 3 Cumulative Percentage of Absolute Inter-Eye Vault Differences. Considering (Average RE and LE Vault) Low Vault <250, Intermediate 250 to 750 µm and High Vault >750 µm |
Discussion
This study aimed to analyse vault differences produced by an ICL in fellow eyes, showing similar anterior segment anatomy, implanted with a similar ICL (type, size and power), operated with the same surgical technique and assessed using the same imaging device, in order to determine an expected range of vault inter-eye differences. All included cases were given clinical discharge and had the vault assessed on average three months after surgery where the vault has been reported to be stable in the short term follow-up.18,30 The results showed that on average, fellow eyes had similar vaults. This concurs with previous findings where no difference in vault was found between eyes implanted with ICL with and without central hole.17 However, the vault ΔE exhibited a range of ±240µm, with 53.7% of the cases showing differences higher than 100 µm and more than half of the cases having differences higher than the repeatability coefficient for vault measurements (58.8 µm).22 The vault ΔE and the range of variability found are in agreement with Schmidinger et al18 who reported a mean absolute vault ΔE of 74 ± 70 µm which is compared to the 97.7 ± 77 µm found in the present study.
The range of vault ΔE observed may be associated to several factors. One of them may regard to the resting position of the lens’ haptics in the posterior chamber. Recently, Zhang et al using UBM in a series of cases implanted with ICL showed that 32.1% of the eyes ended with the haptics resting on the ciliary body and 21.6% on ciliary sulcus.16 By having the haptics rest in different positions it creates different compression forces that in turn will affect forward bulging of the lens, thus the vault. A lens with the haptics resting in sulcus may present a lower compression, due to a larger sulcus-to-sulcus distance compared to a lens with the haptics positioned on the ciliary body. Also, the position of the ICL optic plane will vary depending on whether the lens haptics rests on the ciliary sulcus or on the ciliary body since both resting planes are axially separated by approximately 500µm.31 The variability in the ICL haptics resting position has been shown in other studies as Choi et al32 and Elshafei et al15 who reported 64.7% and 78.9% of their lenses resting on ciliary sulcus respectively, and Kojima et al33 35.3% resting on the ciliary body. Zhang et al advanced as an explanation for the variability in haptics position the inability to intraoperatively image the haptics and place them in the desired position.16 Another factor contributing to inter-eye differences in vault is the variability in the posterior chamber anatomy such as the presence of unilateral iridociliary cysts. In a recent study, Li et al reported a prevalence of cysts of 36.1% in patients operated with myopic ICL; from these, approximately 80% were unilateral.34 Although the central vault did not differ between eyes with and without cysts, the average vault in eyes presenting cysts was 100 μm higher than in the eyes without cysts. Considering these arguments, the current vault prediction formulas7–11 which are solely based on preoperative biometry and lens parameters, may require the introduction of additional factors characterizing the postoperative resting position of the lens. Currently, not considering the postoperative position of the ICL nor particular characteristics in the posterior chamber anatomy, the prediction of the vault in eyes with similar anatomy is limited to 81%.
The amount of vault difference (absolute vault ΔE) increased with vault magnitude (R=0.31), as can be seen by the increasing averages in absolute vault ΔE 50.0 ± 50.2, 85.1 ± 75.5 and 137.4 ± 71.4 µm for the low (<250 µm), intermediate (250 to 750 µm) and high (>750 µm) respectively. Lee et al showed that the main determinant for the vault is the amount of horizontal compression (difference between lens size and transverse size of the eye) to which the lens is exposed (Correlation between HC and vault R=0.50, data not shown).9 In this study, absolute vault ΔE was significantly correlated with the amount of HC (R=0.27). Lenses presenting stronger compressions (generally higher vaults) may be more susceptible to external forces that will influence the bulging of the lens. These effects on the lens’ shape will tend to be smaller in eyes presenting lower compressions because in these cases the lenses will tend to maintain their intrinsic sagittal depth. The association between HC and vault ΔE may also indicate that at the time of positioning an ICL with higher compression (i.e., where the transverse size of the eye is considerably smaller than the ICL size) the ICL may position the haptics in different structures due to the reduced space and as discussed previously, this may lead to differences in vault between the two eyes.
Light induced variations in pupil size have been shown to affect the vault magnitude.13,35 More recently Gonzalez-Lopez et al reported that smaller (<250 µm) vaults were less affected (122 µm of variation) by light induced myosis compared to vaults above 500 µm (211µm), when the pupils constricted by approximately 2.5 mm.14 The effect of pupil myosis was also investigated under the influence of accommodation showing no significant variation in the vault.5,36 In the present study, the effect of pupil size was investigated either by the average pupil size and inter-eye pupil size difference, and it was proven that neither was associated with the amount of vault ΔE. Therefore, the influence of physiological variations in pupil size plays a minor role in explaining the vault differences between fellow eyes.
The inter-eye differences presented here have implications at the time of deciding the ICL size for the second operated eye, especially for the cases where the first operated eye has a vault below 250 µm or above 750 µm. From the seven cases classified as having low vault two patients presented vault ΔE higher than 100 µm. Considering the minimum recommended vault size of 100 µm4 and a vault in the first operated eye of 125 µm, approximately 25% of the patients would be at risk of presenting a vault smaller than 100 µm in the second eye. In the opposite direction, considering 1000 µm as the upper safety boundary for the vault,4 a patient with a vault in the first operated eye close to 850 µm has a 30% chance of having a vault equal or above 1000 µm in the second eye. These estimations can be used by the surgeon to modify the size of the ICL in the second eye or maintain the lens size but implant the ICL vertically. The rotation of the ICL to the vertical meridian has been shown to reduce the vault by 384 ± 217µm, but this approach is limited to spherical lenses.37 Using a conservative approach to minimise the chance of vaults <100µm and >1000µm, in case of bilateral surgery, if the first operated eye has a vault smaller than 260 µm, the closest higher ICL size should be considered for implantation in the second eye; in the opposite direction if the first operated eye has a vault higher than 720 µm, then the closest smaller ICL size should be used. When ordering the lenses, the surgeon should order the sizes recommended by the nomogram, but warn the patient that the second eye surgery depends on the effect of the ICL sizing in the first operated eye.
One limitation of this study is the inability to relate the vault ΔE with the lens resting position. This analysis would be helpful in understanding the influence of the lens resting position in the vault.15,38 Regardless of this limitation, the study reports the range of differences observed in a population with postoperative stable follow-up and using the standard technique for vault assessment. It provides information about the inter-eye variability in vault and addresses the need for further research regarding the association between vault and postoperative anatomy. A second limitation in this study was the use of a single vault per eye instead of an average of three measurements. This limitation could have been overcome in a prospective study and would have helped to dilute small differences in the scan position and improve the accuracy of vault measurement. A single measurement, however is the standard clinical protocol in our clinical setting therefore reflecting the retrospective nature of the study. Despite this, the maximum differences measured in vault between fellow eyes are four times higher than the repeatability of vault measurements using the AS-OCT.22
Conclusion
This study showed that eyes with similar anterior segment anatomy, when implanted with a similar ICL, may present differences in vault size as high as 240 µm. This value can be used as a reference by surgeons to interpret the significance of a difference in vault, i.e. fellow eyes with vault differences higher than this reference may require closer monitoring. Also, the findings can guide the clinician regarding the sizing of the lens in the second operated eye. Finally, this range of variability can assist in explaining the lack of accuracy in vault prediction formulas based on preoperative biometric parameters and vault predictions should be done considering a 240 µm uncertainty window.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Sanders DR, Doney K, Poco M. United States food and drug administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology. 2004;111(9):1683–1692.
2. Sanders DR, Schneider D, Martin R, et al. Toric implantable collamer lens for moderate to high myopic astigmatism. Ophthalmology. 2007;114(1):54–61. doi:10.1016/j.ophtha.2006.08.049
3. Reinstein DZ, Lovisolo CF, Archer TJ, Gobbe M. Comparison of postoperative vault height predictability using white-to-white or sulcus diameter–based sizing for the visian implantable collamer lens. J Refract Surg. 2013;29(1):30–35. doi:10.3928/1081597X-20121210-02
4. Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059–1077. doi:10.2147/OPTH.S111620
5. Lindland A, Heger H, Kugelberg M, Zetterstrom C. Vaulting of myopic and toric implantable collamer lenses during accommodation measured with visante optical coherence tomography. Ophthalmology. 2010;117(6):1245–1250. doi:10.1016/j.ophtha.2009.10.033
6. Fernandez-Vigo JI, Macarro-Merino A, Fernandez-Vigo C, et al. Effects of implantable collamer lens V4c placement on iridocorneal angle measurements by fourier-domain optical coherence tomography. Am J Ophthalmol. 2016;162:43–52 e1. doi:10.1016/j.ajo.2015.11.010
7. Hernández-Matamoros JL, Prieto J, Prieto Garrido FLGE. Preliminary results of ICL implantation using UBM and regression models. In: ASCRS Symposium on Cataract, IOL and Refractive Surgery. San Diego, California, USA; 2007.
8. Dougherty PJ, Rivera RP, Schneider D, Lane SS, Brown D, Vukich J. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13–18. doi:10.1016/j.jcrs.2010.07.014
9. Lee DH, Choi SH, Chung ES, Chung TY. Correlation between preoperative biometry and posterior chamber phakic visian implantable collamer lens vaulting. Ophthalmology. 2012;119(2):272–277. doi:10.1016/j.ophtha.2011.07.047
10. Zheng QY, Xu W, Liang GL, Wu J, Shi JT. Preoperative biometric parameters predict the vault after ICL implantation: a Retrospective Clinical Study. Ophthalmic Res. 2016;56(4):215–221. doi:10.1159/000446185
11. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Implantable collamer lens sizing method based on swept-source anterior segment optical coherence tomography. Am J Ophthalmol. 2018;187:99–107. doi:10.1016/j.ajo.2017.12.015
12. Lee H, Kang DSY, Choi JY, et al. Analysis of pre-operative factors affecting range of optimal vaulting after implantation of 12.6-mm V4c implantable collamer lens in myopic eyes. BMC Ophthalmol. 2018;18(1):163. doi:10.1186/s12886-018-0835-x
13. Lee H, Kang SY, Seo KY, et al. Dynamic vaulting changes in V4c versus V4 posterior chamber phakic lenses under differing lighting conditions. Am J Ophthalmol. 2014;158(6):1199–1204 e1. doi:10.1016/j.ajo.2014.08.020
14. Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Vila-Arteaga J, Beltran J, Baviera J. Dynamic assessment of light-induced vaulting changes of implantable collamer lens with central port by swept-source OCT: Pilot Study. Transl Vis Sci Technol. 2018;7(3):4. doi:10.1167/tvst.7.3.4
15. Elshafei AM, Genaidy MM, Moharram HM. In vivo positional analysis of implantable collamer lens using ultrasound biomicroscopy. J Ophthalmol. 2016;2016:4060467. doi:10.1155/2016/4060467
16. Zhang J, Luo HH, Zhuang J, Yu KM. Comparison of anterior section parameters using anterior segment optical coherence tomography and ultrasound biomicroscopy in myopic patients after ICL implantation. Int J Ophthalmol. 2016;9(1):58–62.
17. Kamiya K, Shimizu K, Ando W, Igarashi A, Iijima K, Koh A. Comparison of vault after implantation of posterior chamber phakic intraocular lens with and without a central hole. J Cataract Refract Surg. 2015;41(1):67–72. doi:10.1016/j.jcrs.2014.11.011
18. Schmidinger G, Lackner B, Pieh S, Skorpik C. Long-term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology. 2010;117(8):1506–1511. doi:10.1016/j.ophtha.2009.12.013
19. Guber I, Bergin C, Perritaz S, Majo F. Correcting interdevice bias of horizontal white-to-white and sulcus-to-sulcus measures used for implantable collamer lens sizing. Am J Ophthalmol. 2016;161:116–25 e1. doi:10.1016/j.ajo.2015.09.037
20. Ang M, Chong W, Tay WT, et al. Anterior segment optical coherence tomography study of the cornea and anterior segment in adult ethnic South Asian Indian eyes. Invest Ophthalmol Vis Sci. 2012;53(1):120–125. doi:10.1167/iovs.11-8386
21. Zhang XF, Li M, Shi Y, Wan XH, Wang HZ. Repeatability and agreement of two anterior segment OCT in myopic patients before implantable collamer lenses implantation. Int J Ophthalmol. 2020;13(4):625–631. doi:10.18240/ijo.2020.04.15
22. Yang Y, Wan T, Yin H, Yang Y, Wu F, Wu Z. Comparative study of anterior segment measurements using 3 different instruments in myopic patients after ICL implantation. BMC Ophthalmol. 2019;19(1):1–8.
23. Goldsmith JA, Li Y, Chalita MR, et al. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology. 2005;112(2):238–244. doi:10.1016/j.ophtha.2004.09.019
24. Baikoff G, Bourgeon G, Jodai HJ, Fontaine A, Lellis FV, Trinquet L. Pigment dispersion and artisan phakic intraocular lenses: crystalline lens rise as a safety criterion. J Cataract Refract Surg. 2005;31(4):674–680. doi:10.1016/j.jcrs.2004.09.034
25. Umurhan Akkan JC, Tuncer K, Elbay A. Postsurgical cystoid macular edema following posterior chamber toric phakic intraocular lens implantation surgery: a case report. Case Rep Ophthalmol. 2015;6(2):223–227. doi:10.1159/000437013
26. Reinstein DZ, Archer TJ, Silverman RH, Rondeau MJ, Coleman DJ. Correlation of anterior chamber angle and ciliary sulcus diameters with white-to-white corneal diameter in high myopes using artemis VHF digital ultrasound. J Refract Surg. 2009;25(2):185–194. doi:10.3928/1081597X-20090201-03
27. Kawamorita T, Uozato H, Kamiya K, Shimizu K. Relationship between ciliary sulcus diameter and anterior chamber diameter and corneal diameter. J Cataract Refract Surg. 2010;36(4):617–624. doi:10.1016/j.jcrs.2009.11.017
28. Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33(1):7–14. doi:10.1111/opo.12009
29. Armstrong RA. When to use the bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):7–14. doi:10.1111/opo.12131
30. Kamiya K, Shimizu K, Kawamorita T. Changes in vaulting and the effect on refraction after phakic posterior chamber intraocular lens implantation. J Cataract Refract Surg. 2009;35(9):1582–1586. doi:10.1016/j.jcrs.2009.03.052
31. Sugiura T, Kaji Y, Tanaka Y. Anatomy of the ciliary sulcus and the optimum site of needle passage for intraocular lens suture fixation in the living eye. J Cataract Refract Surg. 2018;44(10):1247–1253. doi:10.1016/j.jcrs.2018.07.017
32. Choi K, Chung SE, Chung TY, Chung ES. Ultrasound biomicroscopy for determining visian implantable contact lens length in phakic IOL implantation. J Refract Surg. 2007;23(4):362–367. doi:10.3928/1081-597X-20070401-08
33. Kojima T, Maeda M, Yoshida Y, et al. Posterior chamber phakic implantable collamer lens: changes in vault during 1 year. J Refract Surg. 2010;26(5):327–332. doi:10.3928/1081597X-20090617-11
34. Li Z, Xu Z, Wang Y, Liu Q, Chen B. Implantable collamer lens surgery in patients with primary iris and/or ciliary body cysts. BMC Ophthalmol. 2018;18(1). doi:10.1186/s12886-018-0935-7
35. Lindland A, Heger H, Kugelberg M, Zetterström C. Changes in vaulting of myopic and toric implantable collamer lenses in different lighting conditions. Acta Ophthalmol. 2012;90(8):788–791. doi:10.1111/j.1755-3768.2011.02224.x
36. Lee H, Kang DSY, Ha BJ, et al. Effect of accommodation on vaulting and movement of posterior chamber phakic lenses in eyes with implantable collamer lenses. Am J Ophthalmol. 2015;160(4):710–6 e1. doi:10.1016/j.ajo.2015.07.014
37. Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Beltran J, Llovet F, Baviera J. Optimization of the lens sizing for the second eye based on the vault obtained in the first eye in bilateral myopic collamer phakic intraocular lens surgery. Arch Soc Esp Oftalmol. 2018;93(8):368–374. doi:10.1016/j.oftal.2018.04.002
38. Zhang X, Chen X, Wang X, Yuan F, Zhou X. Analysis of intraocular positions of posterior implantable collamer lens by full-scale ultrasound biomicroscopy. BMC Ophthalmol. 2018;18(1):114. doi:10.1186/s12886-018-0783-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.