Back to Journals » Research and Reports in Tropical Medicine » Volume 14
Insights into Magnetic Resonance Imaging Findings in Central Nervous System Paracoccidioidomycosis: A Comprehensive Review
Authors Costa RS , Hygino da Cruz LC Jr, de Souza SR, Ventura N, Corrêa DG
Received 19 April 2023
Accepted for publication 29 July 2023
Published 3 August 2023 Volume 2023:14 Pages 87—98
DOI https://doi.org/10.2147/RRTM.S391633
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Rangel de Sousa Costa,1 Luiz Celso Hygino da Cruz Jr,2 Simone Rachid de Souza,3 Nina Ventura,1 Diogo Goulart Corrêa2
1Department of Radiology, Paulo Niemeyer State Brain Institute, Rio de Janeiro, RJ, Brazil; 2Department of Radiology, Clínica de Diagnóstico por Imagem (CDPI)/DASA, Rio de Janeiro, RJ, Brazil; 3Department of Pathology, Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Correspondence: Diogo Goulart Corrêa, Department of Radiology, Clínica de Diagnóstico por Imagem (CDPI)/DASA, Avenida das Américas, 4666, 302A, 303, 307, 325, 326, Rio de Janeiro, RJ, Brazil, Tel +55 21 993843617, Email [email protected]
Abstract: Paracoccidioidomycosis (PCM) is a infection caused by the thermodimorphic fungus Paracoccidioides spp. (P. lutzii and, mainly, P. brasiliensis). This infection predominantly affects rural male workers aged between 30 and 50 years old who deal with soil on daily activities. Clinically, the disease is classified as acute/subacute phase, which evolves rapidly, secondary to dissemination of the fungus through to the phagocytic-mononuclear system, leading to fever, weight loss, and anorexia, associated with hepatosplenomegaly and lymphadenopathy, which can be complicated with suppuration and fistulization; and chronic phase, which corresponds to 74% to 95% of symptomatic cases, with a common pulmonary involvement. Central nervous system involvement is almost always a characteristic of the chronic form. Inhalation is the most common route of primary infection, usually affecting the lungs, forming the primary complex. From the primary complex, hematogenic dissemination can occur to any organ, including the brain and spinal cord. Although PCM of the central nervous system diagnosis is usually based on histopathological analysis and the imaging features are not specific for PCM, computed tomography and magnetic resonance imaging can demonstrate evidences of granuloma, abscess, meningitis, or a combination of these lesions, contributing to a preoperative diagnosis, especially when considered in conjunction with epidemiology. In this article, we review the pathophysiology, clinical manifestations and imaging aspects of neuro-PCM.
Keywords: paracoccidioidomycosis, neuro-PCM, magnetic resonance imaging, granuloma, meningitis
Introduction
Paracoccidioidomycosis (PCM) is a fungal infection caused by the thermodimorphic fungus Paracoccidioides spp., which primarily inhabits the soil and is endemic in Latin America.1 The disease was first described in 1908 by a Brazilian physician, Adolfo Lutz, and affects at least 10 million people, from Mexico to Argentina.2,3 This fungus is slow-growing and forms colonies that produce infective propagules, called conidia.4 The soil and plants are the primary habitats, and human infections seem more prevalent in regions where clayey soil is more abundant. Infection rates are estimated at 10% of the population in endemic areas, but only up to 2% of infected individuals will develop disseminated forms of the disease.3 There are two main species of pathogenic Paracoccidioides: P. brasiliensis and P. lutzii. P. brasiliensis is composed of a complex of at least five phylogenetic species: S1a, S1b, PS2, PS3 and PS4.4,5 P. lutzii encompasses only one single species.4
PCM predominantly affects rural male workers aged between 30 and 50 years old, who deal with contaminated soil by the fungus on daily activities, usually related with work, such as farming, earthworks, soil preparation, gardening and transportation of vegetable products.1,6 Inhalation is the most common route of infection. Conidia transforms into oval to round yeast that primarily infect the lungs. PCM may also affect a variety of other organs and systems, including the brain and spinal cord.7
Typically, the neurological involvement of PCM is rare and it occurs in the chronic form of the disease, usually associated with significant sequelae and high mortality. Neurological manifestations of PCM are unspecific, depending on the lesion location. PCM can present in several different forms in the central nervous system, including granuloma, abscess, meningitis and a combination of these presentations. The spinal cord can also be affected, mostly with a pseudotumoral presentation.8
Several methods have been helpful to diagnose PCM. The definitive gold standard diagnosis is based on identification of the fungus on microscopy, culture, or histology,9 but imaging may help with diagnostic suspicion and to guide the biopsy site. The infection can also be diagnosed by a positive intradermal reaction to specific antigens and/or necropsy findings of latent fungi, but this is not routinely performed.4
The most characteristic pattern of neuro-PCM on brain magnetic resonance imaging (MRI) is single or multiple pseudotumoral lesions, with hypointense signal on T2-weighted imaging and peripheral contrast-enhancement, due to granulomas, but other granulomatous diseases may show similar findings.8 Despite not having a pathognomonic aspect on neuroimaging exams, imaging aspects of neuro-PCM, in correlation with clinical aspects, epidemiological data and chest imaging exams can be important for an early diagnosis, which may in turn address to a positive impact on patient prognosis.10 In this article, we review the pathophysiology, clinical manifestations and imaging aspects of neuro-PCM, in conventional and advanced imaging sequences, with a focus on the contribution that neuroimaging can provide to the final diagnosis.
Pathophysiology
The most common route of infection is through inhalation, after which the conidia transform to yeast. From its natural habitat, the fungus is inhaled and penetrates the host, usually through the lungs or exceptionally through the skin, due to a lesion caused by an infected wound. Then, the infectious agent can be destroyed or multiply, to produce an inoculation lesion, and be drained to regional lymph nodes, forming the primary complex. From the primary complex, hematogenic dissemination can occur to any organ (metastatic foci).11 During this period, there may be no clinical signs of infection, but the sensitization and an immunospecific response may be detected through a positive response for paracoccidioidin intradermal test. Then, different scenarios may be seen. First, the disease may regress, forming sterile scars. It can also regress, with the maintenance of viable fungi and formation of quiescent foci (including the metastatic foci); or it may progress, with the appearance of clinical signs and symptoms of the disease.4,11
The primary site of infection is usually the lung, but it is often subclinical. A reactivation may occur years after the primary infection, manifesting mainly in the lungs, but other organs, such as mucous and cutaneous tissues, lymph nodes, adrenal glands and the central nervous system can also be affected.12,13 It is important to remember that PCM is not contagious from person to person, because the fungus remains in the yeast form at body temperature.13
The control of the infection or manifestation of the disease depends on the host’s cellular immune response, in which T lymphocytes play an important role.14 Most infected individuals living in endemic areas will not develop any illness. These individuals exhibit a T-helper (Th)-1 immune response pattern, characterized by the release of cytokines that activate macrophages, CD4+ T lymphocytes, and CD8+ T lymphocytes, forming compact granulomas and controlling the fungal replication.15,16 However, dormant forms of the fungus may still exist inside these granulomas. The individuals who develop the disease most likely had deficient Th-1 responses to the fungus, developing Th-2 and Th-9 immune response patterns, not forming compact granulomas, and activating B lymphocytes, leading to formation of high levels of specific antibodies, hypergammaglobulinemia and eosinophilia.17
Although some patients manifest the disease isolated in the central nervous system, usually, when the brain or spinal cord are affected, the patient has a disseminated disease. To affect the central nervous system, the fungus disseminates from a primary focus (which usually is the lung), through hematological and/or lymphatic route, or also by contiguity.18
Clinical Manifestations
After settling in the lungs, the fungus can spread to any organ, where it may or may not produce symptoms. Clinically, PCM can be classified in I) infection, which may be asymptomatic; II) disease, which is symptomatic, and is subclassified in acute/subacute form (juvenile), and chronic form (adult); and III) residual form, which is the sequelae of the disease.11 Some individuals develop the symptoms of PCM years after the primary infection, sometimes when the contact with the contaminated soil has stopped, complicating the diagnosis.4
The acute/subacute form of the disease is responsible for 5–25% of the cases, predominantly affecting children, adolescents and young adults between 30 and 40 years old, equally distributed between genders. Typically, the clinical symptoms evolve rapidly, secondary to dissemination to the phagocytic-mononuclear system, leading to fever, weight loss and anorexia, associated with hepatosplenomegaly and lymphadenopathy, which can be complicated with suppuration and fistulization. Cutaneous or mucosal lesions can also be seen. Eosinophilia is a prominent laboratory finding. Neurological involvement is rare in the acute/subacute form.4,11
The chronic form corresponds to 74% to 95% of symptomatic PCM cases, typically manifesting between 30 and 60 years old, predominantly in men (men to women ratio: 22:1).4 Apparently, women are less likely to develop chronic PCM because estrogens inhibit the transformation of the aspirated conidia into yeast and modulate the immune response against the fungus.19,20 The symptoms of the chronic form begin slowly and may persist beyond 6 to 12 months before the diagnosis. In 90% of patients with this form of PCM, pulmonary involvement is observed. The mucosa of the upper aerodigestive tract and the skin are also commonly affected.4
Involvement of the central nervous system by PCM is almost always a characteristic of the chronic form, and it is associated with a high mortality and significant sequelae. In 21% of the cases, the onset of neurological symptoms appears before the onset of systemic symptoms. Whereas in 33% neurological symptoms appear simultaneously and in 46% they appeared after the onset of the systemic symptoms.18 The frequency of neurological involvement in PCM is extremely variable, but recent evidences demonstrated that it is higher than previously thought, reaching up to 27% of patients.21 A necropsy study demonstrated central nervous system involvement in 36% of cases.22
Symptoms of PCM in the central nervous system can be secondary to pseudotumoral lesions with intraparenchymal granulomas, or abscesses, and/or meningitis, secondary to lepto- and pachymeningeal inflammation, which usually occur in the skull base.23–25 The most common neurological symptoms, in decreasing order of frequency, are motor weakness, headache, seizures, ataxia, cognitive deficit and sensory alterations. Myelopathy is rarely seen.8
Although not usually related to immunosuppressive diseases, such as human immunodeficiency virus (HIV)-infection, cases of PCM as an opportunistic infection have been reported. PCM may be an occasional opportunistic infection in patients with impaired cellular immune response, often due to reactivation of residual lesions of a previous infection. Immunosuppressed patients usually develop disseminated forms of the disease, with a younger age at presentation, a greater proportion of women and higher mortality.26,27 In patients with solid organ transplantations, chronic PCM has been described mainly in kidney transplants. PCM in immunocompromised heart and liver transplant patients has also been reported.28
Diagnosis
The definitive diagnosis of PCM is made by visualization or isolation of the fungus from biopsy or necropsy. Although brain biopsy can confirm the diagnosis, it is an invasive procedure, and the lesion may be located in a region where the biopsy cannot be safely performed.13 Therefore, the diagnosis of neuro-PCM often originates from evidence of PCM in other organs. This is important, since almost 90% of patients with central nervous system PCM have evidence of the disease in other organs, especially the lungs.18
Cerebrospinal fluid (CSF) analysis reveals normal glucose, increased protein level, with increased gamma globulin, due to blood brain barrier breakdown and/or intrathecal antibody production. Hypercellularity can also be seen, with a predominance of monocytes and lymphocytes.13,18 Glycoprotein gp43, a major antigenic component of P. brasiliensis, as well as antibodies against this antigen can be found in the CSF. Detection of these glycoprotein and antibodies in CSF can be helpful in the diagnosis and treatment follow up.29,30 Antibodies anti-gp43 can be found in the CSF of 63% of patients with acute and chronic PCM, reflecting intrathecal production. Enzyme-Linked Immunosorbent Assay anti-gp43 of the CSF has high positive and negative predictive values, and it can aid in the diagnosis and treatment follow up.13 A probable PCM diagnosis can be performed in a patient with clinical manifestations compatible with PCM and positive antibodies for P. brasiliensis/P. lutzii.4 However, direct microscopic examination and culture for P. brasiliensis are usually negative in the CSF.31
Histopathological analysis of PCM exhibits the formation of granulomas, containing epithelioid cells and multinucleated giant cells, which may contain fungi in the cytoplasm. Necrosis may be present and be extensive, containing numerous fungal structures. Granulomas may appear in a compact and well-defined form, exhibiting fibrosis and infiltration of lymphocytes and plasmocytes in their periphery, and sometimes eosinophils; or in a looser form, with few epithelioid cells and giant cells, in the midst of edema and infiltration of neutrophils, plasmocytes, lymphocytes, macrophages and eosinophils, permeating a large number of fungal structures.32,33
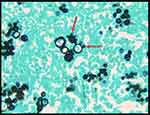
The fungus appears as rounded, yeast-like cells, with bilayered membrane, with or without single or multiple budding, from 4 to 40 µm in its largest diameter. Multiple exosporulation is characteristic of the fungus, giving the classic pilot’s wheel or “Mickey Mouse” head appearance (Figure 1).32,33 Although fungal structures can be sometimes visualized on routine hematoxylin and eosin staining, methenamine silver nitrate stains (Grocott-Gomori) and Schiff’s Periodic Acid (PAS) are employed to demonstrate the microorganism and confirm the diagnosis.34 The diagnosis of confirmed PCM is performed when the patient presents with clinical features compatible with the disease, as well as elements suggestive of P. brasiliensis or P. lutzii infection in body secretions or fluids, through microscopy analysis or culture, and/or tissue sample, through histopathological analysis.4
Imaging Features
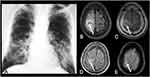
Imaging exams, especially computed tomography (CT) and MRI, are important for the diagnosis. Essentially, neuroimaging can demonstrate evidences of granuloma, abscess, meningitis, or a combination of these lesions (Table 1). However, the imaging features are not specific for PCM and these imaging findings have a long list of differential diagnosis, including other granulomatous diseases, other causes of abscesses, and meningitis.23,35,36 Although the diagnosis is based on histopathological analysis, depending on the clinical suspicion and epidemiological history, correlation with chest CT may help to narrow the differential diagnosis, since neuro-PCM is usually associated with pulmonary involvement. Typically, chest CT reveals several different patterns of abnormalities, such as ground-glass opacities, consolidations, interlobular septal thickening, nodules, masses, cavitation with or without internal septa and fibrosis. The most common imaging pattern of pulmonary PCM is a combination of two or more of these lesions in the same exam (Figure 2).37–39
 |
Table 1 Different Types of Neuro-PCM Presentation, Their Imaging Aspects and Main Differential Diagnoses |
Granuloma
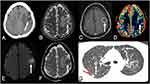
Granulomas are the most common presentation of neuro-PCM, which is characterized by single or multiple lesions in different parts of the central nervous system, without calcifications, with perilesional edema, and mass effect. On CT, this lesion is hypodense, with irregular margins, irregular peripheral contrast enhancement of varying thickness, associated with perilesional edema.10,33 Some lesions may have a hyperdense periphery with a hypodense center, and rarely, the lesions may be totally hyperdense, due to hemorrhages.36,40 The lesions are most commonly located in the infratentorial compartment, mainly in the cerebellum,8 and 53% of the cases present with two or more granulomas.10 Non communicating hydrocephalus can also be seen, mainly when the lesions are located in the cerebellum, due to fourth ventricle compression. However, hydrocephalus may also be due to leptomeningeal involvement in the basal cisterns, obstructing cerebrospinal fluid flow.10
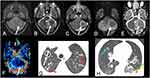
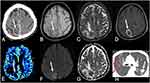
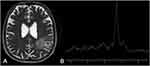
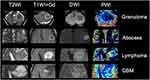
On MRI, granulomas typically present with a predominant hypointense signal on T2-weighted imaging, secondary to increased iron deposition, due to fungal elements and hemorrhages. On T1-weighted imaging, the lesions have hypointense signal intensity, sometimes they may present a slightly peripheral hyperintense signal, with peripheral or nodular gadolinium-enhancement (Figures 2 and 3).21,41,42 These lesions may or may not present restricted diffusion (Figure 4).8 Perfusion-weighted imaging (PWI) can demonstrate reduced relative cerebral blood volume (rCBV), on dynamic susceptibility contrast, and a slow and progressive ascending curve on dynamic contrast enhanced (DCE) (Figure 2).23,36 On magnetic resonance spectroscopy, there are increased lipids peak, suggesting intense necrosis, and decreased N-acetylaspartate and choline peaks, along with the absence of succinate, acetoacetate and alanine peaks (Figure 5).43 Recently, hippocampal sclerosis associated with PCM has also been described as a neuroimaging manifestation of this disease, associated with granulomas.44
The differential diagnosis of this lesion includes primary brain neoplasms, especially high-grade gliomas and metastasis. PWI, DWI and spectroscopy may help to differentiate between granulomas and neoplasms, since high-grade neoplasms and metastasis in general present with increased rCBV, restricted diffusion in their solid portions, associated with increased choline peak.45 Also, high grade neoplasms usually demonstrate multiple intratumoral susceptibility signal, due to intralesional hemorrhages, calcifications or tumoral neovascularity.46 However, a granuloma secondary to PCM cannot be differentiated from other granulomatous diseases, such as tuberculosis and sarcoidosis.23
Abscess Pattern
PCM can also simulate a pyogenic abscess. Brain CT shows a nonspecific hypodense lesion with a low attenuation central portion, with an iso- or hyperdense ring, which presents peripheral contrast-enhancement, surrounded by edema. MRI demonstrates abscesses as an expansive lesion with central hypointense signal on T1-weighted imaging (but slightly hyperintense related to cerebrospinal fluid), central hyperintense signal on T2-weighted imaging and fluid-attenuated inversion recovery (FLAIR), and a thin peripheral capsule, that has hypointense signal on T2-weighted imaging. The peripheral capsule also presents gadolinium-enhancement.47 The central portion of the lesion has restricted diffusion, which is related to the pus inside the cavity (Figure 6).48,49 On susceptibility-weighted imaging (SWI), the “dual ring sign” can be demonstrated, especially in lesions greater than 2 cm. It is characterized as two concentric rims at the margin of the lesion, with the outer rim hypointense and the inner rim hyperintense, which was previously thought to be specific of pyogenic abscess, but can be seen in other diseases.36,50 PWI also demonstrates reduced rCBV.
It may be difficult or even impossible to differentiate a pyogenic abscess from a fungal abscess by imaging alone. Spectroscopy can identify an isolated lipid peak in abscesses due to PCM,5 which is not expected in a pyogenic abscess. On the other hand, pyogenic abscesses may demonstrate peaks of amino acids (0.9 ppm), acetoacetate (1.9 ppm), and succinate (2.4 ppm).51 Trehalose, which appears as multiple peaks ranging from 3.6 ppm to 3.8 ppm in spectroscopy, is specific for fungal infection, although not highly sensitive.52
An abscess can also be differentiated from a necrotic high-grade glioma or metastasis, through PWI and DWI. While high-grade neoplasms and metastasis in general present increased rCBV and restricted diffusion in their solid peripheral portion, abscesses do not present increased rCBV and have restricted diffusion in their central necrotic portion.45 Moreover, the capsule in abscesses usually is thin and regular, whereas in neoplasms, the peripheral solid portion is thick and irregular (Figure 7).
Meningitis
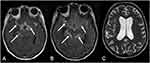
Meningitis related to PCM was first described in 1943.53 It can be associated with granulomas, or more rarely, an isolated finding. Also, the meningeal inflammation can be localized, particularly in the basal cisterns, or diffuse.18 Neuroimaging may demonstrate diffuse pachymeningeal or leptomeningeal enhancement, sometimes associated with hydrocephalus or cortical nodules. MRI is more sensitive than CT to detect these alterations and to monitor the treatment response.21,23,54 Furthermore, post-contrast-FLAIR is even more sensitive than post-contrast-T1-weighted imaging to detect meningeal enhancement, and it should be performed in suspected cases (Figure 8).55 The differential diagnosis of this pattern is any infectious or inflammatory meningitis, especially those that affect preferentially the basal cisterns, such as tuberculosis, syphilis and sarcoidosis.23,56,57
Spinal Cord
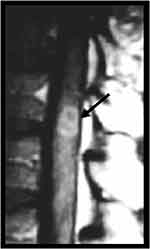
Spinal cord involvement in PCM is very rare, occurring in 4% of neuro-PCM cases and in only 0.6% of all PCM patients. The thoracic portion of the spinal cord is most commonly affected. Clinically, patients may present signs of transverse myelitis.23 MRI can demonstrate enlargement of the spinal cord, with an intramedullary expansive lesion, that shows hypointense signal on T2-weighted imaging, gadolinium-enhancement and peripheral edema, similar findings of a granuloma (Figure 9).58–61 The differential diagnosis for this kind of lesion is intramedullary neoplasms, such as ependymoma and astrocytoma, as well as pseudotumoral infectious and inflammatory lesions, such as tuberculosis and sarcoidosis.
Conclusion
Therefore, imaging can reveal evidences of granuloma, and more rarely abscess, meningitis, and/or a combination of these patterns in neuro-PCM, in the brain and spinal cord. Considering the imaging aspects, together with clinical and epidemiological data, one can suggest the possibility of PCM, and is crucial for an accurate early diagnosis and has a positive impact on patient treatment and prognosis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peçanha PM, Peçanha-Pietrobom PM, Grão-Velloso TR, et al. Paracoccidioidomycosis: what we know and what is new in epidemiology, diagnosis, and treatment. J FungiBasel). 2022;8(10):1098. doi:10.3390/jof8101098
2. Canteros CE. Paracoccidioidomicosis: crónica de una enfermedad olvidada [Paracoccidioidomycosis: chronicle of a neglected disease]. Medicina. 2018;78(3):180–184. Spanish.
3. Travassos LR, Taborda CP, Colombo AL. Treatment options for paracoccidioidomycosis and new strategies investigated. Expert Rev Anti Infect Ther. 2008;6(2):251–262. doi:10.1586/14787210.6.2.251
4. Shikanai-Yasuda MA, Mendes RP, Colombo AL, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop. 2017;50(5):715–740. doi:10.1590/0037-8682-0230-2017
5. Turissini DA, Gomez OM, Teixeira MM, McEwen JG, Matute DR. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet Biol. 2017;106:9–25. doi:10.1016/j.fgb.2017.05.007
6. Terçarioli GR, Bagagli E, Reis GM, et al. Ecological study of Paracoccidioides brasiliensis in soil: growth ability, conidia production and molecular detection. BMC Microbiol. 2007;7(1):92. doi:10.1186/1471-2180-7-92
7. Marques-da-silva SH, Rodrigues AM, de Hoog GS, Silveira-Gomes F, Camargo ZP. Occurrence of Paracoccidioides lutzii in the Amazon region: description of two cases. Am J Trop Med Hyg. 2012;87(4):710–714. doi:10.4269/ajtmh.2012.12-0340
8. de Oliveira VF, Magri MMC, Levin AS, Silva GD. Systematic review of neuroparacoccidioidomycosis: the contribution of neuroimaging. Mycoses. 2023;66(2):168–175. doi:10.1111/myc.13525
9. Silva Ferreira C, de Castro Ribeiro EM, Miranda Goes A, Mello Silva BD. Current strategies for diagnosis of paracoccidioidomycosis and prospects of methods based on gold nanoparticles. Future Microbiol. 2016;11:973–985. doi:10.2217/fmb-2016-0062
10. Gasparetto EL, Liu CB, de Carvalho Neto A, Rogacheski E. Central nervous system paracoccidioidomycosis: imaging findings in 17 cases. J Comput Assist Tomogr. 2003;27(1):12–17. doi:10.1097/00004728-200301000-00003
11. Franco M, Montenegro MR, Mendes RP, Marques SA, Dillon NL, Mota NG. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop. 1987;20(2):129–132. doi:10.1590/s0037-86821987000200012
12. Brummer E, Castaneda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6(2):89–117. doi:10.1128/CMR.6.2.89
13. de Almeida SM. Central nervous system paracoccidioidomycosis: an overview. Braz J Infect Dis. 2005;9(2):126–133. doi:10.1590/s1413-86702005000200002
14. Benard G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia. 2008;165(4–5):209–221. doi:10.1007/s11046-007-9065-0
15. Benard G, Romano CC, Cacere CR, Juvenale M, Mendes-Giannini MJ, Duarte AJ. Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine. 2001;13(4):248–252. doi:10.1006/cyto.2000.0824
16. Oliveira SJ, Mamoni RL, Musatti CC, Papaiordanou PM, Blotta MH. Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: comparison with infected and non-infected controls. Microbes Infect. 2002;4(2):139–144. doi:10.1016/s1286-4579(01)01521-0
17. de Castro LF, Ferreira MC, da Silva RM, Blotta MH, Longhi LN, Mamoni RL. Characterization of the immune response in human paracoccidioidomycosis. J Infect. 2013;67(5):470–485. doi:10.1016/j.jinf.2013.07.019
18. de Almeida SM, Queiroz-Telles F, Teive HA, Ribeiro CE, Werneck LC. Central nervous system paracoccidioidomycosis: clinical features and laboratorial findings. J Infect. 2004;48(2):193–198. doi:10.1016/j.jinf.2003.08.012
19. Shankar J, Restrepo A, Clemons KV, Stevens DA. Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev. 2011;24(2):296–313. doi:10.1128/CMR.00062-10
20. Martinez R. New trends in paracoccidioidomycosis epidemiology. J Fungi. 2017;3(1):1. doi:10.3390/jof3010001
21. Magalhães AC, Bacheschi LA, Caramelli P, et al. Paracoccidioidomicose do sistema nervoso central: estudo de cinco casos por ressonância magnética [Paracoccidioidomycosis of the central nervous system: study of 5 cases by magnetic resonance]. Rev Hosp Clin Fac Med Sao Paulo. 1993;48(2):94–97. Portuguese.
22. Franco M, Mendes RP, Moscardi-Bacchi M, Rezkallah-Iwasso M, Montenegro MR. Paracoccidioidomycosis. Baillières Clinl Trop Med Commun Dis. 1989;4:185–220.
23. Rosa Júnior M, Baldon IV, Amorim AFC, et al. Imaging paracoccidioidomycosis: a pictorial review from head to toe. Eur J Radiol. 2018;103:147–162. doi:10.1016/j.ejrad.2018.03.026
24. Francesconi F, Francesconi Do Valle AC, Silva MT, Costa RL, Carregal E, Talhari S. International issues: meningoencephalitis due to Paracoccidioides brasiliensis. Neurology. 2008;71(21):e65–e67. doi:10.1212/01.wnl.0000335266.60637.c3
25. Elias J
26. Martinez R. Epidemiology of paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo. 2015;19(Suppl 19):11–20. doi:10.1590/S0036-46652015000700004
27. de Almeida Jr. JN Jr, Peçanha-Pietrobom PM, Colombo AL. Paracoccidioidomycosis in immunocompromised patients: a literature review. J Fungi. 2018;5(1):2. doi:10.3390/jof5010002
28. Pereira FV, Alves KPO, Altemani AM, Reis F. Disseminated paracoccidioidomycosis with skull and mandible involvement in a heart transplant recipient. Rev Soc Bras Med Trop. 2022;55:e0110. doi:10.1590/0037-8682-0110-2022
29. Gardizani TP, Della Coletta AM, Romagnoli GG, et al. 43 kDa glycoprotein (gp43) from Paracoccidioides brasiliensis induced IL-17A and PGE2 production by human polymorphonuclear neutrophils: involvement of TLR2 and TLR4. J Immunol Res. 2019;2019:1790908. doi:10.1155/2019/1790908
30. de Almeida SM, Queiroz-Telles F, Doi EM, Ono M, Werneck LC. Anti-gp43 antibodies in the cerebrospinal fluid of patients with central nervous system involvement by paracoccidioidomycosis. Am J Clin Pathol. 2002;118(6):864–868. doi:10.1309/H2LU-UX28-7QHN-V5H3
31. Salina MA, Shikanai-Yasuda MA, Mendes RP, Barraviera B, Mendes Giannini MJ. Detection of circulating Paracoccidioides brasiliensis antigen in urine of paracoccidioidomycosis patients before and during treatment. J Clin Microbiol. 1998;36(6):1723–1728. doi:10.1128/JCM.36.6.1723-1728.1998
32. Góes AM, Silva LSS, Araújo AS, Cruz SG, Siqueira WC, Pedroso ERP. Paracoccidioidomicose (doença de Lutz-Splendore-Almeida): etiologia, epidemiologia e patogênese [Paracoccidioidomycosis disease (Lutz-Splendore-Almeida): etiology, epidemiology, and pathogenesis]. Rev Med Minas Gerais. 2014;24(1):61–66.
33. Filho GB. Bogliolo – Patologia [Bogliolo - Pathology].
34. Palmeiro M, Cherubini K, Yurgel LS. Paracoccidioidomicose – revisão da Literatura [Paracoccidioidomycosis – Literature Review]. Sci Med. 2005;15(4):274–278.
35. Rodacki MA, De Toni G, Borba LA, Oliveira GG. Paracoccidioidomycosis of the central nervous system: CT findings. Neuroradiology. 1995;37(8):636–641. doi:10.1007/BF00593377
36. Rosa Júnior M, Amorim AC, Baldon IV, et al. Paracoccidioidomycosis of the central nervous system: CT and MR imaging findings. Am J Neuroradiol. 2019;40(10):1681–1688. doi:10.3174/ajnr.A6203
37. de Morais RQ, Salomon MFB, Corbiceiro WCH, de Melo ASA, Corrêa DG. Imaging contribution for the diagnosis of disseminated paracoccidioidomycosis. Int J Infect Dis. 2020;101:206–209. doi:10.1016/j.ijid.2020.09.1482
38. Barreto MM, Marchiori E, Amorim VB, et al. Thoracic paracoccidioidomycosis: radiographic and CT findings. Radiographics. 2012;32(1):71–84. doi:10.1148/rg.321115052
39. Costa AN, Benard G, Albuquerque AL, et al. The lung in paracoccidioidomycosis: new insights into old problems. Clinics. 2013;68(4):441–448. doi:10.6061/clinics/2013(04)02
40. da Silva CE, Cordeiro AF, Gollner AM, Cupolilo SM, Quesado-Filgueiras M, Curzio MF. Paracoccidioidomicose do sistema nervoso central: relato de caso [Paracoccidioidomycosis of the central nervous system: case report]. Arq Neuropsiquiatr. 2000;58(3A):741–747. Portuguese. doi:10.1590/s0004-282x2000000400024
41. Reis F, Collier PP, Souza TF, et al. Neuroparacoccidioidomycosis (NPCM): magnetic resonance imaging (MRI) findings. Mycopathologia. 2013;175(1–2):181–186. doi:10.1007/s11046-012-9607-y
42. da Rocha AJ, Maia AC
43. Faria AV, Dabus GC, Zanardi VA, Cendes F. Proton magnetic resonance spectroscopy and magnetic resonance imaging findings in a patient with central nervous system paracoccidioidomycosis. J Neuroimaging. 2004;14(4):377–379. doi:10.1177/1051228404267053
44. Rosa-JÚnior M, Grenfell MLR, Peçanha PM. Hippocampal sclerosis in paracoccidioidomycosis. Arq Neuropsiquiatr. 2020;78(6):384. doi:10.1590/0004-282X20200015
45. Currie S, Fatania K, Matthew R, et al. A comprehensive clinical review of adult-type diffuse glioma incorporating the 2021 World Health Organization classification. Neurographics. 2022;12:43–70. doi:10.3174/ng.2100034
46. Hsu CC, Watkins TW, Kwan GN, Haacke EM. Susceptibility-weighted imaging of glioma: update on current imaging status and future directions. J Neuroimaging. 2016;26(4):383–390. doi:10.1111/jon.12360
47. Abud LG, Queiroz RM, Abud TG. Concomitant pulmonary and central nervous system paracoccidioidomycosis with cerebellar abscess. Rev Soc Bras Med Trop. 2015;48(6):789. doi:10.1590/0037-8682-0259-2015
48. Ebisu T, Tanaka C, Umeda M, et al. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996;14(9):1113–1116. doi:10.1016/s0730-725x(96)00237-8
49. Riechelmann RS, Rodrigues LH, Avelar TM, et al. Isolated neuroparacoccidioidomycosis as a pseudotumoral lesion in the absence of systemic disease. Surg Neurol Int. 2020;11:151. doi:10.25259/SNI_224_2020
50. Toh CH, Wei KC, Chang CN, et al. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. Am J Neuroradiol. 2012;33(8):1534–1538. doi:10.3174/ajnr.A2986
51. Pal D, Bhattacharyya A, Husain M, Prasad KN, Pandey CM, Gupta RK. In vivo proton MR spectroscopy evaluation of pyogenic brain abscesses: a report of 194 cases. Am J Neuroradiol. 2010;31(2):360–366. doi:10.3174/ajnr.A1835
52. Duarte SBL, Oshima MM, Mesquita JVDA, Do Nascimento FBP, de Azevedo PC, Reis F. Magnetic resonance imaging findings in central nervous system cryptococcosis: comparison between immunocompetent and immunocompromised patients. Radiol Bras. 2017;50(6):359–365. doi:10.1590/0100-3984.2016.0017
53. Pedro RDJ, Branchini ML, de Lucca RS, da Silveira ML, Facure NO, Amato Neto V. Paracoccidioidomicose de sistema nervoso central. A propósito de dois casos [Paracoccidioidomycosis of the central nervous system. Apropos of 2 cases]. Rev Inst Med Trop Sao Paulo. 1980;22(5):269–274. Portuguese.
54. Lorenzoni PJ, Chang MR, Paniago AM, Salgado PR. Meningite paracoccidioidomicótica: relato de caso [Paracoccidioidomycosis meningitis: case report]. Arq Neuropsiquiatr. 2002;60(4):1015–1018. Portuguese. doi:10.1590/s0004-282x2002000600025
55. Roozpeykar S, Azizian M, Zamani Z, et al. Contrast-enhanced weighted-T1 and FLAIR sequences in MRI of meningeal lesions. Am J Nucl Med Mol Imaging. 2022;12(2):63–70.
56. Corrêa DG, de Souza SR, Freddi TAL, et al. Imaging features of neurosyphilis. J Neuroradiol. 2023;50(2):241–252. doi:10.1016/j.neurad.2023.01.003
57. Kandemirli SG, Bathla G. Neuroimaging findings in rheumatologic disorders. J Neurol Sci. 2021;427:117531. doi:10.1016/j.jns.2021.117531
58. França AFEDC, Velho PENF, Reis F. Spinal cord and cutaneous involvement in paracoccidioidomycosis. Rev Soc Bras Med Trop. 2021;54:e0115. doi:10.1590/0037-8682-0115-2021
59. Almeida TAL, Mallmann AB, Crusius PS, et al. Medullary paracoccidioidomycosis treated successfully with oral itraconazole. Arquivos Brasileiros de Neurocirurgia. 2016;35(04):352–356. doi:10.1055/S-0035-1571267
60. Do Valle AC, Skacel M, Costa RL, Ribeiro CT, Montagna NA, da Cruz LC. A case report of intraspinal paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo. 1998;40(3):203–207. doi:10.1590/s0036-46651998000300014
61. Pacheco RA, Arruda WO, Hunhevicz SC, Tsubouchi MH, Torres LF. Thoracic intraspinal paracoccidioidomycosis. Case report. Arq Neuropsiquiatr. 1996;54(3):474–478. doi:10.1590/s0004-282x1996000300018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.