Back to Journals » International Journal of Nanomedicine » Volume 19
Innovations in Breaking Barriers: Liposomes as Near-Perfect Drug Carriers in Ischemic Stroke Therapy
Authors Zhang Q , Huang S , Liu X, Wang W, Zhu Z , Chen L
Received 31 January 2024
Accepted for publication 13 April 2024
Published 23 April 2024 Volume 2024:19 Pages 3715—3735
DOI https://doi.org/10.2147/IJN.S462194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Qiankun Zhang,* Songze Huang,* Xiaowen Liu, Wei Wang, Zhihan Zhu, Lukui Chen
Department of Neurosurgery, Southern Medical University Hospital of Integrated Traditional Chinese and Western Medicine, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lukui Chen, Department of Neurosurgery, Southern Medical University Hospital of Integrated Traditional Chinese and Western Medicine, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China, Email [email protected]
Abstract: Liposomes, noted for their tunable particle size, surface customization, and varied drug delivery capacities, are increasingly acknowledged in therapeutic applications. These vesicles exhibit surface flexibility, enabling the incorporation of targeting moieties or peptides to achieve specific targeting and avoid lysosomal entrapment. Internally, their adaptable architecture permits the inclusion of a broad spectrum of drugs, contingent on their solubility characteristics. This study thoroughly reviews liposome fabrication, surface modifications, and drug release mechanisms post-systemic administration, with a particular emphasis on drugs crossing the blood-brain barrier (BBB) to address lesions. Additionally, the review delves into recent developments in the use of liposomes in ischemic stroke models, offering a comparative evaluation with other nanocarriers like exosomes and nano-micelles, thereby facilitating their clinical advancement.
Keywords: liposome, BBB, ischemic stroke, drug carrier, liposome-based engineering
Graphical Abstract:
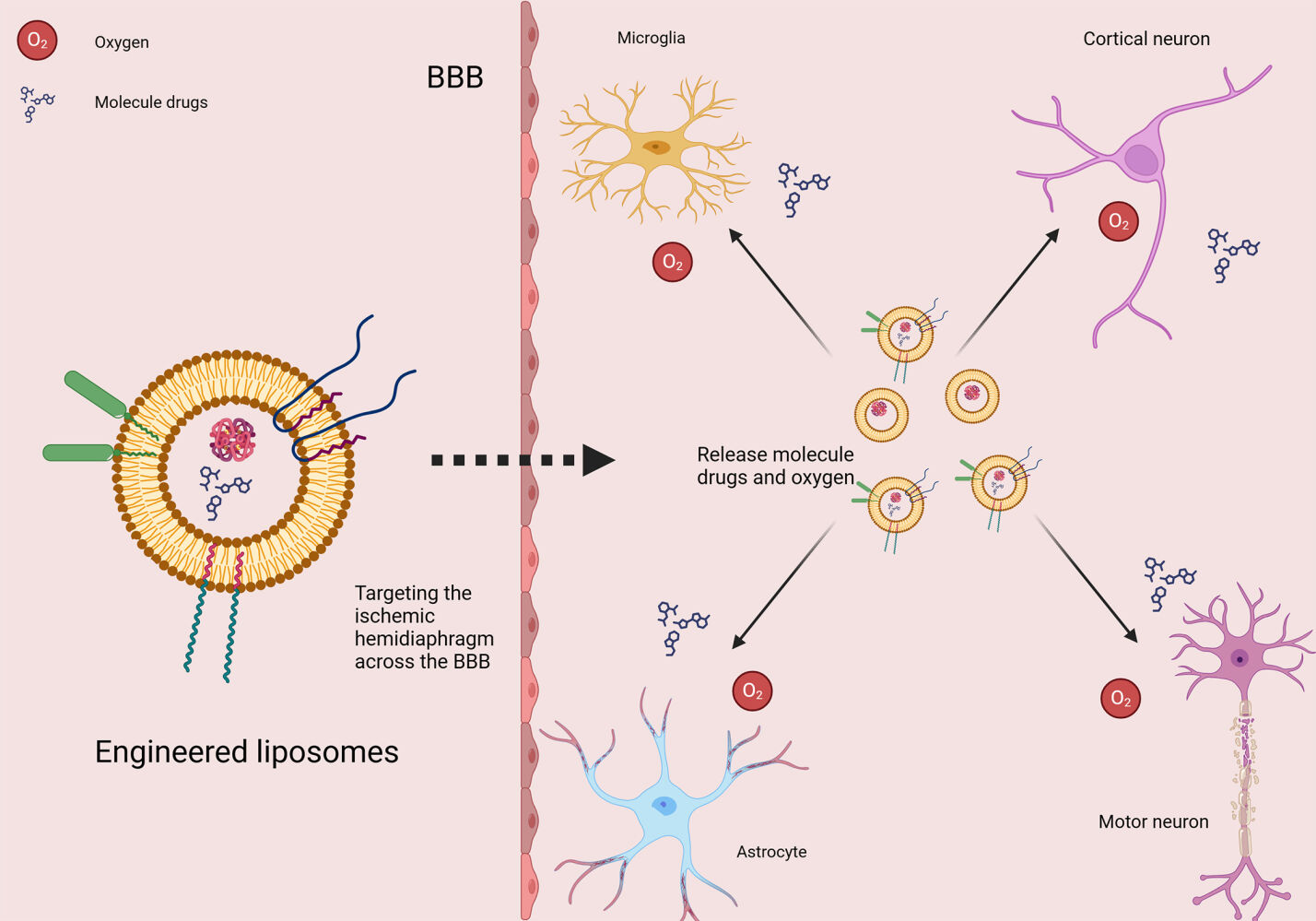
Introduction
Ischemic stroke, a leading cause of global morbidity and mortality, presents significant treatment challenges due to the complex cerebral vascular network and the BBB. Advances in nanotechnology have spurred the development of novel therapeutic strategies, notably liposomes, as promising nanocarriers for drug delivery.1,2 This review aims to explore the promising new area of liposome technology, focusing on its potential applications in the treatment of ischemic stroke.
Liposomes, spherical vesicles formed from one or more phospholipid bilayers, are distinguished by their unique drug-delivery capabilities. Their adjustable particle size and extensive surface modifiability are vital for their effectiveness.3 This size adaptability allows precise control over the release and distribution of drugs, enhancing targeted delivery while minimizing systemic side effects.4,5 Furthermore, customizing liposome surfaces with specific groups or peptides enhances their targeting precision, ensuring accurate site-specific action and evading lysosomal degradation.
A notable strength of liposomes is their adaptability to various drugs.6 Depending on the drug’s solubility, they can encapsulate hydrophilic drugs in their aqueous core and hydrophobic drugs in the lipid bilayer. This adaptability extends the range of therapeutic agents effectively delivered to ischemic brain regions.
Crucial to their clinical relevance is the ability of liposomes to cross the BBB post-systemic administration and reach ischemic lesions. This review examines the principles of liposome synthesis, surface modification techniques, and the complex drug release mechanisms following BBB penetration. It also comprehensively compares liposomes with other nanocarriers like exosomes, nano micelles, and cyclodextrins, highlighting their respective advantages and limitations.
In conclusion, this review focuses on the latest applications and advancements of liposomes in ischemic stroke models, aiming to illuminate their potential for clinical translation. By objectively evaluating liposomes against other nanocarriers, the review underscores their suitability as drug delivery vehicles and advocates for their prospective inclusion in standard ischemic stroke treatment protocols.
Physiological Changes Following the Ischemic Stroke Process
Etiology and Pathogenesis
Ischemic stroke, a significant concern in cerebrovascular pathology, often results from chronic or acute ischemic episodes and affects a broad demographic (Figure 1). Its etiology includes various factors.7,8 Vascular corresponding diseases positively correlate with the incidence of ischemic stroke.9–11 Central to this is thrombosis, characterized by plaque accumulation in cerebral arteries due to coagulation abnormalities, high cholesterol levels, or genetic factors.12,13 Embolism, a major contributing factor, involves the migration of a thrombus from regions such as the heart to the cerebral vasculature.14,15 Additionally, arterial stenosis and systemic hypovolemia, which decrease cerebral blood flow, are significant contributors to ischemic events.
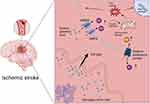 |
Figure 1 Mechanisms of neuronal injury after stroke. Created with BioRender.com. |
Neuronal Vulnerability and Metabolic Disruption
Ischemic events lead to a shortage of critical elements (oxygen, glucose) essential for neuron survival, as neurons are susceptible to such deprivations.16 Under normal conditions, cerebral blood flow is between 50 and 60 mL/100 g of tissue per minute. However, ischemia can decrease this to as low as 20 mL/100 g of tissue per minute, which is inadequate for maintaining neuronal function and consequently results in the shutdown of neural circuits.17 This reduction in blood flow disrupts neuronal metabolism, leading to acidosis, ionic imbalance, and cellular swelling. Extended periods of ischemia force metabolism to switch to anaerobic pathways, causing an increase in lactic acid (exceeding 20–25 μmol/g) and a drop in neuronal pH from 7.0 to approximately 6.0.18–20 Such changes aggravate swelling and can lead to irreversible neuronal damage. Additionally, hypoxia in the infarct zone impairs mitochondrial NADH oxidation, contributing further to acidosis and neuronal injury.21,22
Ionic and Molecular Alterations
Cerebral ischemia alters intracellular ions, notably the extracellular accumulation of K+ and glutamate, disrupting ionic gradients and increasing neuronal and astrocyte depolarization.23–25 Excess glutamate triggers receptors like NMDA and AMPA, causing calcium, sodium, and water influx into neurons.26,27 This influx activates enzymes (metalloproteases, lipases, nucleases), producing free radicals, arachidonic acid, and nitric oxide. These processes damage mitochondrial membranes and organelles in neurons, releasing excess free radicals and causing localized neuronal death.28 Additionally, excess free radicals stimulate inflammatory cytokines (TNF-α, IL-1, IL-5, TGF-β), exacerbating ischemia and promoting neuronal death through oxidative and inflammatory pathways.29
Traditional Remedies and Challenges
Clinical Treatment Strategies
Antiplatelet and Thrombolysis
Ischemic stroke management predominantly utilizes thrombolytic drugs and antiplatelet therapy as central interventions (Figure 2).30–32 Tissue plasminogen activator (tPA), administered intravenously, is the foremost thrombolytic agent.33 It catalyzes the conversion of plasminogen to plasmin, dissolving fibrin in blood clots. The effectiveness of tPA is contingent on early administration, ideally within 4.5 hours post-stroke onset, with evidence indicating its success in reestablishing cerebral blood flow and improving patient outcomes.34,35
 |
Figure 2 The advantages and disadvantages of some stroke treatment methods. By Figdraw. |
Intracerebral haemorrhage (ICH), hemorrhagic transformation (HT), and increased fatality rates are concerns associated with delayed tPA delivery.36–38 Concerns also arise from its neurotoxic effects, such as intensified ischemia-induced glutamate release and neuronal damage, increased leukocyte infiltration, microglial activation, and free radical generation in ischemic brain regions.39,40 TPA is also known to activate matrix metalloproteinases (MMPs), adversely affecting the integrity of the blood-brain barrier.41,42 Research is exploring the potential of agents like Pyrrolidine dithiocarbamate (PDTC), Bryostoxin, and Progesterone to mitigate tPA-induced hemorrhage and modulate inflammatory and oxidative responses.43–45 However, the efficacy of these agents remains to be confirmed in clinical settings.
Antiplatelet and Anticoagulant Therapies
Antiplatelet and anticoagulant medications are crucial in both acute ischemic stroke management and secondary prevention.46,47 Antiplatelet drugs, including aspirin, clopidogrel, and dipyridamole, prevent platelet aggregation, an essential process in thrombus formation.48 These medications are mainly used to avoid recurrent strokes. In contrast, anticoagulants such as warfarin, dabigatran, and rivaroxaban target the coagulation cascade, making them suitable for strokes associated with atrial fibrillation or cardiac embolism.49,50 Careful administration of these medications is critical for reducing future thrombotic episodes.
In primary and secondary stroke prevention, aspirin and clopidogrel are often administered in combination during the acute phase to enhance their effectiveness. However, their extended use carries an increased risk of bleeding.51 Future research aims to develop personalized antiplatelet regimens to balance the benefits of stroke prevention with associated risks, thereby advancing towards safer and more effective stroke management strategies.
Laboratory Treatment Strategies: Neuroprotective Agents
Developing neuroprotective treatments for acute ischemic stroke is imperative to protect the goal of protecting the brain both before and during reperfusion. These approaches aim to widen the therapeutic window and enhance patient recovery outcomes.52 A significant portion of neuroprotective research remains in the experimental stage, with minimal potential for application in clinical settings.
Ischemic stroke triggers hypoxia-induced disruptions in neuronal energy metabolism, ATP depletion, and ion pump malfunction, leading to the collapse of fundamental cellular functions. The reintroduction of blood flow, or reperfusion, aggravates this condition by generating an overabundance of free radicals, causing mitochondrial and cellular membrane damage.53,54 Recent studies emphasize the significance of mitochondrial autophagy in stroke, demonstrating that mitochondrial reactive oxygen species (mtROS) and DNA (mtDNA) can instigate NLRP1 and NLRP3 inflammasome activation via the p38/NF-κB pathway, culminating in pyroptotic cell death.
Several neuroprotective agents are being investigated for their potential to address different aspects of ischemic injury, such as excitotoxicity, oxidative stress, and inflammation. For example, Nimodipine, a calcium channel blocker, can alleviate the reduction of extracellular calcium ions and inhibit post-ischemic blood-brain barrier disruption when given intravenously.55 However, the challenge in targeting NMDA receptors (NMDARs) lies in their contradictory roles in neuron survival and death, necessitating a selective approach to inhibit their detrimental effects.56 In the apoptosis domain, emerging neuroprotective agents like NOX inhibitors (eg, LR134, LR143) are being studied alongside established Caspase-3 inhibitors and Bcl-2 enhancers.57
Clinical trials have not yet proven that neuroprotective medicines effectively enhance functional recovery in stroke patients despite substantial theoretical support for this approach to stroke therapy. This disparity highlights the challenges of translating laboratory research into practical patient treatments.
Challenges to Current Conventional Therapies
Despite progress in stroke treatment, substantial challenges remain in refining conventional therapies (Figure 3). The limited therapeutic window for thrombolytic agents restricts their application to a select group of patients. Additionally, the heightened risk of hemorrhagic complications with thrombolytics and anticoagulants demands careful patient selection and ongoing monitoring. The intricate pathophysiology of stroke further complicates therapeutic approaches, as treatments effective for one stroke subtype may not be beneficial for others. Furthermore, the absence of definitive proof for the effectiveness of neuroprotective agents underscores the challenge of transforming laboratory findings into clinical success.58 Addressing these obstacles necessitates continued research and innovation in stroke therapeutics.
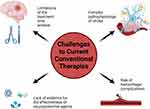 |
Figure 3 The challenges to current conventional therapies in stroke. Created with BioRender.com. |
To summarize, the pharmacological approaches used in stroke treatment today are intended to address different aspects of stroke pathogenesis. Improving patient outcomes in stroke care requires constant improvement of these treatments, supported by a thorough comprehension of the difficulties they present.
Liposome-Based Engineering in Ischemic Stroke Therapeutics
The field of liposome engineering is a complex area of scientific research that involves using artificial liposomes, which are vesicular structures similar to cellular membranes. The primary objective of liposome engineering is to enhance the efficient transportation of therapeutic agents, genetic materials, and other biomedical substances, particularly in the context of ischemic stroke. This review thoroughly examines the fundamental principles essential to liposome engineering, exploring the complex nature of this dynamic domain (refer to Figure 4).
 |
Figure 4 Multiple effective strategies for engineering liposomes. Created with BioRender.com. |
Liposome Structure and Composition
Liposomes, with their distinctive vesicular structure enclosed by a lipid bilayer membrane, possess an aqueous core and typically measure under 200 nanometers in diameter. These vesicles are synthesized using various methods, including ethanol injection, thin-film hydration, and extrusion techniques.59,60 Their applications span a wide range in the pharmaceutical industry, from anti-cancer and anti-fungal treatments to vaccine formulations.61,62 The evolution of stroke involves various pathological environmental scenarios, demanding tailored therapeutic interventions. As such, in the field of stroke therapy, the design of liposomes requires specific customization to suit the particular needs of the condition (Figure 5).
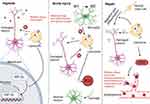 |
Figure 5 Utilizing liposomes for stroke treatment: strategies for varied progression phases. Created with BioRender.com. |
Surface Engineering of Liposomes for Neurotherapeutic Applications
Liposome surface engineering involves adding polymers or polyethylene glycol (PEG) for stability and reduced immunogenicity. Functionalization with ligands or antibodies enables targeted delivery, which is crucial for bypassing the BB and enhancing stroke treatment efficacy.63,64
The primary rationale behind the surface modification of liposomes for neurological applications is to surmount the challenges posed by the BBB.65 The BBB, a physiological barrier constituted by brain microvascular endothelial cells, astrocytes, and neurons, effectively prevents most drugs from entering the central nervous system, thereby maintaining neural stability. However, this barrier also impedes the efficient delivery of therapeutics for neurological conditions, often leading to suboptimal therapeutic efficacy or increased side effects.66 Consequently, developing liposomal drug delivery systems capable of traversing the BBB emerges as a viable strategy.67–69
The foremost goal in advancing therapeutic interventions is achieving targeted drug delivery. Strategic surface modifications of liposomes facilitate this approach to ensure delivery to specific cellular or tissue targets, thereby reducing unintended effects. The following points elaborate on this concept.
Stealth Liposome Technology
Stealth liposomes, coated with polymers such as PEG, evade immune detection and prolong bloodstream circulation. Initially designed to mimic red blood cells, these liposomes utilize gangliosides and sialic acid derivatives for reduced clearance.70
The incorporation of hydrophilic polymers and glycolipids reduces macrophage phagocytosis, exemplified by PEGylated liposomes with asialo-erythropoietin (aEPO), improving circulation time and targeted delivery in brain ischemia/reperfusion (I/R) injury.71,72 The modification of PEG-Lip with AEPO involved the post-insertion of a dioleoyl phosphatidylethanolamine (DSPE)-PEG2000-AEPO conjugate. This conjugate was synthesized through a chemical reaction between AEPO and the lysine residues of DSPE-PEG using N-hydroxysuccinimide. Liposomal encapsulation of AEPO was shown to extend its circulation time in blood and increase its accumulation in I/R-affected regions. As a result, intravenous administration of AEPO-modified PEG-Lip immediately after I/R was significantly more effective in mitigating cerebral cell damage than free AEPO given 24 hours post-I/R.73 However, the “accelerated blood clearance” (ABC) phenomenon presents challenges in repeated dosing due to altered pharmacokinetics.74
Ligand Conjugation Technology
Attaching ligands such as antibodies or aptamers onto liposome surfaces is a pivotal strategy to enhance affinity for specific receptors on target cells, thereby facilitating targeted drug delivery. The significance of Paired immunoglobulin-like receptor B (PirB) in the setting of ischemic stroke cannot be overstated. PirB restricts neuronal plasticity and prevents the formation of neural projections.75,76 Ligand attachment on liposomes, like antibodies or aptamers, enhances specificity for target cells. For example, PEG-modified liposomes delivering soluble PirB (sPirB) promote neuronal repair post-stroke.77
Liposomes co-extruded with microglia-derived macrophage membranes also show improved brain targeting, pharmacokinetics, and therapeutic efficacy in stroke models.78 Yue Zhao’s team developed dual-modified liposomes (T7 peptide and stroke-homing peptide), demonstrating enhanced BBB penetration and ischemic region targeting in MCAO rats.79 These findings suggest that ligand-mediated active targeting liposomes can strongly interact with molecules highly expressed in ischemic areas, thereby potentially increasing neuroprotectants’ delivery efficiency and therapeutic efficacy.
Environmentally Sensitive Modifications Technology
Integrating of environmentally responsive molecules, like pH-sensitive components, into liposomes facilitates selective therapeutic agent release in target tissues’ acidic conditions.80 This technique significantly enhances drug efficacy.
pH-Sensitive Liposomal Modifications
pH-sensitive liposomes are designed to respond to acidity variations in different tissue environments. They remain stable in neutral pH (like in the bloodstream) and rapidly release their payload in acidic environments, typical in diseased tissues such as cancer or ischemic stroke areas.81,82 These liposomes efficiently target pathological sites with differing pH levels from healthy tissues by incorporating specific lipids or polymers that change conformation or destabilize under acidic conditions. For instance, DC-Cholesterol-based liposomes exhibit pH-responsive behavior, releasing drugs in acidic tumor environments and enhancing cytotoxicity against cancer cells.83
Innovative approaches like t-PA-incorporated, ROS-scavenging, and pH-responsive nanoparticles have been developed in stroke treatment, improving bioavailability and extending the therapeutic window.84 These liposomes disintegrate in ischemic stroke environments, releasing their contents for synergistic treatment.
Notable constraints hamper the prevailing therapeutic methodologies for the diagnosis of ischemic stroke patients. For instance, CT scans lack the precision to identify ischemic strokes during their initial stages accurately. Moreover, while MRI can furnish comprehensive imagery, its utilization is marred by extensive scanning durations and its unsuitability for patients with metallic implants. Additionally, neither technique is adept at monitoring the dynamic microenvironmental alterations at the site of the lesion in a contemporary manner. Furthermore, extant research delineates that ischemic tissue characteristically manifests a diminished pH value relative to healthy tissue, attributable to the expedited glycolysis in the infarcted region, culminating in lactate accumulation.85,86 In response, researchers have pioneered the development of a pH-responsive NIR fluorescent probe, BOD@Lip, specifically engineered to gauge the magnitude of ischemic stroke. This groundbreaking strategy seeks to enhance the precision and immediacy in assessing the brain areas impacted by ischemia, utilizing the probe’s distinctive pH sensitivity to discern microenvironmental fluctuations indicative of ischemic conditions.87 BOD@Lip is proficient in permeating the blood-brain barrier, concentrating in areas affected by stroke, and responding to the aberrant acidic milieu by discharging its liposomal contents, thereby precipitating a variance in fluorescence signals. Monitoring these fluorescence metrics enables the real-time evaluation of the disease’s trajectory. Concurrently, the advent of pH-responsive liposomes for drug encapsulation, which discharge Ipidacrine within the infarcted locale, markedly attenuates neural damage after an ischemic stroke. This methodology not only ensures the targeted delivery of therapeutic agents but also amplifies the treatment efficacy for stroke by facilitating medication release in alignment with the specific biochemical environs of the afflicted tissue.88
Temperature-Sensitive Liposomal Modifications
These modifications leverage temperature differences between diseased and normal tissues. Lipids or polymers with phase transition temperatures near body temperature are used, enabling phase transitions or hydrolysis under elevated temperatures for drug release. PEG-PNIPAM-modified liposomes, for example, undergo a hydrogel-to-sol transition at 37°C, enhancing drug permeability.89
In light of the deficiencies associated with existing thrombolytic agents, researchers have endeavored to achieve precise delivery of sufficient thrombolytics directly to the site of the thrombus while significantly reducing the risk of systemic exposure and complications. In one study, alteplase was encapsulated within temperature-sensitive liposomes, which are designed to gradually release their contents at temperatures above 40°C gradually.90 The investigation underscored the necessity for thrombolytics to swiftly localize to the infarcted area post-injection, with a real-time rate that can be adjusted according to the therapeutic progression. Consequently, researchers devised polyethylene glycol (PEG)-modified thermosensitive magnetic liposomes, employing magnetic guidance to transport rtPA to the thrombus site directly. Controlled release of the drug was then achieved through a temperature-triggered mechanism under the influence of an external magnetic field’s thermomagnetic effects.91 In recent years, photothermal-responsive modification strategies have emerged, predominantly relying on temperature-sensitive liposomes where thermosensitive phospholipids disintegrate under near-infrared light irradiation, thus discharging their payload.92 Therefore, the core mechanism remains anchored in the use of thermosensitive liposome modifications.
Enzyme-Sensitive Liposomal Modifications
In targeted medication administration, enzyme-sensitive liposomes leverage the elevated expression levels of specific enzymes within tumour tissues. The introduction of enzyme-sensitive peptides or polymers onto the surface of liposomes results in enzymatic cleavage, hence initiating the release of drugs. For example, PEG-lipid materials sensitive to MMPs are specifically engineered to release therapeutic agents when MMPs are overexpressed in tumour tissues.93
Drug Carrier Engineering of Liposomes for Neurotherapeutic Applications
Liposomes serve as critical drug carriers, encapsulating pharmaceuticals within their lipid bilayers or aqueous cores.94 Customizing liposomal properties, such as size, shape, and charge, is key for modulating drug release kinetics and protecting encapsulated agents. Surface modification of liposomes enhances their targeting capabilities and functional effects.95
Carrying Laboratory Therapeutic Agents for Ischemic Stroke
The concept of deploying liposomes as vehicles for drug delivery in the treatment of ischemic stroke has a longstanding history. Initially, hemoglobin served as the inaugural drug carrier in this context. The primary objective was not to direct hemoglobin towards the ischemic brain regions. This approach stems from the recognition that the periphery of ischemic strokes is characterized by a pronounced lack of oxygen. This approach stems from recognizing that a pronounced lack of oxygen characterizes the periphery of ischemic strokes. Therefore, liposomes have been employed to deliver hemoglobin (Hb) to reoxygenate the cells in ischemic brain areas.96 Studies on rat models of stroke have shown that liposomal Hb could selectively infiltrate the ischemic core, while sparing the healthy brain tissue. This precision in delivery facilitated improved oxygen levels and resulted in a reduction of infarct size. Additionally, this method notably decreased inflammation and ameliorated cognitive impairments.97 The extent and mode of neuronal cell death following ischemia are closely associated with the intracellular levels of adenosine triphosphate (ATP). However, the pharmacological utility of ATP is constrained by its poor cellular permeability and rapid hydrolysis by extracellular enzymes. Delivering ATP via liposomes to the ischemic penumbra could improve cell metabolism in low-oxygen environments, potentially aiding neovascularization and brain function recovery post-stroke.98
Beyond the initial use of natural hemoglobin for oxygen transport to areas affected by stroke, subsequent innovation has been inspired by existing clinical drugs. This inspiration arises from the recognition that the efficiency of gastrointestinal absorption of drugs, coupled with the eliminatory effects of the liver, often leads to less-than-optimal outcomes in clinical drug application. Consequently, researchers have started to utilize liposome-based delivery systems to transport clinical drugs, aiming to bypass these issues and enhance therapeutic efficacy. Research has demonstrated that neutral and negatively charged liposomes can target ischemic regions, enhancing statin drug accumulation. This suggests a potential for improved blood-brain barrier traversal.99
Over the last decade, the field has seen an extensive emergence of neuroprotective compounds. However, the efficacy of specific neuroprotective agents is limited by their inability to target the regions affected by ischemic stroke. This remarkable increase in innovative developments has prompted numerous researchers to explore the integration of these agents into liposomes, targeting the treatment of ischemic stroke. Liu et al pioneered the creation of liposomes that are modified with Vascular Cell Adhesion Molecule-1 (VCAM-1) to target ischemic brain tissues specifically. Encapsulating Cytidine-5’-diphosphocholine (CDPC) within these specialized liposomes, they achieved enhanced delivery efficiency to areas affected by ischemia, thereby markedly reducing cerebral infarction sizes.100,101 Incorporating neurotrophic factors like bFGF into liposomes has shown promising results. Intranasal administration in rodent models significantly reduced infarction volume and enhanced recovery.102 Subsequent investigations have advanced the engineering of drug delivery systems to cater to the distinct demands of various pathologies. Iron oxide nanoparticles (IONPs) are distinguished by their inherent magnetic attributes and capacity to induce photothermal effects. Upon targeted delivery to designated areas, liposomes incorporating IONPs enable the quantitative release of their contents through laser irradiation. Modifications with IONPs have augmented the efficacy of liposomes in drug delivery, particularly in photothermal therapy, showcasing enhanced therapeutic outcomes.103
Furthermore, the use of liposomes for delivering neuroprotective agents such as FK506 and cyclosporine A (CsA) has been explored. When encapsulated in liposomes, these agents have shown a reduction in oxidative stress markers and a decrease in cell death during ischemic reperfusion injury in animal models. Liposomal formulations can lower the minimum effective dose of these drugs, highlighting the potential of liposomes to improve drug bioavailability and reduce side effects.104
Carrying Clinical Therapeutic Agents for Ischemic Stroke
In the treatment of ischemic stroke, the clinical use of medications is primarily focused on thrombolytic drugs and antiplatelet therapy. However, due to the associated risks of increased cerebral hemorrhage and higher mortality rates, researchers have considered employing alternative strategies to improve outcomes. Among these, some researchers have combined the classic drug tPA with fasudil-modified liposomes to transport it into the brain, thereby extending the therapeutic window of tPA thrombolysis and ameliorating neural damage in treating of ischemic stroke. Compared to monotherapy, even with delayed administration of tPA, combination therapy has demonstrated more potent neuroprotective effects.105 Additionally, researchers have explored the introduction of gases into liposomal drug delivery systems for stroke treatment. Xenon (Xe) possesses unique capabilities to protect brain tissue without causing side effects, and its combination therapy with rtPA can improve neurological function recovery in acute stroke cases.106
Moreover, a “repurposing of old drugs” strategy has emerged in the laboratory after the past several years, where EPO, an existing drug known for promoting angiogenesis, has been shown to significantly reduce the area of neural infarction following cerebral ischemic injury when carried by liposomes injected into MACO model mice.73 While the body of research remains limited, these initial ideas mark a commendable beginning. They are poised to lay the vital groundwork for using liposomes in the clinical management of stroke. Even more, impaired neurological functions for patients with stroke have been explored to restore in recent years.107
A Series of Materials Obtained from Engineered Liposomes for Stroke Treatment
Enhancing BBB Permeability Through Liposomes
When the ischemic stroke occurs, the BBB undergoes significant alterations, and notably increased its permeability. This change is chiefly attributed to the altered functionality of brain endothelial cells (BECs) and tight junction (TJ) proteins.108 The enhanced permeability post-stroke is linked to the degradation of the extracellular matrix by enzymes like matrix metalloproteinases, release of inflammatory mediators, and infiltration of leukocytes.109,110 In this context, liposomes, with their nanoscale size and stability, offer a promising approach for drug delivery in ischemic stroke therapy. A comparison between them is shown in (Table 1).
 |
Table 1 Synthesis, Classification and Characteristics of Various Engineered Liposome on Ischemic |
Passive Targeting Strategies in Liposomal Delivery
Innovations in Drug Encapsulation Using Liposomes
Researchers have innovatively employed liposomes for drug encapsulation, aiming to augment drug stability and metabolism within the systemic circulation, thereby facilitating passive targeting by disrupting the BBB. For instance, Michael R. Arul et al have utilized liposomes for the encapsulation of 5S-(3-Bromophenyl)-1,3-dihydro-2H-Benzofuro[3,2-e]-1,4-diazepin-2-one (5BDBD), targeting the brain passively. This 5BDBD compound inhibits the purinergic receptor P2X4, thereby ameliorating post-stroke damage and contributing to neuroprotection.127 In a parallel study, Yang Li et al amalgamated ginkgolide B (GB) with docosahexaenoic acid (DHA) to formulate a lipophilic GB-DHA complex. Encapsulated within nanoliposomes (Lipo @ GB-DHA), this complex demonstrated enhanced stability and effectively concentrated GB in the infarct region of rat brains in a middle cerebral artery occlusion (MCAO) model.114 Reju George Thomas and collaborators also synthesized nanoparticles by conjugating atorvastatin with polyethylene glycol (PEG), achieving effective accumulation of lipstatin at cerebral injury sites in rats through passive targeting.118
Integration of Liposomes with Naturally Targeted Cell Membranes
In a novel approach, some researchers have integrated liposomes with naturally targeted cell membranes, harnessing the innate targeting capabilities of specific cells. This methodology ensures the stable delivery of encapsulated drugs, both pre- and post-fusion with native cell membranes. Xingping Quan et al encapsulated tissue plasminogen activator (tPA) within liposomes and integrated these into platelet membranes, exploiting the natural targeting properties of platelets for delivering thrombolytic agents in a mouse model of ischemic stroke.128
Strategies to Enhance Blood-Brain Barrier Permeability
Similarly, Kai Wang et al demonstrated that hydroxyurea (HYD) modulates the expression of tight junction proteins in brain microvascular endothelial cells following oxygen-glucose deprivation (OGD), thereby enhancing liposome penetration across brain endothelial layers in vitro. Anticipating HYD’s potential to increase BBB permeability during acute stroke phases, they engineered targeted liposomes fused with neutrophil-like cell membranes for precise delivery to inflamed brain microvascular endothelial cells.119 Yu Long et al modified liposomes with monocyte-macrophage membranes, facilitating passage through the BBB for targeted delivery.78
Advancements in Surface Modification for Enhanced Targeting
Beyond passive targeting and cell membrane fusion, researchers have delved into the surface modification of liposomes with peptides or molecules to augment targeting and evasion capabilities. Jia Hou et al affixed the c(RGDyK) peptide to liposomes, aiming at monocytes and neutrophils, expressing integrin αvβ1 at high levels. This strategy capitalizes on the propensity of these cells to converge at stroke cores and penumbras, thus facilitating the delivery of therapeutic molecules to injury sites through secondary targeting.129 In a similar vein, Hongdan Lu, Yan Yan Chen, and others have modified liposomes with this peptide to enhance drug targeting.117,130
Enhancing BBB Crossing and Precision Delivery
Moreover, the surface modification of liposomes with transferrin (TF) has shown to facilitates BBB crossing.131 Qian Bai et al attached dodecylbenzene sulfonamide (p-DBSN) with endoplasmic reticulum-targeting functions to liposomes.132 Yue Zhao et al engineered liposomes with the HAIYPRH (T7) and CLEVSRKNC peptides for precise drug delivery to ischemic brain tissue, exploiting T7’s specific binding to the transferrin receptor (TfR) for BBB crossing and CLEVSRKNC’s preferential localization in ischemic tissue.79
Targeting Strategies for Clinical Translations
Focusing on acute, high-risk diseases like ischemic stroke, Xingping Quan et al noncovalently bonded cryogenic shock platelets (CsPILs) to liposomes for thrombus targeting.133 Michael Sun and other researchers also investigated targeting thrombosis by modifying liposomes with platelet-binding (PBP) and fibrin-binding (FBP) peptides, assessing their respective efficacies.134 Shanbo Sun et al utilized CREKA peptides, known for their microthrombus targeting effects, for drug delivery.115 In earlier studies, the modification of liposome surfaces with AEPO demonstrated efficacy in targeting ischemic regions via EPO receptor binding.120
Comparative Analysis of Nanoparticles in Drug Delivery
Given the need of drug delivery mechanisms, there is a pivotal demand for drug carriers to possess reduced particle sizes but also extended stability in circulation times. The advent of nanoparticle drug carriers marks a significant milestone throughout the continuum of research in this field. These carriers are diverse and categorizable into bionic, synthetic, and inorganic nanoparticles based on the raw materials used for their synthesis.135 Among them, liposomes, as a prevalent form of nanoparticle drug carrier, frequently serve as a benchmark for comparison with other types of nanoparticles (Table 1).
Particle Size Variability
Liposomes, synthesized via thin-film hydration followed by ultrasonication and extrusion, offer a wide range of customizable particle sizes from 5µm down to 100nm. This flexibility in size selection is enhanced by using a liposome extruder, which, through a polycarbonate membrane, produces liposomes with a discrete coefficient of less than 0.1 and uniform particle sizing. In contrast, as natural extracellular vesicles, exosomes exhibit a more homogeneous and fixed size range, typically between 40–100nm. Similarly, the size of nanomicelles is inherently fixed and challenging to alter, as it is contingent on the synthetic raw materials used in their formation. Compared with exosomes and nanomicelles, liposomes have the advantages of diverse selection, flexibility and controllability regarding particle size.
Stability in Circulation and Storage
Liposomes demonstrate notable stability in blood circulation, attributed to their phospholipid bilayer structure, akin to cellular membranes. Furthermore, liposomes can be stored stably at room temperature or 4°C for several months, a feature facilitated by their lack of nucleotide content. In contrast, exosomes require more stringent storage conditions to maintain their therapeutic efficacy, as inappropriate temperatures can lead to the deactivation of their contents. Nano micelles exhibit less stability in blood circulation than liposomes, given their self-assembled nature.
Engineering Flexibility
Both liposomes and nanomicelles allow for precise surface engineering to meet specific therapeutic needs. For nanomicelles, this involves designing long and short chains. A notable example is the work by Zhenhua Wang et al,136 who achieved targeted lysosomal escape and mitochondrial targeting using long and short PEG chains combined with ROS-responsive groups. In contrast, exosomes, being cell-derived extracellular vesicles, present challenges in engineering due to their inherent biological complexity (Figure 6).
 |
Figure 6 The contrast of engineered nanoparticles: Liposomes, Exosomes and nanomicelles. By Figdraw. |
Liposomes for Stroke Treatment via Different Administration Routes
Exploring different administration routes for liposomal drug delivery is crucial in stroke treatment due to the distinct pharmacokinetic profiles and therapeutic outcomes they offer. Intravenous delivery ensures rapid systemic distribution but may pose challenges when crossing the BBB. Although gastrointestinal degradation and bioavailability limit oral administration, it is patient-friendly. Intranasal delivery presents a promising route by potentially bypassing the BBB and directly targeting the central nervous system, offering a targeted approach for neuroprotection and enhanced therapeutic efficacy in stroke management.
Intravenous (IV) Delivery
Intravenous (IV) administration of liposomes for ischemic stroke treatment offers critical advantages, including immediate systemic availability and high bioavailability, ensuring rapid therapeutic action, which is vital in acute conditions. Although much of the literature does not include the keyword “Intravenous Delivery”, the method section describes its administration via intravenous injection. Notably, the liposomal drug delivery mentioned previously is mainly through the intravenous route. This route facilitates precise drug dosing, even for drugs with short action durations, and allows for the administration of large or hypertonic solutions, which is crucial for managing certain complications of stroke.
Increasing the local concentration of drugs, makes it possible to reduce the side effects of the carried drugs on other non-target cells throughout the body. Some drugs have a low oral absorption rate and are quickly cleared from the body. For example, Ginkgolide B, as a classic candidate drug for stroke recovery, has a very low utilization rate when injected intravenously due to its poor water solubility and lipid solubility. However, using liposomes as carriers can extend its circulation time in the body. Research shows that this method can significantly reduce the volume of infarction and substantially improve the neurological damage in MCAO rats after reperfusion 2 hours later.114
While offering potential benefits, this treatment is accompanied by several significant risks. These include the irreversible nature of the drug’s action upon administration, the possibility of tissue necrosis in cases of extravasation, and the occurrence of severe adverse effects. Furthermore, the necessity of maintaining strict aseptic conditions cannot be overstated, given the elevated risk of bacterial contamination.
Oral Administration
The strategy of oral administration of liposome-encapsulated peptides and proteins necessitates a deeper exploration into optimizing liposome stability and bioavailability. This approach prompts an innovative rethinking of liposome formulation, focusing on the resilience of liposomes to the digestive tract rigors.137
N-Butylphthalide (NBP), characterized by its poor water solubility and oral bioavailability, poses significant challenges for widespread oral application. To address this, researchers have designed a liposome delivery system incorporating sodium cholate (CA-liposomes) as a biosurfactant, significantly improving NBP’s oral absorption through both paracellular and transcellular pathways across intestinal epithelia. This method ensures rapid cerebral delivery and exhibits neuroprotective effects in treating ischemic stroke, as demonstrated in both in vivo and in vitro studies.138
Oral liposome administration for stroke offers substantial benefits, such as increased drug solubility, bioavailability, and precise cerebral targeting, potentially enhancing neuroprotection. Nonetheless, it faces challenges like variable absorption rates, complex formulation processes, and the necessity for stable liposome integrity within the gastrointestinal tract. Balancing these elements is crucial for optimizing therapeutic efficacy and minimizing potential limitations.
Intranasal Delivery
The nasal cavity’s unusual architecture enables direct brain administration of medicines bypassing the BBB via olfactory and trigeminal nerve pathways. This intranasal administration is non-invasive, safe, and rapid, circumventing first-pass metabolism and enhancing cerebral bioavailability. Regarded as a promising approach for central nervous system disorders, recent studies have demonstrated that intranasal delivery of liposomes containing bFGF significantly ameliorates neurological deficits and reduces stroke infarction volumes in mice, showcasing substantial improvements in spontaneous motor activities and neurofunctional recovery.102 Not only that, but researchers have proposed a novel strategy for targeted cerebral ischemia therapy by co-encapsulating Notoginsenoside (PNS) and Ginsenoside Rg3 (Rg3) within liposomes. These liposomes are then blended with macrophage membranes to target ischemic areas in the brain, utilizing the natural homing abilities of macrophages to inflammation sites. This innovative approach aims to enhance the delivery and efficacy of therapeutic compounds directly to the areas of the brain affected by stroke.139
Intranasal delivery of liposomes for stroke therapy presents notable advantages, including bypassing the BBB for direct brain targeting, potentially quicker therapeutic onset, and reduced systemic side effects. However, challenges include the limited capacity for drug loading, potential variability in dosing due to mucociliary clearance, and the need for formulations that can effectively navigate the nasal environment to reach the brain. This delivery route requires careful consideration of formulation and dosing to ensure efficacy and safety.
Discussion and Conclusion
This comprehensive review has illuminated the pivotal role of liposome-based drug delivery systems in ischemic stroke therapy. The evolution of liposome technology, characterized by its biocompatibility, versatility in drug encapsulation, and ability to traverse the BBB, marks a significant leap forward in the quest for effective treatments. Through the encapsulation of both hydrophilic and hydrophobic drugs, liposomes offer a multifaceted approach to addressing the intricate pathophysiology of ischemic stroke, extending the range of potential therapeutic agents that can be effectively deployed.
The innovative modifications of liposome surfaces, including the incorporation of targeting ligands and stimuli-responsive components, have demonstrated remarkable efficacy in enhancing drug delivery to ischemic brain tissue. These advancements ensure the precision of therapeutic interventions and minimize systemic side effects, aligning with the critical requirements of stroke therapy. Furthermore, integrating diagnostic and therapeutic functions within multifunctional liposomes epitomizes the promising horizon of theranostics in managing ischemic stroke, paving the way for more personalized and dynamic treatment strategies.
Despite these promising developments, several challenges remain in the clinical translation of liposome-based therapies. The scalability of production, regulatory hurdles, and considerations of cost-effectiveness are among the key obstacles that must be addressed to realize the full potential of liposomes in clinical settings. It necessitates a concerted effort from researchers, clinicians, and regulatory bodies to navigate these challenges and optimize the therapeutic efficacy of liposomes.
In conclusion, liposome-based drug delivery systems present a promising avenue for advancing ischemic stroke therapy. As we continue to unravel the complexities of liposome technology and its applications, the focus should remain on enhancing these systems’ precision, efficacy, and safety. Collaborative research efforts are essential to overcome the existing barriers and fully harness the potential of liposomes, ultimately improving the outcomes for patients suffering from ischemic stroke.
Abbreviations
ABC, Accelerated blood clearance; AEPO, Asialo-erythropoietin; AMPA, Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ATP, Adenosine Triphosphate; BBB, Blood-brain barrier; Bcl-2, B-cell lymphoma-2; BECs, Brain endothelial cells; bFGF, Basic Fibroblast Growth Factor; CDPC, Cytidine-5’-diphosphocholine; CREKA, Cysteinyl-arginyl-glutamyl-lysyl-alanyl; CsPLTs, Cryo-shocked platelets; DC-Cholesterol, 3-[N-(N’,N’-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride; DHA, Docosahexaenoic acid; DSPE, Dioleoyl phosphatidylethanolamine; DSPE-PEG, 1.2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; EPO, Erythropoietin; FBP, Fibrin-binding peptide; GB, Ginkinolide B; GM1, Monosialoganglioside; Hb, Hemoglobin; HT, Hemorrhagic transformation; HYD, Hydroxyurea; I/R, Ischemia/reperfusion; ICH, Intracerebral hemorrhage; IL-1, Interleukin-1; IL-5, Interleukin-5; IONPs, Iron Oxide Nanoparticles; LCST, Lower critical solution temperature; mtDNA, Mitochondrial DNA; mtROS, Mitochondrial reactive oxygen species; M / Ns, Monocytes and neutrophils; MCAO, Middle cerebral artery occlusion; MMP, Matrix metalloproteinase; MMP-2, Matrix Metalloproteinase 2; MMPs, Matrix metalloproteinases; NADH, Nicotinamide adenine dinucleotide; NMDA, N-methyl-D-aspartate; NMDARs, N-methyl-D-aspartate receptors; NOX, Nicotinamide adenine dinucleotide phosphate oxidase; NLRP1, NLR family pyrin-domain-containing protein 1; NLR, NLRP3 family pyrin-domain-containing protein 3; Nrf2, Nuclear Factor E2-Related Factor 2; OGD, Oxygen glucose deprivation; P2X4, Purinergic Receptor P2X, Ligand-Gated Ion Channel, 4; PBP, Platelet-binding peptide; p-DBSN, Dodecylbenzene sulfonamide; PDTC, Pyrrolidine dithiocarbamate; PEG, Polyethylene glycol; PEG-Lip, PEGylated liposomes; PEG-PNIPAM, Polyethylene glycol-poly(N-isopropylacrylamide); pH, Potential of hydrogen; PirB, Paired immunoglobulin-like receptor B; PPC, Peptide/cholesterol; PS, Phosphatidylserine; ROS, Reactive oxygen species; SHp, Strokehoming peptide; sPirB, Soluble Paired immunoglobulin-like receptor B; TF, Transferrin; TGF-β, Transforming Growth Factor beta; TJ, Tight junction; TNF-α, Tumor Necrosis Factor-alpha; tPA, Tissue plasminogen activator; VCAM-1, Vascular Cell Adhesion Molecule-1; VLA-4, Very Late Antigen-4; 5BDBD, 5-(3-Bromophenyl)-1,3-dihydro-2H-Benzofuro[3,2-e]-1,4-diazepin-2-one.
Acknowledgment
We thank Fig.Draw (https://www.figdraw.com/) for editing Figures 2 and 6. Graphical abstract, Figures 1, 3, 4 and 5 were created with Biorender.com. We thank Dr. Yifan Bao for helping us obtain the copyright for the images drawn on the Biorender.com. Also we thank Dr. Ahmed Waqas for professional language polishing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Key R&D Program of China (2022YFA1104900 & 2022YFA1104904), the Innovation Team Project (2023KCXTD007) and the Special project in key areas of Guangdong Province (2021ZDZX2011), and the President Foundation of the Integrated Hospital of Traditional Chinese Medicine of Southern Medical University (1202101003).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Nayab DE, Din FU, Ali H, et al. Nano biomaterials based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: an up-to-date perspective. J Nanobiotechnology. 2023;21(1):477. doi:10.1186/s12951-023-02250-1
2. Zhu ZH, Jia F, Ahmed W, et al. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen Res. 2023;18(2):404–409. doi:10.4103/1673-5374.346466
3. Sisubalan N, Shalini R, Ramya S, Sivamaruthi BS, Chaiyasut C. Recent advances in nanomaterials for neural applications: opportunities and challenges. Nanomedicine. 2023;18(26):1979–1994. doi:10.2217/nnm-2023-0261
4. Xing J, Liu D, Zhou G, et al. Liposomally formulated phospholipid-conjugated novel near-infrared fluorescence probe for particle size effect on cellular uptake and biodistribution in vivo. Colloids Surf B Biointerfaces. 2018;161:588–596. doi:10.1016/j.colsurfb.2017.11.033
5. Polak R, Lim RM, Beppu MM, Pitombo RN, Cohen RE, Rubner MF. Liposome-loaded cell backpacks. Adv Healthc Mater. 2015;4(18):2832–2841. doi:10.1002/adhm.201500604
6. Filipczak N, Pan J, Yalamarty S, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22. doi:10.1016/j.addr.2020.06.022
7. Ding Q, Liu S, Yao Y, Liu H, Cai T, Han L. Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology. 2022;98(3):e279–e290. doi:10.1212/WNL.0000000000013115
8. Ahmed W, Kuniyan MS, Jawed AM, Chen L. Engineered extracellular vesicles for drug delivery in therapy of stroke. Pharmaceutics. 2023;15(9):2173. doi:10.3390/pharmaceutics15092173
9. Georgakis MK, Gill D, Rannikmäe K, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139(2):256–268. doi:10.1161/CIRCULATIONAHA.118.035905
10. Chen C-Y, Lin P-T, Wang Y-H, et al. Etiology and risk factors of intracranial hemorrhage and ischemic stroke in young adults. J Chin Med Assoc. 2021;84(10):930–936. doi:10.1097/JCMA.0000000000000598
11. Tian D-Y, Fan D-S. Risk factors, regional disparity and trends of ischemic stroke etiologic subtypes. Chin Med J. 2018;131(2):127–129. doi:10.4103/0366-6999.222332
12. Ogata J, Masuda J, Yutani C, Yamaguchi T. Mechanisms of cerebral artery thrombosis: a histopathological analysis on eight necropsy cases. J Neurol Neurosurg Psychiatry. 1994;57(1):17–21. doi:10.1136/jnnp.57.1.17
13. Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112(9):3555–3562. doi:10.1182/blood-2008-04-144758
14. Zöller B, Sundquist J, Sundquist K, Ohlsson H. The risk for venous thromboembolism and cardiometabolic disorders in offspring from thrombosis-prone pedigrees. J Thromb Haemost. 2024;22(3):775–784. doi:10.1016/j.jtha.2023.11.024
15. Feske SK. Ischemic Stroke. Am J Med. 2021;134(12):1457–1464. doi:10.1016/j.amjmed.2021.07.027
16. Liu J, Wang Y, Akamatsu Y, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi:10.1016/j.pneurobio.2013.11.004
17. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. doi:10.1016/S1474-4422(19)30078-X
18. Jokivarsi KT, Gröhn HI, Gröhn OH, Kauppinen RA. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magn Reson Med. 2007;57(4):647–653. doi:10.1002/mrm.21181
19. Larkin JR, Foo LS, Sutherland BA, Khrapitchev A, Tee YK. Magnetic Resonance pH imaging in stroke - combining the old with the new. Front Physiol. 2021;12:793741. doi:10.3389/fphys.2021.793741
20. Mukherjee S, Sikdar SK. Intracellular activation of full-length human TREK-1 channel by hypoxia, high lactate, and low pH denotes polymodal integration by ischemic factors. Pflugers Arch. 2021;473(2):167–183. doi:10.1007/s00424-020-02471-5
21. Dénes A, Ferenczi S, Kovács KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8:164. doi:10.1186/1742-2094-8-164
22. Bui TA, Jickling GC, Winship IR. Neutrophil dynamics and inflammaging in acute ischemic stroke: a transcriptomic review. Front Aging Neurosci. 2022;14:1041333. doi:10.3389/fnagi.2022.1041333
23. Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation. 2010;7:74. doi:10.1186/1742-2094-7-74
24. Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174(1):35–43. doi:10.1093/aje/kwr051
25. Johnson LS, Mattsson N, Sajadieh A, Wollmer P, Söderholm M. Serum potassium is positively associated with stroke and mortality in the large, population-based malmö preventive project cohort. Stroke. 2017;48(11):2973–2978. doi:10.1161/STROKEAHA.117.018148
26. András IE, Deli MA, Veszelka S, Hayashi K, Hennig B, Toborek M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab. 2007;27(8):1431–1443. doi:10.1038/sj.jcbfm.9600445
27. Song M, Yu SP. Ionic regulation of cell volume changes and cell death after ischemic stroke. Transl Stroke Res. 2014;5(1):17–27. doi:10.1007/s12975-013-0314-x
28. Ren C, Guingab-Cagmat J, Kobeissy F, et al. A neuroproteomic and systems biology analysis of rat brain post intracerebral hemorrhagic stroke. Brain Res Bull. 2014;102:46–56. doi:10.1016/j.brainresbull.2014.02.005
29. Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26(8):884–892. doi:10.1179/016164104X2357
30. Jolugbo P, Ariëns R. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke. 2021;52(3):1131–1142. doi:10.1161/STROKEAHA.120.032810
31. Tsivgoulis G, Katsanos AH, Sandset EC, et al. Thrombolysis for acute ischaemic stroke: current status and future perspectives. Lancet Neurol. 2023;22(5):418–429. doi:10.1016/S1474-4422(22)00519-1
32. Diener HC, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol. 2020;75(15):1804–1818. doi:10.1016/j.jacc.2019.12.072
33. Li S, Pan Y, Wang Z, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled Phase II study. Stroke Vasc Neurol. 2022;7(1):47–53. doi:10.1136/svn-2021-000978
34. Atchaneeyasakul K, Desai S, Malhotra K, et al. Intravenous tPA delays door-to-puncture time in acute ischemic stroke with large vessel occlusion. J Stroke Cerebrovasc Dis. 2021;30(6):105732. doi:10.1016/j.jstrokecerebrovasdis.2021.105732
35. Shah S, Liang L, Kosinski A, et al. Safety and Outcomes of Intravenous tPA in Acute Ischemic Stroke Patients With Prior Stroke Within 3 Months: findings From Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2020;13(1):e006031. doi:10.1161/CIRCOUTCOMES.119.006031
36. Wang R, Zhu Y, Liu Z, et al. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood. 2021;138(1):91–103. doi:10.1182/blood.2020008913
37. Charidimou A, Pasi M. Microbleeds evolution and remote hemorrhage post-tPA: ”Red meets white” revisited. Neurology. 2019;92(7):307–308. doi:10.1212/WNL.0000000000006933
38. Shi K, Zou M, Jia DM, et al. tPA mobilizes immune cells that exacerbate hemorrhagic transformation in stroke. Circ Res. 2021;128(1):62–75. doi:10.1161/CIRCRESAHA.120.317596
39. Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator. J Cereb Blood Flow Metab. 2004;24(9):945–963. doi:10.1097/01.WCB.0000137868.50767.E8
40. Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52(3):1572–1579. doi:10.1007/s12035-014-8952-x
41. Tsuji K, Aoki T, Tejima E, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954–1959. doi:10.1161/01.STR.0000177517.01203.eb
42. Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603. doi:10.1161/01.cir.0000046451.38849.90
43. Wang Z, Shan W, Cao J, Wintermark M, Huang W, Zuo Z. Early administration of pyrrolidine dithiocarbamate extends the therapeutic time window of tissue plasminogen activator in a male rat model of embolic stroke. J Neurosci Res. 2018;96(3):449–458. doi:10.1002/jnr.24186
44. Tan Z, Lucke-Wold BP, Logsdon AF, et al. Bryostatin extends tPA time window to 6 h following middle cerebral artery occlusion in aged female rats. Eur J Pharmacol. 2015;764:404–412. doi:10.1016/j.ejphar.2015.07.035
45. Li Q, Han X, Lan X, et al. Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol Dis. 2017;108:173–182. doi:10.1016/j.nbd.2017.08.011
46. Wisløff T, Hamidi V, Ringerike T, Harboe I, Klemp M. Intravenous Thrombolytic Treatment After Acute Stroke and Secondary Antithrombotic Prevention Treatment (Antiplatelet and Anticoagulant Treatment) After Stroke. Oslo, Norway; 2010.
47. Bala MM, Celinska-Lowenhoff M, Szot W, et al. Antiplatelet and anticoagulant agents for secondary prevention of stroke and other thromboembolic events in people with antiphospholipid syndrome. Cochrane Database Syst Rev. 2020;10(10):CD012169. doi:10.1002/14651858.CD012169.pub3
48. Jia YM, Ge PX, Zhou H, et al. Vicagrel enhances aspirin-induced inhibition of both platelet aggregation and thrombus formation in rodents due to its decreased metabolic inactivation. Biomed Pharmacother. 2019;115:108906. doi:10.1016/j.biopha.2019.108906
49. Kamel H, Healey JS. Cardioembolic Stroke. Circ Res. 2017;120(3):514–526. doi:10.1161/CIRCRESAHA.116.308407
50. Adams PC, Cohen M, Chesebro JH, Fuster V. Thrombosis and embolism from cardiac chambers and infected valves. J Am Coll Cardiol. 1986;8(6 Suppl B):76B–87B. doi:10.1016/s0735-1097(86)80009-2
51. Kamarova M, Baig S, Patel H, et al. Antiplatelet use in ischemic stroke. Ann Pharmacother. 2022;56(10):1159–1173. doi:10.1177/10600280211073009
52. Ovbiagele B, Kidwell CS, Starkman S, Saver JL. Neuroprotective agents for the treatment of acute ischemic stroke. Curr Neurol Neurosci Rep. 2003;3(1):9–20. doi:10.1007/s11910-003-0031-z
53. Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738 Suppl):A7–14. doi:10.1038/399a007
54. Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–275. doi:10.1016/j.redox.2018.03.002
55. Lazarewicz JW, Pluta R, Puka M, Salinska E. Diverse mechanisms of neuronal protection by nimodipine in experimental rabbit brain ischemia. Stroke. 1990;21(12 Suppl):1.
56. Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 2018;11(1):15. doi:10.1186/s13041-018-0357-8
57. Wang Z, Zhou Z, Wei X, et al. Therapeutic potential of novel twin compounds containing tetramethylpyrazine and carnitine substructures in experimental ischemic stroke. Oxid Med Cell Longev. 2017;2017:7191856. doi:10.1155/2017/7191856
58. Luo Y, Tang H, Li H, Zhao R, Huang Q, Liu J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur J Med Chem. 2019;162:132–146. doi:10.1016/j.ejmech.2018.11.014
59. Karunakaran B, Gupta R, Patel P, et al. Emerging trends in lipid-based vaccine delivery: a special focus on developmental strategies, fabrication methods, and applications. Vaccines (Basel). 2023;11(3):661. doi:10.3390/vaccines11030661
60. Has C, Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J Liposome Res. 2020;30(4):336–365. doi:10.1080/08982104.2019.1668010
61. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
62. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi:10.3390/pharmaceutics9020012
63. Bondì ML, Di Gesù R, Craparo EF. Lipid nanoparticles for drug targeting to the brain. Methods Enzymol. 2012;508:229–251. doi:10.1016/B978-0-12-391860-4.00012-4
64. Joshi S, Singh-Moon R, Wang M, et al. Cationic surface charge enhances early regional deposition of liposomes after intracarotid injection. J Neurooncol. 2014;120(3):489–497. doi:10.1007/s11060-014-1584-1
65. Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. doi:10.1016/j.jconrel.2017.12.015
66. de Lange E, Hammarlund Udenaes M. Understanding the blood-brain barrier and beyond: challenges and opportunities for novel CNS therapeutics. Clin Pharmacol Ther. 2022;111(4):758–773. doi:10.1002/cpt.2545
67. Kashyap K, Shukla R. Drug delivery and targeting to the brain through nasal route: mechanisms, applications and challenges. Curr Drug Deliv. 2019;16(10):887–901. doi:10.2174/1567201816666191029122740
68. Agrawal M, Saraf S, Saraf S, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139–177. doi:10.1016/j.jconrel.2018.05.011
69. Crowe TP, Greenlee M, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi:10.1016/j.lfs.2017.12.025
70. Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988;85(18):6949–6953. doi:10.1073/pnas.85.18.6949
71. Price CD, Yang Z, Karlnoski R, Kumar D, Chaparro R, Camporesi EM. Effect of continuous infusion of asialoerythropoietin on short-term changes in infarct volume, penumbra apoptosis and behaviour following middle cerebral artery occlusion in rats. Clin Exp Pharmacol Physiol. 2010;37(2):185–192. doi:10.1111/j.1440-1681.2009.05257.x
72. He M, Kittur FS, Hung CY, et al. A novel plant-produced asialo-rhuEPO protects brain from ischemic damage without erythropoietic action. Transl Stroke Res. 2022;13(2):338–354. doi:10.1007/s12975-021-00943-z
73. Ishii T, Asai T, Oyama D, et al. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160(1):81–87. doi:10.1016/j.jconrel.2012.02.004
74. Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105(3):305–317. doi:10.1016/j.jconrel.2005.04.003
75. Deng B, Li L, Gou X, et al. TAT-PEP enhanced neurobehavioral functional recovery by facilitating axonal regeneration and corticospinal tract projection after stroke. Mol Neurobiol. 2018;55(1):652–667. doi:10.1007/s12035-016-0301-9
76. Llorens F, Gil V, Del Río JA. Emerging functions of myelin-associated proteins during development, neuronal plasticity, and neurodegeneration. FASEB J. 2011;25(2):463–475. doi:10.1096/fj.10-162792
77. Wang J, Zhang Y, Xia J, et al. Neuronal PirB upregulated in cerebral ischemia acts as an attractive theranostic target for ischemic stroke. J Am Heart Assoc. 2018;7(3):e007197. doi:10.1161/JAHA.117.007197
78. Long Y, Xiang Y, Liu S, et al. Macrophage membrane modified baicalin liposomes improve brain targeting for alleviating cerebral ischemia reperfusion injury. Nanomedicine. 2022;43:102547. doi:10.1016/j.nano.2022.102547
79. Zhao Y, Jiang Y, Lv W, et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71. doi:10.1016/j.jconrel.2016.04.038
80. Li C, Li Z, Gong X, et al. Acidic tumor microenvironment-sensitive liposomes enhance colorectal cancer therapy by acting on both tumor cells and cancer-associated fibroblasts. Nanoscale. 2021;13(23):10509–10525. doi:10.1039/d1nr01506k
81. Abri Aghdam M, Bagheri R, Mosafer J, et al. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J Control Release. 2019;315:1–22. doi:10.1016/j.jconrel.2019.09.018
82. Kim Y, Oh KT, Youn YS, Lee ES. pH-sensitive twin liposomes containing quercetin and laccase for tumor therapy. Biomacromolecules. 2022;23(9):3688–3697. doi:10.1021/acs.biomac.2c00571
83. Rayamajhi S, Marchitto J, Nguyen T, Marasini R, Celia C, Aryal S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf B Biointerfaces. 2020;188:110804. doi:10.1016/j.colsurfb.2020.110804
84. Mei T, Kim A, Vong LB, et al. Encapsulation of tissue plasminogen activator in pH-sensitive self-assembled antioxidant nanoparticles for ischemic stroke treatment - Synergistic effect of thrombolysis and antioxidant. Biomaterials. 2019;215:119209. doi:10.1016/j.biomaterials.2019.05.020
85. Katsura K, Ekholm A, Asplund B, Siesjö BK. Extracellular pH in the brain during ischemia: relationship to the severity of lactic acidosis. J Cereb Blood Flow Metab. 1991;11(4):597–599. doi:10.1038/jcbfm.1991.109
86. Menyhárt Á, Zölei-Szénási D, Puskás T, et al. Spreading depolarization remarkably exacerbates ischemia-induced tissue acidosis in the young and aged rat brain. Sci Rep. 2017;7(1):1154. doi:10.1038/s41598-017-01284-4
87. Yao S, He C, Yuan P, et al. Real-time objective evaluation of the ischemic stroke through ph-responsive fluorescence imaging. Adv Healthc Mater. 2023;12(9):e2201981. doi:10.1002/adhm.202201981
88. Kikuchi T, Fukuta T, Agato Y, et al. Suppression of cerebral ischemia/reperfusion injury by efficient release of encapsulated ifenprodil from liposomes under weakly acidic pH conditions. J Pharm Sci. 2019;108(12):3823–3830. doi:10.1016/j.xphs.2019.09.006
89. Filippov SK, Bogomolova A, Kaberov L, et al. Internal Nanoparticle Structure of Temperature-Responsive Self-Assembled PNIPAM-b-PEG-b-PNIPAM Triblock Copolymers in Aqueous Solutions: NMR, SANS, and Light Scattering Studies. Langmuir. 2016;32(21):5314–5323. doi:10.1021/acs.langmuir.6b00284
90. Saxena V, Gacchina Johnson C, Negussie AH, Sharma KV, Dreher MR, Wood BJ. Temperature-sensitive liposome-mediated delivery of thrombolytic agents. Int J Hyperthermia. 2015;31(1):67–73. doi:10.3109/02656736.2014.991428
91. Hsu HL, Chen JP. Preparation of thermosensitive magnetic liposome encapsulated recombinant tissue plasminogen activator for targeted thrombolysis. North-Holland. 2017. doi:10.1016/j.jmmm.2016.10.122
92. Refaat A, Del Rosal B, Palasubramaniam J, et al. Near-infrared light-responsive liposomes for protein delivery: towards bleeding-free photothermally-assisted thrombolysis. J Control Release. 2021;337:212–223. doi:10.1016/j.jconrel.2021.07.024
93. Wan Y, Han J, Fan G, Zhang Z, Gong T, Sun X. Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials. 2013;34(12):3020–3030. doi:10.1016/j.biomaterials.2012.12.051
94. Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1372. doi:10.3390/molecules27041372
95. Liu Y, Castro Bravo KM, Liu J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horiz. 2021;6(2):78–94. doi:10.1039/d0nh00605j
96. Rabinovici R, Rudolph AS, Ligler FS, Yue TL, Feuerstein G. Liposome-encapsulated hemoglobin: an oxygen-carrying fluid. Circ Shock. 1990;32(1):1–17.
97. Kawaguchi AT, Fukumoto D, Haida M, Ogata Y, Yamano M, Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke. 2007;38(5):1626–1632. doi:10.1161/STROKEAHA.106.467290
98. Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis. 2010;16:2882–2890.
99. Campos-Martorell M, Cano-Sarabia M, Simats A, et al. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int J Nanomed. 2016;11:3035–3048. doi:10.2147/IJN.S107292
100. Sun R, Shang J, Yan X, et al. VCAM1 drives vascular inflammation leading to continuous cortical neuronal loss following chronic cerebral hypoperfusion. J Alzheimers Dis. 2023;91(4):1541–1555. doi:10.3233/JAD-221059
101. Liu H, Jablonska A, Li Y, et al. Label-free CEST MRI detection of citicoline-liposome drug delivery in ischemic stroke. Theranostics. 2016;6(10):1588–1600. doi:10.7150/thno.15492
102. Zhao Y-Z, Lin M, Lin Q, et al. Intranasal delivery of bFGF with nanoliposomes enhances in vivo neuroprotection and neural injury recovery in a rodent stroke model. J Control Release. 2016;224:165–175. doi:10.1016/j.jconrel.2016.01.017
103. Park T, Amatya R, Min KA, Shin MC. Liposomal iron oxide nanoparticles loaded with doxorubicin for combined chemo-photothermal cancer therapy. Pharmaceutics. 2023;15(1):292. doi:10.3390/pharmaceutics15010292
104. Alkaff SA, Radhakrishnan K, Nedumaran AM, Liao P, Czarny B. Nanocarriers for stroke therapy: advances and obstacles in translating animal studies. Int J Nanomed. 2020;15:445–464. doi:10.2147/IJN.S231853
105. Fukuta T, Asai T, Yanagida Y, et al. Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J. 2017;31(5):1879–1890. doi:10.1096/fj.201601209R
106. Peng T, Booher K, Moody MR, et al. Enhanced Cerebroprotection of Xenon-Loaded Liposomes in Combination with rtPA Thrombolysis for Embolic Ischemic Stroke. Biomolecules. 2023;13(8):1256. doi:10.3390/biom13081256
107. Huang H, Chen L, Chopp M, et al. The 2020 Yearbook of Neurorestoratology. Journal of Neurorestoratology. 2021;9(1):1–12. doi:10.26599/JNR.2021.9040002
108. Al-Ahmady ZS. Selective drug delivery approaches to lesioned brain through blood brain barrier disruption. Expert Opin Drug Deliv. 2018;15(4):335–349. doi:10.1080/17425247.2018.1444601
109. Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. doi:10.3389/fneur.2013.00032
110. Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol. 2011;26(9):1193–1198. doi:10.1177/0883073811408610
111. Smith DA, Vaidya SS, Kopechek JA, et al. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol. 2010;36(1):145–157. doi:10.1016/j.ultrasmedbio.2009.08.009
112. Shekhar H, Bader KB, Huang S, et al. In vitro thrombolytic efficacy of echogenic liposomes loaded with tissue plasminogen activator and octafluoropropane gas. Phys Med Biol. 2017;62(2):517–538. doi:10.1088/1361-6560/62/2/517
113. Asahi M, Rammohan R, Sumii T, et al. Antiactin-targeted immunoliposomes ameliorate tissue plasminogen activator-induced hemorrhage after focal embolic stroke. J Cereb Blood Flow Metab. 2003;23(8):895–899. doi:10.1097/01.WCB.0000072570.46552.DF
114. Li Y, Zhang M, Li S, et al. Selective ischemic-hemisphere targeting Ginkgolide B liposomes with improved solubility and therapeutic efficacy for cerebral ischemia-reperfusion injury. Asian J Pharm Sci. 2023;18(2):100783. doi:10.1016/j.ajps.2023.100783
115. Sun S, Lv W, Li S, et al. Smart liposomal nanocarrier enhanced the treatment of ischemic stroke through neutrophil extracellular traps and cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway inhibition of ischemic penumbra. ACS Nano. 2023;17(18):17845–17857. doi:10.1021/acsnano.3c03390
116. Kakehata J, Yamaguchi T, Togashi H, et al. Therapeutic potentials of an artificial oxygen-carrier, liposome-encapsulated hemoglobin, for ischemia/reperfusion-induced cerebral dysfunction in rats. J Pharmacol Sci. 2010;114(2):189–197. doi:10.1254/jphs.10115fp
117. Chen YY, Gong ZC, Zhang MM, Huang ZH. Brain-targeting emodin mitigates ischemic stroke via inhibiting AQP4-mediated swelling and neuroinflammation. Transl Stroke Res. 2023. doi:10.1007/s12975-023-01170-4
118. Thomas RG, Kim JH, Kim JH, Yoon J, Choi KH, Jeong YY. Treatment of ischemic stroke by atorvastatin-loaded PEGylated liposome. Transl Stroke Res. 2024;15(2):388–398. doi:10.1007/s12975-023-01125-9
119. Wang K, Zhou W, Jin X, et al. Enhanced brain delivery of hypoxia-sensitive liposomes by hydroxyurea for rescue therapy of hyperacute ischemic stroke. Nanoscale. 2023;15(27):11625–11646. doi:10.1039/d3nr01071f
120. Ishii T, Asai T, Fukuta T, et al. A single injection of liposomal asialo-erythropoietin improves motor function deficit caused by cerebral ischemia/reperfusion. Int J Pharm. 2012;439(1–2):269–274. doi:10.1016/j.ijpharm.2012.09.026
121. Wen Y, Zhang Z, Cai Z, Liu B, Wu Z, Liu Y. Ligustrazine-loaded borneol liposome alleviates cerebral ischemia-reperfusion injury in rats. ACS Biomater Sci Eng. 2022;8(11):4930–4941. doi:10.1021/acsbiomaterials.2c00847
122. Wu S, Liao D, Li X, et al. Endogenous oleoylethanolamide crystals loaded lipid nanoparticles with enhanced hydrophobic drug loading capacity for efficient stroke therapy. Int J Nanomed. 2021;16:8103–8115. doi:10.2147/IJN.S344318
123. Fukuta T, Asai T, Sato A, et al. Neuroprotection against cerebral ischemia/reperfusion injury by intravenous administration of liposomal fasudil. Int J Pharm. 2016;506(1–2):129–137. doi:10.1016/j.ijpharm.2016.04.046
124. Yu S, Li D, Shi A, et al. Multidrug-loaded liposomes prevent ischemic stroke through intranasal administration. Biomed Pharmacother. 2023;162:114542. doi:10.1016/j.biopha.2023.114542
125. Zhao Y, Xin Z, Li N, et al. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic Biol Med. 2018;124:1–11. doi:10.1016/j.freeradbiomed.2018.05.082
126. Guo X, Jin X, Han K, et al. Iron promotes neurological function recovery in mice with ischemic stroke through endogenous repair mechanisms. Free Radic Biol Med. 2022;182:59–72. doi:10.1016/j.freeradbiomed.2022.02.017
127. Arul MR, Alahmadi I, Turro DG, et al. Fluorescent liposomal nanocarriers for targeted drug delivery in ischemic stroke therapy. Biomater Sci. 2023;11(24):7856–7866. doi:10.1039/d3bm00951c
128. Quan X, Han Y, Lu P, et al. Annexin V-modified platelet-biomimetic nanomedicine for targeted therapy of acute ischemic stroke. Adv Healthc Mater. 2022;11(16):e2200416. doi:10.1002/adhm.202200416
129. Hou J, Yang X, Li S, et al. Accessing neuroinflammation sites: monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci Adv. 2019;5(7):eaau8301. doi:10.1126/sciadv.aau8301
130. Lu H, Li S, Dai D, et al. Enhanced treatment of cerebral ischemia-Reperfusion injury by intelligent nanocarriers through the regulation of neurovascular units. Acta Biomater. 2022;147:314–326. doi:10.1016/j.actbio.2022.05.021
131. Bai M, Cui N, Liao Y, et al. Astrocytes and microglia-targeted Danshensu liposomes enhance the therapeutic effects on cerebral ischemia-reperfusion injury. J Control Release. 2023;364:473–489. doi:10.1016/j.jconrel.2023.11.002
132. Bai Q, Han Y, Khan S, et al. A novel endoplasmic reticulum-targeted metal-organic framework-confined ruthenium (Ru) Nanozyme regulation of oxidative stress for central post-stroke pain. Adv Healthc Mater. 2024;13(2):e2302526. doi:10.1002/adhm.202302526
133. Quan X, Liang X, Ding Y, et al. Cryo-Shocked platelet coupled with ros-responsive nanomedicine for targeted treatment of thromboembolic disease. ACS Nano. 2023;17(7):6519–6533. doi:10.1021/acsnano.2c11865
134. Sun M, Miyazawa K, Pendekanti T, et al. Combination targeting of ‘platelets + fibrin’ enhances clot Anchorage efficiency of nanoparticles for vascular drug delivery. Nanoscale. 2020;12(41):21255–21270. doi:10.1039/d0nr03633a
135. Mishra D, Hubenak JR, Mathur AB. Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J Biomed Mater Res A. 2013;101(12):3646–3660. doi:10.1002/jbm.a.34642
136. Wang Z, Pan J, Yuan R, Chen M, Guo X, Zhou S. Shell-sheddable polymeric micelles alleviate oxidative stress and inflammation for enhanced ischemic stroke therapy. Nano Lett. 2023;23(14):6544–6552. doi:10.1021/acs.nanolett.3c01567
137. Mutoh T, Mutoh T, Taki Y, Ishikawa T. Therapeutic potential of natural product-based oral nanomedicines for stroke prevention. J Med Food. 2016;19(6):521–527. doi:10.1089/jmf.2015.3644
138. Zhang A, Li J, Wang S, et al. Rapid and improved oral absorption of N-butylphthalide by sodium cholate-appended liposomes for efficient ischemic stroke therapy. Drug Deliv. 2021;28(1):2469–2479. doi:10.1080/10717544.2021.2000678
139. Liu T, Wang Y, Zhang M, et al. The optimization design of macrophage membrane camouflaging liposomes for alleviating ischemic stroke injury through intranasal delivery. Int J Mol Sci. 2024;25(5):2927. doi:10.3390/ijms25052927
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
