Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Inhibition of Key Glycolytic Enzyme Hexokinase 2 Ameliorates Psoriasiform Inflammation in vitro and in vivo
Authors Zhuang L, Ma W , Jiao J
Received 15 August 2023
Accepted for publication 24 October 2023
Published 9 November 2023 Volume 2023:16 Pages 3229—3239
DOI https://doi.org/10.2147/CCID.S435624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Le Zhuang,1 Weiyuan Ma,2 Jing Jiao1
1Department of Dermatology, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong Province, People’s Republic of China; 2Department of Dermatology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong Province, People’s Republic of China
Correspondence: Jing Jiao, Department of Dermatology, Central Hospital Affiliated to Shandong First Medical University, No. 105, Jiefang Road, Lixia District, Jinan, Shandong Province, 250013, People’s Republic of China, Tel +8618100389680, Fax +8653185695114, Email [email protected] Weiyuan Ma, Department of Dermatology, Affiliated Hospital of Weifang Medical University, No. 2428, Yuhe Road, Kuiwen District, Weifang, Shandong Province, 261035, People’s Republic of China, Tel +861865362629, Fax +865363081201, Email [email protected]
Purpose: Epidermal keratinocytes with an abnormal glucose metabolism have been identified in psoriasis. Hexokinase 2 (HK2) is a crucial enzyme involved in glycolytic metabolic pathways. However, the expression of HK2 and its potential therapeutic effects in psoriasis remains unclear. This study aimed to investigate the expression pattern of HK2 and evaluate its therapeutic effects in psoriasis.
Patients and Methods: A gene expression dataset (GSE121212) downloaded from the Gene Expression Omnibus (GEO) database was used to examine the expression of HK2 in psoriasis. HK2 RNA and protein expression were investigated in psoriasis vulgaris (n=5) and healthy (n=5) samples. Immunohistochemistry for HK2 was performed on psoriasis vulgaris (n=22) and healthy skin (n=10) samples. Additionally, HaCaT cells were treated with M5 (interleukin [IL]-17A, tumor necrosis factor-α, IL-1α, IL-22, and Oncostatin-M) to induce a psoriatic inflammation cell model. A mouse model of psoriatic inflammation was established using topical 5% imiquimod cream. Psoriasis-like cells and mouse models were treated with the HK2 inhibitor 3-bromopyruvate (3-BrPA). Cell proliferation, glucose consumption, and lactate production were assessed. Furthermore, the activation of nuclear factor-kappa B (NF-Kb) and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) was investigated using Western blot analysis.
Results: According to the GEO dataset, HK2 expression was significantly elevated in psoriasis. Upregulation of HK2 in psoriatic tissues was confirmed by quantitative real-time polymerase chain reaction and Western blotting. The immunohistochemistry score for HK2 was higher in psoriatic lesions than in healthy skin. 3-BrPA inhibited the proliferation and glycolysis of M5-stimulated HaCaT cells. Topical 3-BrPA ameliorated imiquimod-induced psoriasis-like dermatitis. Activation of NF-kB and NLRP3 was downregulated by 3-BrPA treatment.
Conclusion: Our study revealed that the glycolytic enzyme HK2 was upregulated in psoriasis and that the HK2 inhibitor 3-BrPA exhibited therapeutic effects in psoriasis cell and mouse models.
Keywords: psoriasis, glucose metabolism, glycolysis, proliferation, 3-bromopyruvate
Introduction
Psoriasis is a common, debilitating systemic disease that significantly impacts healthcare systems globally.1 It is a systemic inflammatory disease accompanied by a heavy burden of physical and mental-related comorbidities.2 Epidermal hyper-proliferation and inflammation are critical features of psoriasis.3,4 Psoriasis is characterized by the excessive production of cytokines derived from immune cells, which activate multiple signaling pathways and transcription factors.5 Activation of essential pathways such as the nuclear factor-kappa B (NF-κB) and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) pathways leads to the proliferation of epidermal keratinocytes, ultimately worsening psoriasis.6 The proliferation speed of keratinocytes in psoriatic skin lesions is significantly higher than that of normal keratinocytes in healthy skin.7
Aerobic glycolysis is a nontraditional glucose metabolism process observed in proliferating cells with sufficient oxygen.8 During this process, glucose is consumed and split into lactate and pyruvate to meet the energy requirements of proliferating cells.9 In psoriasis, the rapid proliferation of keratinocytes and their high energy demand lead to an enhanced glycolytic energy supply. These dysregulated metabolic pathways are associated with the development of psoriasis.10,11 Recent studies have shown that therapeutic agents targeting glycolysis-related molecules have potential therapeutic value in psoriasis in laboratory experiments (in vitro) and living organisms (in vivo).12–14
Hexokinase (HK) is the key enzyme responsible for the initial and rate-limiting steps of the glycolytic metabolic pathway. It catalyzes the phosphorylation of glucose into glucose-6-phosphate.15 In mammals, four isoforms of HK have been identified: HK1, HK2, HK3, and HK4.16,17 HK1 is the predominant form expressed in most tissues, while HK2 is selectively expressed in a few normal tissues, such as embryonic tissues. HK3 and HK4 have more limited expression and are less characterized.17 Notably, HK2 is significantly upregulated in various epithelial cancers and inflammatory diseases.18 Its overexpression contributes to metabolic rewiring towards aerobic glycolysis in neoplastic cells, and genetic ablation of HK2 in mouse models has shown inhibition of cell proliferation.17 Additionally, HK2 has been implicated in activating the NLRP3 inflammasome through glycolysis in multiple inflammatory diseases, including atherosclerosis, type 2 diabetes, obesity, and rheumatoid arthritis.19 Considering the upregulation of glycolysis metabolism in psoriasis and the relatively restricted expression of HK2 in normal tissues, targeting HK2 may hold potential as a therapeutic approach for psoriasis. However, the expression patterns and therapeutic effects of HK2 in psoriasis have not been investigated.
This study aimed to investigate the expression pattern of HK2 in psoriatic lesions. Additionally, we sought to determine the therapeutic effects and anti-inflammatory mechanisms of the HK2 inhibitor 3-bromopyruvate (3-BrPA) in an M5-stimulated keratinocyte and imiquimod (IMQ)-induced psoriatic inflammation mouse models.
Materials and Methods
Clinical Sample
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the ethics committee of the Affiliated Hospital of Weifang Medical University. Informed consent was obtained from all study participants. Psoriatic (n = 5) and healthy skin (n = 5) samples were collected for Western blotting immediately after biopsy. Immunohistochemistry was performed on paraffin-embedded tissue samples from 22 patients with psoriasis and 10 healthy controls. The diagnoses of all patients were confirmed histopathologically.
Cell Lines and Cell Culture
The human skin keratinocyte cell line HaCaT was obtained from the American Type Culture Collection. The cells were cultured in a humidified environment at 37°C with 5% CO2 and 95% air. High-glucose Dulbecco’s Modified Eagle Medium (12100046, Gibco, China) supplemented with 10% fetal bovine serum (10099141C, Gibco, Australia) was used as the cell culture medium.
Induction of Psoriatic Cell Model and Psoriatic Animal Model
HaCaT cells were treated with a cocktail of cytokines, referred to as M5, consisting of interleukin (IL)-17A, tumor necrosis factor-alpha (TNF-α), IL-1α, IL-22, and oncostatin-M at a final concentration of 10 ng/mL (Peprotech), to induce psoriatic inflammation.20
Two-month-old mice were shaved and depilated using a depilatory paste to construct the psoriatic mouse model (IMQ mice). The shaved dorsal skin of mice was treated with 5% IMQ cream (Aldara, 3M Pharmaceuticals) daily for 5 days. Photographs of the dorsal skin were recorded daily.
For treatment with 3-BrPA, a well-documented inhibitor of HK2, M5-stimulated HaCaT cells were treated with 3-BrPA (50μM) or sterile water for 24 h. IMQ-treated mice were randomized to receive either 3-BrPA (100μL of 1 mg/mL), 1% tacrolimus cream, or a vehicle control topically. 3-BrPA treatment was applied topically daily for 4 days, immediately after 5 days of IMQ treatment.
Immunohistochemistry
Paraffin-embedded tissue samples were cut into 5-μm sections. The sections were deparaffinized in xylene, and antigen retrieval was performed by heating at 121°C for 5 min in a buffer containing 10 mM Tris and 1.0 mM ethylenediaminetetraacetic acid. After cooling to room temperature, the sections were blocked with 3% bovine serum albumin (Sigma, A9418). Primary antibodies, HK2 (1:500, Thermo, RB-9052-P0) were incubated overnight at 4°C. Signal detection was performed using a two-step test kit (ZSbio, PV9000), followed by hematoxylin counterstaining (solarbio, G1150). The stained sections were examined under a microscope, and the immunohistochemistry scores (0 for negative, 1 for mildly positive, 2 for moderately positive, and 3 for strongly positive) were independently evaluated by two authors.
Western Blotting
Tissue and cell lysis were performed using Radioimmunoprecipitation Assay Lysis Buffer (89901, Thermo Science™, USA). The lysates were then loaded onto a 4–15% Tris-Glycine gel (#P0057A; BeyoGel™, Shanghai, China) and transferred to nitrocellulose membranes (#170-4159; Bio-Rad, USA). These membranes were incubated overnight at 4°C with primary antibodies against the following proteins: HK2 (1:2000, Thermo, RB-9052-P0), pNF-κB p65 (1:500, Santa Cruz, SC-70907), TNF-α (1:1000, Santa Cruz, SC-53253), NLRP3 (1:1000, Santa Cruz, SC-53253), caspase-1 (1:1000, CST, 4877), IL-1β (1:1000, Thermo, MA5-11803), and β-actin (1:1000, CST, 3661, 3662). After washing three times with tris-buffered saline/Tween, the membranes were incubated with Horseradish peroxidase-conjugated secondary antibody for 1 h, and detection was performed using an electrochemiluminescence system (NEL104001EA; PerkinElmer, USA). Protein content was quantified using a Bicinchoninic Acid Protein Assay Kit (23227; Thermo Pierce, USA).
RNA Extraction, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using a Qiagen RNeasy Plus Mini Kit (Qiagen GmbH, 74134). Subsequently, cDNA was generated using a PrimeScriptTM RT Master Mix kit (Takara, RR036A). Real-time quantitative polymerase chain reaction (qPCR) was performed using the Applied Biosystems 7500 Real-Time PCR System (Applied Biosciences, USA) and PowerUp SYBR Green mixture (Invitrogen, A25742).
Cell Proliferation Assay
For the cell proliferation assay, HaCaT cells were subjected to various treatments. A suspension medium containing 5000 cells (100μL) was seeded on 96 well plates with three replicate wells. Then, 20μL of CCK-8 assays (Beyotime, C0038) at a concentration of 5 mg/mL was added to each well every 24, 48, 72, and 96 h. The mixture was incubated for 2 h at 37°C. The absorbance of each well was measured at 450 nm using a microplate reader (iMark Reader, Bio-Rad). Cell cycle distribution was measured using flow cytometry (BD Biosciences) after staining with propidium iodide.
Metabolite Measurements and Enzyme-Linked Immunosorbent Assay Analysis
The glucose and lactate concentrations in the cell culture supernatant were measured using colorimeter test kits (BioVision, K686 and K627) following the manufacturer’s instructions. The absorbance was monitored at 450 nm using a microtiter plate reader (iMark Reader, Bio-Rad, USA).
The skin tissues of the mice and the supernatants of HaCaT cells were collected. According to the manufacturer’s instructions, the TNF-α, IL-17, IL-22, CXCL-1, CCL-20, and IL-1β levels were evaluated using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems).
Statistical Analysis
Statistical significance for categorical data was tested using the two-tailed Fisher’s exact test, while the Mann–Whitney or Kruskal–Wallis tests were performed for continuous data. Statistical significance was set at p<0.05. Graphical representations and statistical analyses were performed using GraphPad Prism 8.00.
Results
HK2 Expression is Upregulated in Psoriasis Lesions
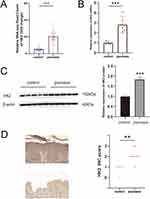
We analyzed an RNA-seq gene expression dataset (GSE121212) downloaded from the Gene Expression Omnibus website to examine the expression of HK2 in psoriatic and healthy skin samples. The fold change in HK2 RNA expression was upregulated in psoriasis (Figure 1A). Furthermore, we validated the upregulation of HK2 by qPCR and Western blot analyses (Figures 1B and C). Immunohistochemical staining confirmed the upregulation of HK2 in psoriasis, with predominant expression observed in the psoriatic epidermis (Figure 1D).
3-BrPA Suppresses M5-Stimulated HaCaT Cell Proliferation and Glycolysis
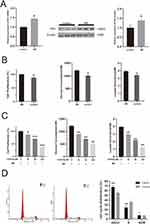
We utilized five different cytokines (IL-17A, TNF-α, IL-1α, IL-22, and Oncostatin-M) to induce psoriatic inflammation in HaCaT cells. The upregulation of HK2 in M5-stimulated cells was confirmed at mRNA and protein levels (Figure 2A). Cells treated with the cytokines exhibited increased proliferation and glycolysis compared to those in the control group (Figure 2B). Given the confirmed upregulation of HK2 in psoriatic lesions and cell models, we hypothesized that inhibiting HK2 could be a critical and clinically actionable therapy for psoriasis. To test this hypothesis, we investigated the effects of the pharmacological inhibition of HK2 in vitro. M5-stimulated HaCaT cells were treated with 3-BrPA, a well-known HK2 inhibitor. We observed significant suppression of cell proliferation in the 3-BrPA-treated group (Figure 2C). In addition, glucose consumption and lactate production were significantly downregulated (Figure 2C). Furthermore, flow cytometry confirmed that 3-BrPA induced G1 phase repression, limiting cell proliferation (Figure 2D).
3-BrPA Suppressed the Activation of the NF-kB Pathway and NLRP3 Inflammasome and Expression of Chemokines
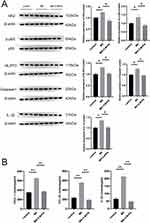
Given the importance of the NF-κB signaling pathway and NLRP3 inflammasome in psoriasis-related inflammatory responses, our study aimed to investigate the potential impact of 3-BrPA on these pathways. We conducted Western blot analysis to assess NLRP3 and NF-kB signaling molecule changes. As expected, the expression levels of NLRP3 and NF-kB were significantly elevated in the M5-stimulated HaCaT cells (Figure 3A); however, administration of 3-BrPA effectively reversed these effects (Figure 3A). Furthermore, we analyzed chemokines that may be abnormally increased and activated by the NF-κB signaling pathway and the NLRP3 inflammasome in HaCaT cells. Specifically, we examined the expression of CXCL-1, CCL-20, and IL-1β in keratinocytes and quantified the levels of these chemokines in the medium supernatant using ELISA. The results demonstrated a significant increase in CXCL-1, CCL-20, and IL-1β expressions in M5-induced HaCaT cells. However, after treatment with 3-BrPA, the elevated CXCL-1, CCL-20, and IL-1β expression was significantly reduced (Figure 3B).
Topical Application of 3-BrPA Attenuates IMQ-Induced Psoriasiform Inflammation in Mice
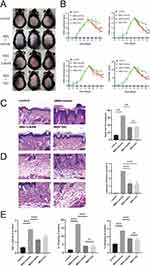
A mouse model of IMQ-induced psoriatic inflammation was established to investigate the therapeutic effects of 3-BrPA in vivo (Figure 4A). Five days after the IMQ application, the mice displayed typical symptoms of scaling, thickening, erythema, and total Psoriasis Area and Severity Index (PASI) score (Figure 4B). We applied 3-BrPA topically to the dorsal skin and measured the thickness of the epidermis. As expected, the groups treated with 3-BrPA showed significant improvements in scaling, thickening, and erythema compared to the model group (Figure 4B). We assessed the inhibitory effect of 3-BrPA on keratinocyte proliferation by examining epidermal thickness on hematoxylin and eosin staining slides (Figure 4C). The control group had an epidermal thickness of 13.07 ± 0.65 μm, while the model group had a thickness of 65.52 ± 3.65 μm. Treatment with 3-BrPA reduced the epidermal thickness to 34.38 ± 2.02 μm, which was equivalent to tacrolimus treatment (33.63 ± 2.38 μm) (Figure 4D). In this study, we investigated the effects of 3-BrPA on inflammation in IMQ-treated mice. Leukocyte infiltration is a crucial characteristic of inflammatory response in psoriasis. Our findings demonstrated that leukocyte infiltration was significantly reduced by 3-BrPA treatment (Figure 4D). Additionally, the concentration of critical proinflammatory cytokines, including TNF-α, IL-17, and IL-22 in the lesion, were downregulated after 3-BrPA treatment (Figure 4E).
Discussion
In this study, we identified elevated levels of the key glycolytic enzyme HK2 in psoriasis. We demonstrated that the HK2 inhibitor 3-BrPA decreased the proliferation and glycolytic activity of M5-stimulated HaCaT cells. Topical application of 3-BrPA alleviated psoriasis in IMQ-treated mice. Further studies revealed that 3-BrPA treatment attenuated the expressions of NF-kB and NLRP3 in psoriasis inflammation. Therefore, our results suggest that blocking HK2 is a potential new therapeutic strategy for psoriasis.
Keratinocyte hyperproliferation and inflammatory cell infiltration are well-known characteristics of psoriasis.21 Current treatment methods include topical therapy, phototherapy, systemic immune modulators, and biological agents to reduce symptoms and improve the quality of life. Psoriasis treatment with systemic immune modulators and biological agents focuses on adjusting the dysregulated immune microenvironment and cytokines.22 Therapies targeting IL-12/IL-23, IL-17, or its receptor (IL-17RA) have shown good therapeutic effects.23 However, the problem of disease relapse has not been solved, and some psoriasis lesions do not respond to these new therapies.24 Therefore, finding new target molecules is still valuable and necessary. It is well-known that keratinocytes are important contributors and main victims of psoriasis.25 Psoriatic keratinocytes exhibit significantly enhanced glucose uptake and glycolytic energy supply.26 Glucose signaling is important in redox metabolism and cytokine induction in keratinocytes.27 Glycolysis, a tightly regulated process, involves several enzymes, including HK2, which plays a crucial role.28 In this study, we found that the key glycolysis enzyme HK2 was elevated in psoriasis, and immunohistochemistry located HK2 mainly expressed in keratinocytes of psoriatic skin.
Previous studies have reported essential molecules involved in glucose metabolism, such as glucose transporter 1 and Pyruvate kinase M2. These molecules have also been found to be dysregulated in psoriatic keratinocytes.13,29 Targeting these key glucose metabolism molecules has been shown to alleviate psoriasis lesions in the IMQ-treated mice model significantly.12 These studies have revealed an increase in the glycolysis pathway in patients with psoriasis, suggesting that targeting the glucose metabolism pathway could be a potential alternative therapy. Consistent with these findings, our results suggested that HK2 plays a significant role in the development of psoriasis.
To verify the expression of HK2 in psoriasis models and assess the therapeutic effect of targeting HK2, we induced psoriatic inflammation-like conditions in HaCaT cells using a cytokine cocktail. This widely reported method of cytokine stimulation successfully generated an inflammatory keratinocyte model that mimicked certain characteristics of psoriatic skin in vitro.30–32 As anticipated, upregulation of HK2 was observed in M5-stimulated HaCaT cells, accompanied by increased cell proliferation and enhanced glycolysis. These findings were consistent with the characteristics of keratinocytes in patients with psoriasis.
Two commonly used inhibitors, 2-Deoxy-D-glucose (2-DG) and 3-BrPA, have also been discovered. 2-DG is a pan-HK inhibitor capable of inhibiting all isoforms of HK, whereas 3-BrPA is an HK2 selective inhibitor.33,34 Consequently, we selected 3-BrPA for subsequent experiments. Treatment with 3-BrPA significantly inhibited the proliferation and glycolysis of M5-stimulated HaCaT cells and reduced HK2 protein levels. The topical administration of 3-BrPA exhibited remarkable therapeutic effects in an IMQ mouse model, reducing the PASI score and attenuating epidermal thickening. Activated keratinocytes with hyperproliferative ability are considered to play a crucial role.35 As an essential marker of keratinocyte proliferation, epidermal thickness notably decreased after 3-BrPA treatment. Thus, 3-BrPA demonstrated significant therapeutic effects in psoriasis-like cells and mouse models.
Furthermore, our study demonstrated that 3-BrPA treatment significantly reduced inflammation in a psoriasis model. Psoriasis is a well-known inflammatory skin disease where NFκB signaling and NLRP3 activation are important modulators.36 NF-kB plays a crucial role in the pathogenesis of psoriasis, potentially triggering inflammation, promoting epidermal hyperproliferation, and exacerbating psoriatic presentation.37 Similarly, the NLRP3 inflammasome is another vital intracellular multiprotein inflammatory pathway that controls the production of proinflammatory cytokines.38 In psoriasis, NLRP3 was specifically found to be activated and expressed in the activated epithelial cells of the psoriatic epidermis.39,40 Given that the critical glycolysis enzyme HK2 has been reported to be involved in the inflammation process in multiple inflammatory diseases,19 and considering that glucose signaling is vital for inflammation processing in keratinocytes,27 we aimed to investigate whether 3-BrPA can attenuate the activation of NF-kB and NLRP3 in psoriatic keratinocytes. In the current study, we observed significant activation of NLRP3 and p-NF-kB in M5-stimulated HaCaT cells and lesions in IMQ-treated mice. Importantly, treatment with 3-BrPA effectively reversed these changes, providing evidence that NF-kB and NLRP3 activation plays a role in the therapeutic action of 3-BrPA in psoriasis. One possible explanation for the cytokine pathway and HK2 interaction may lie in an imbalance in energy sensing leading to inflammasome activation.41 Knockdown of HK in previous study inhibits activation of NLRP3 by extracellular ATP, thereby linking energy sensing to active glycolysis, a prerequisite for NLRP3 inflammasome activation.42 Another possible explanation is that HK is associated with mitochondria through Akt activity. Akt activity decreases during ATP depletion, leading to ATP depletion-mediated activation of NLRP3.43 These mechanisms need to be confirmed experimentally in psoriatic keratinocytes.
Conclusion
Our study demonstrated that the glycolytic enzyme HK2 was upregulated in psoriasis. Furthermore, we found that inhibiting HK2 suppresses M5-stimulated HaCat cell proliferation and improved animal models of IMQ-induced psoriasis-like dermatitis. These findings contribute to our understanding of abnormal glucose metabolism in psoriasis and have potential implications for developing precise treatment options.
Data Sharing Statement
Data supporting this study’s findings are available by correspondence author Weiyuan Ma upon reasonable request.
Funding
Research reported in this publication was supported by Shandong Province Natural Science Foundation (No. ZR2020QH138), the Doctoral Startup Fund of Affiliated Hospital of Weifang Medical University (No. 2021BKQ02), and the Traditional Chinese Medicine Science and Technology Project of Shandong Province (No. 2021Q093).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180. doi:10.3389/fmed.2021.743180
2. Buja A, Miatton A, Cozzolino C, et al. The prevalent comorbidome at the onset of psoriasis diagnosis. Dermatol Ther. 2023;13(9):2093–2105. doi:10.1007/s13555-023-00986-0
3. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi:10.1056/NEJMra0804595
4. Larsen MH, Strumse YS, Andersen MH, et al. Associations between disease education, self-management support, and health literacy in psoriasis. J Dermatolog Treat. 2021;32(6):603–609. doi:10.1080/09546634.2019.1688233
5. Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–272. doi:10.1111/1346-8138.14139
6. Yang BY, Cheng YG, Liu Y, et al. Datura metel L. Ameliorates imiquimod-induced psoriasis-like dermatitis and inhibits inflammatory cytokines production through TLR7/8-MyD88-NF-kappaB-NLRP3 inflammasome pathway. Molecules. 2019;24. doi:10.3390/molecules24112157
7. Pasquali L, Srivastava A, Meisgen F, et al. The keratinocyte transcriptome in psoriasis: pathways related to immune responses, cell cycle and keratinization. Acta Derm Venereol. 2019;99(2):196–205. doi:10.2340/00015555-3066
8. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809
9. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27(1):441–464. doi:10.1146/annurev-cellbio-092910-154237
10. Kang H, Li X, Zhou Q, et al. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br J Dermatol. 2017;176(3):713–722. doi:10.1111/bjd.15008
11. Hamanaka RB, Mutlu GM. PFKFB3, a direct target of p63, is required for proliferation and inhibits differentiation in epidermal keratinocytes. J Invest Dermatol. 2017;137(6):1267–1276. doi:10.1016/j.jid.2016.12.020
12. Zhang Z, Zi Z, Lee EE, et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat Med. 2018;24(5):617–627. doi:10.1038/s41591-018-0003-0
13. Liu YZ, Xu MY, Dai XY, et al. Pyruvate kinase M2 mediates glycolysis contributes to psoriasis by promoting keratinocyte proliferation. Front Pharmacol. 2021;12:765790. doi:10.3389/fphar.2021.765790
14. Hao L, Mao Y, Park J, et al. 2’-Hydroxycinnamaldehyde ameliorates imiquimod-induced psoriasiform inflammation by targeting PKM2-STAT3 signaling in mice. Exp Mol Med. 2021;53(5):875–884. doi:10.1038/s12276-021-00620-z
15. Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(12):2049–2057. doi:10.1242/jeb.00241
16. Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25(34):4777–4786. doi:10.1038/sj.onc.1209603
17. Ciscato F, Ferrone L, Masgras I, et al. Hexokinase 2 in cancer: a prima donna playing multiple characters. Int J Mol Sci. 2021;22(9):4716. doi:10.3390/ijms22094716
18. Yang L, Yan X, Chen J, et al. Hexokinase 2 discerns a novel circulating tumor cell population associated with poor prognosis in lung cancer patients. Proc Natl Acad Sci U S A. 2021;118. doi:10.1073/pnas.2012228118
19. Hughes MM, O’Neill LAJ. Metabolic regulation of NLRP 3. Immunol Rev. 2018;281(1):88–98. doi:10.1111/imr.12608
20. Rabeony H, Petit-Paris I, Garnier J, et al. Inhibition of keratinocyte differentiation by the synergistic effect of IL-17A, IL-22, IL-1alpha, TNFalpha and oncostatin M. PLoS One. 2014;9(7):e101937. doi:10.1371/journal.pone.0101937
21. Zhou X, Chen Y, Cui L, et al. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81. doi:10.1038/s41419-022-04523-3
22. Mohd Noor AA, Azlan M, Mohd Redzwan N. Orchestrated cytokines mediated by biologics in psoriasis and its mechanisms of action. Biomedicines. 2022;10(2):498. doi:10.3390/biomedicines10020498
23. Kutwin M, Migdalska-Sek M, Brzezianska-Lasota E, et al. An analysis of IL-10, IL-17A, IL-17RA, IL-23A and IL-23R expression and their correlation with clinical course in patients with psoriasis. J Clin Med. 2021;10(24):5834. doi:10.3390/jcm10245834
24. Tian D, Lai Y. The relapse of psoriasis: mechanisms and mysteries. JID Innov. 2022;2(3):100116. doi:10.1016/j.xjidi.2022.100116
25. Gao J, Chen F, Fang H, et al. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol Res. 2020;53(1):48. doi:10.1186/s40659-020-00316-0
26. Cibrian D, de la Fuente H, Sanchez-Madrid F. Metabolic pathways that control skin homeostasis and inflammation. Trends Mol Med. 2020;26(11):975–986. doi:10.1016/j.molmed.2020.04.004
27. Wickersham M, Wachtel S, Wong Fok Lung T, et al. Metabolic stress drives keratinocyte defenses against Staphylococcus aureus infection. Cell Rep. 2017;18(11):2742–2751. doi:10.1016/j.celrep.2017.02.055
28. Garcia SN, Guedes RC, Marques MM. Unlocking the potential of HK2 in cancer metabolism and therapeutics. Curr Med Chem. 2019;26(41):7285–7322. doi:10.2174/0929867326666181213092652
29. Hodeib AA, Neinaa YME, Zakaria SS, et al. Glucose transporter-1 (GLUT-1) expression in psoriasis: correlation with disease severity. Int J Dermatol. 2018;57(8):943–951. doi:10.1111/ijd.14037
30. Guilloteau K, Paris I, Pedretti N, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1alpha, and TNF-alpha recapitulates some features of psoriasis. J Immunol. 2010;184(9):5263–5270. doi:10.4049/jimmunol.0902464
31. Duan Q, Wang G, Wang M, et al. LncRNA RP6-65G23.1 accelerates proliferation and inhibits apoptosis via p-ERK1/2/p-AKT signaling pathway on keratinocytes. J Cell Biochem. 2020;121(11):4580–4589. doi:10.1002/jcb.29685
32. Chen C, Yang Z, Yin X, et al. CircEIF5 contributes to hyperproliferation and inflammation of keratinocytes in psoriasis via p-NFkappaB and p-STAT3 signalling pathway. Exp Dermatol. 2022;31(8):1145–1153. doi:10.1111/exd.14565
33. Pajak B, Siwiak E, Soltyka M, et al. 2-Deoxy-d-glucose and its analogs: from diagnostic to therapeutic agents. Int J Mol Sci. 2019;21(1):234. doi:10.3390/ijms21010234
34. Zhang Q, Zhang Y, Zhang P, et al. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer. 2014;5(3–4):100–112. doi:10.18632/genesandcancer.9
35. Ortiz-Lopez LI, Choudhary V, Bollag WB. Updated perspectives on keratinocytes and psoriasis: keratinocytes are more than innocent bystanders. Psoriasis. 2022;12:73–87. doi:10.2147/PTT.S327310
36. Ferrari D, Casciano F, Secchiero P, et al. Purinergic signaling and inflammasome activation in psoriasis pathogenesis. Int J Mol Sci. 2021;22(17):9449. doi:10.3390/ijms22179449
37. Moorchung N, Kulaar JS, Chatterjee M, et al. Role of NF -κB in the pathogenesis of psoriasis elucidated by its staining in skin biopsy specimens. Int J Dermatol. 2014;53(5):570–574. doi:10.1111/ijd.12050
38. Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. doi:10.3390/ijms20133328
39. Ciazynska M, Olejniczak-Staruch I, Sobolewska-Sztychny D, et al. The role of NLRP1, NLRP3, and AIM2 inflammasomes in psoriasis: review. Int J Mol Sci. 2021;22(11):5898. doi:10.3390/ijms22115898
40. Tsuji G, Hashimoto-Hachiya A, Yen VH, et al. Metformin inhibits IL-1beta secretion via impairment of NLRP3 inflammasome in keratinocytes: implications for preventing the development of psoriasis. Cell Death Discov. 2020;6(1):11. doi:10.1038/s41420-020-0245-8
41. Ma Jewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16(5):819–830. doi:10.1016/j.molcel.2004.11.014
42. Moon JS, Hisata S, Park MA, et al. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep. 2015;12(1):102–115. doi:10.1016/j.celrep.2015.05.046
43. Hahn-Windgassen A, Nogueira V, Chen CC, et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280(37):32081–32089. doi:10.1074/jbc.M502876200
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.