Back to Journals » Drug Design, Development and Therapy » Volume 12
Inhibition of ROS-mediated activation Src-MAPK/AKT signaling by orientin alleviates H2O2-induced apoptosis in PC12 cells
Authors Qi S, Feng Z, Li Q, Qi Z, Zhang Y
Received 25 June 2018
Accepted for publication 17 October 2018
Published 16 November 2018 Volume 2018:12 Pages 3973—3984
DOI https://doi.org/10.2147/DDDT.S178217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Qiongyu Guo
Shimei Qi,1,2,* Zunyong Feng,1,3,* Qiang Li,1,2 Zhilin Qi,1,2 Yao Zhang1,2
1Anhui Province Key Laboratory of Active Biological Macro-molecules, Wannan Medical College, Wuhu 241002, People’s Republic of China; 2Department of Biochemistry, Wannan Medical College, Wuhu 241002, People’s Republic of China; 3Department of Forensic Medicine, Wannan Medical College, Wuhu 241002, People’s Republic of China
*These authors contributed equally to this work
Purpose: Reactive oxygen species (ROS) are considered a direct cause of neurodegenerative diseases (NDDs). Drugs developed to target ROS are effective for the treatment of NDDs. Orientin is a pyrone glucoside extracted from Polygonum orientale, and it exhibits many pharmacological activities. In this study, we aimed to determine whether orientin could relieve hydrogen peroxide (H2O2)-induced neuronal apoptosis and to investigate the specific target of orientin.
Materials and methods: In this study, the neuroprotective effect and its possible mechanisms of orientin in mouse pheochromocytoma cell line (PC12) cells stimulated by H2O2, establishing an oxidative stress model, were investigated. And we further tested the role of ROS in the neuroprotective effects of orientin.
Results: Orientin (5–100 µg/mL) did not cause toxicity in PC12 cells but significantly decreased H2O2-induced reduction in PC12 cell viability, cell apoptosis rates, and nuclear condensation. It also inhibited the activation of caspase-3 and degradation of poly(ADP-ribose) polymerase (PARP). Under the stimulation of H2O2, MAPKs (ERK, JNK, and p38), AKT, and Src signaling proteins in PC12 cells were activated in a time-dependent manner. The application of inhibitors that were specific for MAPKs, AKT, and Src effectively alleviated H2O2-induced cell apoptosis. In addition, the Src inhibitor decreased the activation of MAPKs and AKT signaling. More importantly, orientin effectively decreased H2O2-induced phosphorylation of MAPKs, AKT, and Src signaling proteins. Finally, we confirmed that orientin effectively inhibited H2O2-induced accumulation of ROS in cells. In addition, ROS inhibitors blocked the Src-MAPKs/AKT signaling pathway-dependent cell apoptosis stimulated by H2O2.
Conclusion: These results indicate that alleviation of H2O2-induced cell apoptosis by orientin is Src-MAPKs/AKT dependent. Overall, our study confirms that orientin alleviates H2O2-induced cell apoptosis by inhibiting the ROS-mediated activation of Src-MAPKs/AKT signaling.
Keywords: oxidative stress, orientin, neuroprotection, apoptosis, Src, MAPKs, AKT
Introduction
Eukaryotic cells continuously produce free radicals during metabolic processes. The antioxidant defense system eliminates free radicals to maintain the redox balance in cells. Excessive reactive oxygen species (ROS) and an imbalance in the regulation of the antioxidant defense system cause oxidative stress (OS) damage.1 In neuronal cells, OS induced by an imbalance in redox regulation causes severe damage. This neuronal damage and death is a direct cause of Alzheimer’s disease, Parkinson’s disease, and Huntington’s chorea. Excessive ROS attack and break nucleic acids, degrade or inactivate enzymes, induce a melting reaction in polysaccharides, and induce lipid peroxidation in biological membranes. Generally, ROS continue to destroy all biological macromolecules until the cells die.2 Treatment of neurodegenerative diseases (NDDs) in clinics has usually been ineffective; therefore, the development of a treatment strategy to block OS damage in neuronal cells is urgently needed.
Polygonum orientale Linn is a herb widely distributed throughout China, excluding Tibet. In traditional Chinese medicine, P. orientale is used to treat rheumatoid arthritis. Orientin, a pyrone glucoside, extracted from P. orientale Linn (Figure 1A), has anti-inflammatory,3–5 antitumor,6 and anti-oxidative7,8 properties, and alleviates ischemic and hypoxic damage to cardiomyocytes.9,10 Recent in vivo studies have confirmed that orientin alleviates cognitive deficits and OS damage in the Aβ-induced mouse model of Alzheimer’s disease.11 Therefore, orientin has a great potential in the treatment of NDDs similar to Alzheimer’s disease. Although orientin exhibits satisfactory antioxidant activity in many studies, its efficacy in a neuronal damage model stimulated by exogenous peroxides is unclear. Moreover, the specific action mechanism and anti-oxidative targets of orientin have not been elucidated.
In this study, we constructed an OS damage model using exogenous H2O2 and the mouse PC12 neuronal cell line to confirm whether orientin could effectively alleviate OS damage and cell apoptosis in PC12 cells. Next, we investigated the specific mechanism underlying orientin-regulated apoptosis mediated by the activated signal transduction pathways.
Materials and methods
Antibodies and reagents
Orientin, H2O2, N-acetyl-L-cysteine (NAC), 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d] pyrimidine (PP2), and 4-amino-7-phenylpyrazol [3,4-d] pyrimidine (PP3) were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies specifically targeted to caspase-3, PARP, GAPDH, JNK, p38, ERK, AKT, SRC, phospho-JNK, phosphor-p38, phospho-ERK, phospho-AKT, and phospho-Src were purchased from Cell Signaling Technology (Danvers, MA, USA). Fluorophore-labeled secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE, USA). Inhibitors of ERK, p38, JNK, and AKT namely U0126, SB203580, SP600125, and LY294002, respectively, were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
The PC12 cells were obtained from the Kunming Cell Bank of the Chinese Academy of Sciences and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (containing 10% horse serum, 5% fetal bovine serum, and 1% penicillin/streptomycin double antibiotic) at 37°C in a 5% CO2 incubator. The culture medium was replaced every day, and cells were passaged every other day.
Cell viability assay
The PC12 cells were seeded into 96-well plates at 5×104 cells per well 24 hours before treatment. Following treatment with orientin and/or H2O2 for the indicated time periods, cells were incubated with 10 μL of CCK-8 (Dojindo, Kumamoto, Japan) for 2 hours, and absorbance values were measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
DAPI staining
The PC12 cells were seeded into six-well plates. After 24 hours, the cells were incubated with orientin and/or H2O2 at the indicated concentrations for the indicated time. The cells were then fixed in 4% paraformaldehyde for 30 minutes and stained with DAPI (Beyotime, Shanghai, People’s Republic of China) for 1 hour. Cell morphology was observed under an Olympus IX71 inverted microscope.
Measurement of cell apoptosis rate
PC12 cells were cultured into six-well plates at 5×104 cells. Twenty-four hours later, the cells were handled with orientin and/or H2O2 as indicated in the figure legends. Cells were co-stained with Annexin V-FITC and propidium iodide (PI) (Beyotime, Shanghai, People’s Republic of China) for 30 minutes, and cell apoptosis rates were measured using BD FACSVerse flow cytometry (BD Biosciences, San Jose, CA, USA).
Western blotting
Cells were washed with pre-cooled PBS and lysed in cell lysis buffer containing 1% phenylmethylsulfonyl fluoride, a protease inhibitor, on ice for 40 minutes. Cell lysates were collected and mixed with 2× loading buffer (Beyotime, Shanghai, People’s Republic of China), and 20 μL of each sample was subjected to SDS-PAGE. Proteins were transferred to a nitrocellulose membrane, blocked in 5% nonfat milk, incubated with the corresponding primary antibodies at 4°C overnight, and then incubated with fluorophore-labeled secondary antibodies for 1 hour. The results were observed using an Odyssey imaging system (LI-COR Biosciences, Lincoln, NE, USA).
ROS detection
The intracellular accumulation of ROS, including H2O2 and other peroxides, was monitored using the fluorescent probe CM-H2DCFDA. At the end of the treatment, 10 μM of the fluorescent probe CM-H2DCFDA (Invitrogen, Carlsbad, CA, USA) was added and the samples were incubated at 37°C for 30 minutes in each well. Absorbance was measured at 450 nm (excitation) and 535 nm (emission) using a microplate reader.
Statistical analysis
All data are reported as mean±SD. Comparisons between two groups were performed using the t-test and one-way ANOVA. Statistical significance of differences was determined at P<0.05. Statistical analysis of data was performed using SPSS 13.0.
Results
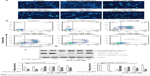
Orientin alleviates H2O2-induced reduction in PC12 cell viability
To confirm that orientin is not toxic to PC12 cells, PC12 cells were incubated with 0, 5, 10, 20, 40, 60, 80, and 100 μg/mL orientin for 24 hours, and cell viability was measured using the CCK-8 method. Results showed that orientin was not toxic to PC12 cells at any of the doses tested (Figure 1B). To identify the toxic dose of H2O2, PC12 cells were incubated with 0, 100, 150, 200, 250, 300, 350, and 400 μM H2O2 for 24 hours. Measurement of cell viability showed that H2O2 caused significant toxicity in cells at concentrations higher than 100 μM (Figure 1C). Therefore, 150 μM H2O2 was used as the toxic dose in the OS damage model in PC12 cells. To evaluate the effects of orientin on the reduction of PC12 cell viability induced by H2O2, PC12 cells were incubated with 0, 5, 10, 20, 40, 60, 80, and 100 μg/mL orientin for 2 hours and then stimulated by 150 μM H2O2 for 24 hours. Measurement of cell viability showed that the reduction in H2O2-induced viability of PC12 cells was significantly suppressed at orientin concentrations higher than 40 μg/mL (Figure 1D). Therefore, orientin at 60, 80, and 100 μg/mL was used as a low, medium, and high dose, respectively, in subsequent experiments.
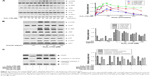
Orientin alleviates H2O2-induced apoptosis in PC12 cells
OS damage causes cells to undergo programmed apoptosis.12 Therefore, we evaluated the function of orientin in the process of H2O2-induced apoptosis in PC12 cells. PC12 cells were preincubated with 60, 80, and 100 μg/mL orientin for 2 hours and then stimulated by 150 μM H2O2 for 24 hours. Subsequently, PC12 cells were stained with DAPI, and nuclear morphology was observed under an inverted fluorescence microscope. The results showed that H2O2 induced DNA condensation and breakage in the nuclei of PC12 cells; however, orientin significantly improved the nuclear morphology and reversed the change induced by H2O2 (Figure 2A). PC12 cells were co-stained with Annexin V-FITC and PI, and apoptosis and survival rates of PC12 cells were measured using flow cytometry. Results showed that orientin significantly decreased the H2O2-induced apoptosis rates in PC12 cells (Figure 2B). Caspase-3 and PARP are indispensable for cell apoptosis. Caspase-3 is the executor of programmed apoptosis, whereas PARP is the substrate of caspase-3. After pre-incubation of PC12 cells with orientin (60, 80, and 100 μg/mL) and stimulation by H2O2 for 24 hours, changes in the level of caspase-3 and PARP proteins were evaluated using Western blotting. The results showed that H2O2 stimulation downregulated caspase-3 precursor (35 kDa), upregulated the cleaved caspase-3 protein (17 kDa), and cleaved PARP protein (117 kDa) into 116 and 85 kDa proteins (Figure 2C). Additionally, orientin reversed caspase-3 activation and PARP degradation stimulated by H2O2 (Figure 2C). These results indicate that orientin effectively blocks H2O2-induced apoptosis in PC12 cells.
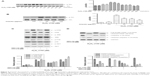
Orientin inhibits MAPK and AKT signaling
The serine/threonine kinase AKT, also known as protein kinase B (PKB), represents an important intersection in multiple signaling pathways; AKT regulates cytokines, growth factors, and oncogenic Ras-activated cell survival signals.13 The mitogen-activated protein kinase (MAPK) signal transduction pathway is deeply involved in the regulation of cell proliferation, differentiation, stress, apoptosis, and survival.14 To verify the activation of signaling pathways in PC12 cells after H2O2 stimulation, PC12 cells were continuously stimulated by 150 μM H2O2 for indicated times, and the phosphorylation of AKT, ERK, JNK, and p38 was measured using Western blotting. The results showed that H2O2 stimulation of PC12 cells led to AKT activation in a time-dependent manner, with the maximum activation at 4 hours (Figure 3A). Activation of MAPKs (ERK, JNK, and p38) was the highest at 1.5 hour. To determine whether orientin decreased the activation of AKT and MAPKs induced by H2O2, PC12 cells were preincubated with 60, 80, and 100 μg/mL orientin for 2 hours, and phosphorylation of AKT and MAPKs was measured after 4 and 1.5 hours of H2O2 stimulation, respectively. The results showed that orientin decreased the H2O2-induced phosphorylation of AKT, ERK, JNK, and p38 in a dose-dependent manner (Figure 3B). Next, PC12 cells were preincubated with inhibitors of AKT (LY294002; 10 μM), ERK (U0126; 10 μM), JNK (SP600125; 50 μM), and p38 (SB203580; 20 μM) for 2 hours and then stimulated by H2O2 for 24 hours. Measurement of the cleavage conditions of caspase-3 and PARP revealed that inhibitors of AKT and MAPKs significantly inhibited H2O2-induced caspase-3 activation and PARP degradation (Figure 3C). These results indicate that the inhibition of H2O2-induced apoptosis by orientin was achieved through the downregulation of AKT phosphorylation and MAPK signal transduction pathways or their upstream signaling.
Orientin inhibits the activity of Src protein
The Src protein family plays important roles in cellular OS.15,16 Our previous studies show that Src protein regulates the activation of AKT/ERK signaling in HeLa cells after stimulation by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).17 To investigate the upstream signaling related to the suppression of AKT and MAPK signaling activation by orientin, PC12 cells were continuously stimulated by 150 μM H2O2 for 6 hours, and the phosphorylation of Src was evaluated using Western blotting. Following H2O2 stimulation, Src was activated in a time-dependent manner, with maximum activation at approximately 45 minutes (Figure 4A). To determine whether orientin decreased the activation of Src signaling, PC12 cells were preincubated with 60, 80, and 100 μg/mL orientin for 2 hours and then stimulated by H2O2 for 45 minutes. Analysis of Src phosphorylation revealed that orientin decreased the H2O2-induced phosphorylation of Src in a dose-dependent manner (Figure 4B). Next, PC12 cells were preincubated with PP2 (Src inhibitor; 10 μmol/L) or PP3 (negative inhibitor) for 2 hours, and the phosphorylation of AKT and MAPKs was measured after 4 and 1.5 hours of H2O2 stimulation, respectively, and the cleavage conditions of caspase-3 and PARP were measured after 24 hours of H2O2 stimulation. The results showed that PP2 significantly inhibited H2O2-induced activation of AKT and MAPK signaling (Figure 4C). Furthermore, AKT/MAPK-dependent caspase-3 cleavage and PARP degradation were also decreased after PP2 treatment. The negative inhibitor PP3 had no effect on the activation of AKT/MAPK and cutting of apoptotic proteins (Figure 4D). These results indicate that inhibition of H2O2-induced cell apoptosis by orientin was achieved through the regulation of Src-mediated activation of AKT/MAPK signaling.
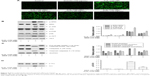
Orientin cleared ROS to decrease Src-MAPK/AKT-dependent cell apoptosis
Excessive exogenous H2O2 stimulates an antioxidant system disorder in cells and reduces the ability to clear endogenous ROS, thus causing excessive production of ROS and consequently OS damage and cell death.18 To determine whether orientin could clear the ROS produced in response to H2O2, PC12 cells were preincubated with 60, 80, and 100 μg/mL orientin for 2 hours and then stimulated by H2O2 for 30 minutes. Analysis of intracellular ROS production using the ROS probe CM-H2DCFDA showed that orientin significantly decreased H2O2-induced ROS production in PC12 cells (Figure 5A). To determine whether H2O2-induced cell apoptosis was due to the increase in the level of intracellular ROS, PC12 cells were incubated with the ROS inhibitor NAC for 2 hours and then stimulated by H2O2 for 24 hours. Measurement of the cleavage conditions of caspase-3 and PARP showed that NAC inhibited caspase-3 activation and PARP degradation (Figure 5C). We further detected the phosphorylation of Src, MAPKs, and AKT after 2 hours NAC pretreatment, followed by H2O2 stimulation for 45 minutes, 1.5 hour, and 4 hours, respectively. Figure 5B and D shows that NAC restrained the activation of Src, MAPKs, and AKT. Overall, these data suggest that orientin alleviates H2O2-induced PC12 cell apoptosis through the inhibition of ROS-mediated activation of Src-MAPK/AKT signaling pathways.
Discussion
NDDs, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and multiple sclerosis, have a long disease course, are difficult to cure, can shorten life span, and cause disability.19 There are many pathological factors in NDDs, including autophagy system disorder,20 unfolded protein response (UPR),21 inflammatory injury,22 apoptosis,23 OS,24 and mitochondrial dysfunction.25 Among these factors, OS and its role in the pathological process of NDDs have received significant attention. Generally, excessive production of free radicals, ROS, and reactive nitrogen species or the deregulation of detoxifying and/or repairing systems causes OS, either individually or together. Therefore, it would be very practical to develop drugs for the clearance of oxidative free radicals or activation of the antioxidant defense system for NDD treatment. This study, for the first time, provides in vitro results showing that through the clearance of H2O2-induced ROS and reduced activation of ROS-dependent Src-MAPK/AKT signaling pathways, orientin protected PC12 cells against H2O2-induced cell apoptosis and oxidative damage.
Many studies have confirmed that H2O2 induces apoptosis in a variety of neuronal cells. The most likely mechanism underlying this observation is that H2O2 excessively consumes anti-oxidases in neuronal cells, resulting in redox balance disorders, and ultimately cell death.26,27 Our results showed that orientin alleviated H2O2-induced apoptosis in PC12 cells via the restoration of cell viability, reduction of cell apoptosis rates, and improvement of nuclear morphology. Caspase-3 is the most crucial end-cleaving enzyme during the process of cell apoptosis; it cleaves and inactivates the DNA repair enzyme PARP, which plays key roles in DNA repair and stimulates the cell apoptosis program.28 Western blotting results showed that orientin blocked caspase-3 activation and PARP degradation. Together, these data suggest that orientin significantly alleviates H2O2-induced apoptosis in PC12 cells.
Many signaling pathways, including the MAPK,29 PI3K/AKT,30 and NF-κB,31 play important roles in the neuronal apoptosis induced by OS. Our experimental results showed that H2O2 stimulated the activation of MAPKs (ERK, JNK, and p38) and AKT signaling pathways in a time-dependent manner, and orientin decreased H2O2-induced phosphorylation of MAPKs and AKT signaling proteins. When cells were incubated with specific inhibitors of ERK, JNK, p38, and AKT, H2O2-induced apoptosis in PC12 cells was inhibited to different degrees. These results indicate that alleviation of H2O2-induced apoptosis by orientin is mediated by the suppression of MAPK/AKT signaling pathways. The Src family proteins usually play a housekeeping role in cell proliferation, differentiation, stress, and apoptosis. Src is usually used as an early indicator of the activation of downstream signaling.32 In this study, H2O2 stimulated the activation of Src in PC12 cells, and Src was activated earlier than MAPKs and AKT. However, orientin decreased H2O2-induced phosphorylation of the Src signaling protein. When PC12 cells were incubated with the Src-specific inhibitor PP2, H2O2-induced activation of MAPKs and AKT signaling was inhibited. More importantly, MAPK/AKT-mediated cell apoptosis was also inhibited by PP2. The above results indicate that alleviation of H2O2-induced apoptosis by orientin is dependent on the Src-MAPK/AKT signaling pathways.
Many studies have previously confirmed that OS induces neuronal apoptosis, which can be mediated by many signaling pathways.33–36 As expected, H2O2 induced PC12 cells to produce a large amount of ROS, and pre-incubation with orientin significantly inhibited H2O2-induced ROS production in PC12 cells. To determine whether Src-MAPK/AKT signaling-dependent cell apoptosis was mediated by ROS in our experimental system, we preincubated cells with ROS-specific scavenger NAC. The results showed that NAC inhibited H2O2-induced activation of Src. Additionally, MAPKs and AKT signaling molecules located downstream of Src were also inhibited. More importantly, H2O2-induced cell apoptosis in PC12 cells was also inhibited by NAC. Overall, orientin alleviated H2O2-induced apoptosis in PC12 cells by inhibiting ROS-mediated activation of Src-MAPK/AKT signaling.
Conclusion
This is the first study showing that orientin alleviates H2O2-induced apoptosis in PC12 cells in vitro. This is probably because by inhibiting H2O2-induced ROS production, orientin further inhibited the activation of Src-MAPK/AKT signaling to alleviate apoptosis induced by OS damage. Thus, we suggest orientin as a potential antioxidant drug for the treatment of NDDs induced by neural OS.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (grant nos 31301171, 81601380, and 81800082); Project supported by the Natural Science Foundation of Anhui Province, China (General Program) (grant no 1508085MH149); Anhui Provincial Key Laboratory Project (grant no 1306C083008); Colleges and Universities Provincial Young Talents Foundation Key Project (grant no 2013SQRL055ZD); Wannan Medical College Start Research Foundation Project (grant no 201223); Active Biological Macromolecules Research Provincial Key Laboratory Project (grant no 1306C083008); Natural Science Research Project of Anhui Colleges and Universities (grant no KJ2016SD59); Colleges and Universities Outstanding Young Talent Support Program Key Projects (grant no gxyqZD2016173); and Wuhu Technology Bureau Production and Research Cooperative Special Foundation (grant no 2013cxy04).
Disclosure
The authors report no conflicts of interest in this work.
References
Liochev SI, Fridovich I. The role of O2.− in the production of HO.: in vitro and in vivo. Free Radic Biol Med. 1994;16(1):29–33. | ||
Burtis WJ, Brady TG, Orloff JJ, et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990;322(16):1106–1112. | ||
Zhou X, Gan P, Hao L, et al. Antiinflammatory effects of orientin-2″-O-galactopyranoside on lipopolysaccharide-stimulated microglia. Biol Pharm Bull. 2014;37(8):1282–1294. | ||
Lee W, Ku SK, Bae JS. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vascul Pharmacol. 2014;62(1):3–14. | ||
Yoo H, Ku SK, Lee T, Bae JS. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation. 2014;37(5):1705–1717. | ||
An F, Wang S, Tian Q, Zhu D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol Lett. 2015;10(4):2627–2633. | ||
An F, Wang S, Yuan D, Gong Y, Wang S. Attenuation of Oxidative Stress of Erythrocytes by Plant-Derived Flavonoids, Orientin and Luteolin. Evid Based Complement Alternat Med. 2016;2016(1):1–8. | ||
An F, Yang G, Tian J, Wang S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen Res. 2012;7(33):2565–2575. | ||
Lu N, Sun Y, Zheng X. Orientin-induced cardioprotection against reperfusion is associated with attenuation of mitochondrial permeability transition. Planta Med. 2011;77(10):984–991. | ||
Liu L, Wu Y, Huang X. Orientin protects myocardial cells against hypoxia-reoxygenation injury through induction of autophagy. Eur J Pharmacol. 2016;776:90–98. | ||
Yu L, Wang S, Chen X, et al. Orientin alleviates cognitive deficits and oxidative stress in Aβ1-42-induced mouse model of Alzheimer’s disease. Life Sci. 2015;121:104–109. | ||
Kupsco A, Schlenk D, Stress O. Oxidative stress, unfolded protein response, and apoptosis in developmental toxicity. Int Rev Cell Mol Biol. 2015;317:1–66. | ||
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. | ||
Symons A, Beinke S, Ley SC. MAP kinase kinase kinases and innate immunity. Trends Immunol. 2006;27(1):40–48. | ||
Pal R, Palmieri M, Loehr JA, et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun. 2014;5(1):4425. | ||
Li LF, Lee CS, Liu YY, et al. Activation of Src-dependent Smad3 signalling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury. Respir Res. 2015;16(1):1–14. | ||
Qi S, Xin Y, Qi Z, et al. HSP27 phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK signaling through interaction with β-arrestin2. Cell Signal. 2014;26(3):594–602. | ||
Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62(9):90–101. | ||
Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104. | ||
Mccray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008;16(1):75–84. | ||
Ross CA, Poirier MA. Opinion: What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol. 2005;6(11):891–898. | ||
Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29(9):518–527. | ||
Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9(8):1059–1096. | ||
Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10 suppl:S18–S25. | ||
Lin MT, Beal MF. Alzheimer’s APP mangles mitochondria. Nat Med. 2006;12(11):1241–1243. | ||
Chen XH, Zhou X, Yang XY, et al. Propofol Protects Against H2O2-Induced Oxidative Injury in Differentiated PC12 Cells via Inhibition of Ca(2+)-Dependent NADPH Oxidase. Cell Mol Neurobiol. 2016;36(4):541–551. | ||
Luo Y, Liu X, Zheng Q, et al. Hydrogen sulfide prevents hypoxia-induced apoptosis via inhibition of an H2O2-activated calcium signaling pathway in mouse hippocampal neurons. Biochem Biophys Res Commun. 2012;425(2):473–477. | ||
Galluzzi L, Kepp O, Kroemer G. Caspase-3 and prostaglandins signal for tumor regrowth in cancer therapy. Oncogene. 2012;31(23):2805–2808. | ||
Moon H, Lee B, Choi G, et al. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci U S A. 2003;100(1):358–363. | ||
Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–312. | ||
Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49(6):681–697. | ||
Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13(1):513–609. | ||
Aminzadeh A, Dehpour AR, Safa M, Mirzamohammadi S, Sharifi AM. Investigating the protective effect of lithium against high glucose-induced neurotoxicity in PC12 cells: involvements of ROS, JNK and P38 MAPKs, and apoptotic mitochondria pathway. Cell Mol Neurobiol. 2014;34(8):1143–1150. | ||
Gao Y, Dong C, Yin J, Shen J, Tian J, Li C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell Mol Neurobiol. 2012;32(4):523–529. | ||
Song Y, Kim HD, Lee MK, et al. Protective effect of centipedegrass against Aβ oligomerization and Aβ-mediated cell death in PC12 cells. Pharm Biol. 2015;53(9):1260–1266. | ||
Kinarivala N, Shah K, Abbruscato TJ, Trippier PC. Passage Variation of PC12 Cells Results in Inconsistent Susceptibility to Externally Induced Apoptosis. ACS Chem Neurosci. 2017;8(1):82–88. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.