Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Inhibition Effect of Physalis angulata Leaf Extract on Viability, Collagen Type I, and Tissue Inhibitor of Metalloproteinase 1 (TIMP-1) but Not Plasminogen Activator Inhibitor-1 (PAI-1) of Keloid Fibroblast Culture
Authors Widiatmoko A , Fitri LE , Endharti AT , Murlistyarini S , Brahmanti H , Yuniaswan AP , Ekasari DP , Rasyidi F, Nahlia NL, Safitri PR
Received 8 June 2023
Accepted for publication 11 August 2023
Published 30 August 2023 Volume 2023:16 Pages 2365—2373
DOI https://doi.org/10.2147/CCID.S425036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Arif Widiatmoko,1,2 Loeki Enggar Fitri,1,3 Agustina Tri Endharti,1,3 Sinta Murlistyarini,1,2 Herwinda Brahmanti,2 Anggun Putri Yuniaswan,2 Dhany Prafita Ekasari,2 Faradiani Rasyidi,2 Nurul Laili Nahlia,2 Putri Rachma Safitri2
1Doctoral Program in Medical Science, Faculty of Medicine Universitas Brawijaya, Malang, East Java, Indonesia; 2Department of Dermatology and Venereology, Faculty of Medicine Universitas Brawijaya, Dr. Saiful Anwar General Hospital, Malang, East Java, Indonesia; 3Department of Parasitology, Faculty of Medicine Universitas Brawijaya, Malang, East Java, Indonesia
Correspondence: Arif Widiatmoko, Department of Dermatology and Venereology, Faculty of Medicine Universitas Brawijaya, Dr. Saiful Anwar General Hospital, Jl. Jaksa Agung Suprapto 2, Malang, East Java, 65111, Indonesia, Tel +62 341340991, Fax +62 341340991, Email [email protected]
Introduction: Keloids are excessive fibroproliferative diseases that are caused by abnormal wound healing. The anti-proliferative activity of Physalis angulata compounds has potential as a keloid therapeutic agent. This study aimed to observe the effects of P. angulata on fibroblast viability and collagen type I, tissue inhibitor of metalloproteinase 1 (TIMP-1), and plasminogen activator inhibitor 1 (PAI-1) levels in human keloid fibroblasts.
Methods: We conducted an experimental study of P. angulata ethanol extract on three primary human keloid fibroblast 3 passage cultures with four replications. Fibroblast viability was measured using the MTT assay after incubation with 3, 5, and 10 μg/mL P. angulata. Concentrations of P. angulata used to observe effects on TIMP-1, PAI-1, and collagen type I levels were 10%, 20%, 30%, and 40% of inhibitory concentration 50 (IC50). The levels of collagen type I, TIMP-1, and PAI-1 were measured by ELISA. Mean comparisons between multiple treatment groups were analyzed using one-way ANOVA followed by post-hoc analysis.
Results: The 10 μg/mL P. angulata group had significantly lower fibroblast viability than the control group (p< 0.05) with an IC50 6.3 μg/mL. The collagen type I level of 10% IC50 (0.63 μg/mL) P. angulata group was lower than control (12.910 vs 47.866 ng/mL) (p=0.042). Level of TIMP-1 in 40% IC50 (2.51 μg/mL) P. angulata group was lower than control (5.350 vs 9.972 ng/mL) (p=0.043). There was no significant difference in the PAI-1 levels.
Conclusion: This study showed the inhibitory effect of 10 μg/mL P. angulata extract on keloid fibroblast viability, with an IC50 of 6.3 μg/mL. This study also showed collagen type-1 and TIMP-1 inhibition, but not PAI-1 inhibition, after P. angulate treatment.
Keywords: Physalis angulata, keloid, fibroblast viability, collagen type I, TIMP-1, PAI-1
Introduction
Keloids are benign fibroproliferative tumors that arise because of an abnormal wound healing process. The incidence of keloids varies depending on race. A retrospective study on the overall incidence of keloids after head or neck surgery showed an incidence rate of 0.1% and 0.8% for White American and African Americans, respectively.1 Keloid impairs the quality of life by causing pain, itchiness, cosmetic impairment, and functional deformity. There is no single effective keloid treatment because of the high recurrence rate.2
Extracellular matrix degradation and deposition imbalance can cause abnormalities in the wound-healing process. Extracellular matrix deposition in keloids is more prominent than ECM degradation. Enhancement of fibroblast activity and collagen synthesis is involved in keloid pathogenesis. Keloid fibrous tissue can spread to the surrounding normal skin. The ability of keloids to spread into adjacent normal skin is different from that of hypertrophic scars.3
Keloid fibroblast has faster proliferation rate and higher collagen type I production compared to normal fibroblast.4 Increase of human keloid fibroblast proliferation and differentiation induced by transforming growth factor β (TGF-β).5 Collagen type I excessive accumulation is found in keloid tissue, which is induced by TGF-β. Percentage of collagen type I in fibroblast keloid was 61% compared to 37% in preputium fibroblast.6 Keloid fibroblast plasminogen activator inhibitor 1 (PAI-1) expression was higher; Urokinase type plasminogen activator (uPA) was lower than normal fibroblasts.4 The level of tissue inhibitor of metalloproteinase 1 (TIMP-1) in keloid tissue was higher than normal skin. Extracellular matrix (ECM) degradation by matrix metalloproteinase 1 (MMP-1) was inhibited by TIMP-1.7
Most single keloid treatments have a high recurrence rate. The combination of surgical procedures and medical therapy resulted in a lower recurrence rate. Medical monotherapies have a high failure rate. Triamcinolone acetonide intralesional injection monotherapy is effective in reducing keloid volume; however, unwanted side effects, such as telangiectasia, acne lesions, and ulceration, can develop.8
Studies on naturally derived compounds in keloid pathogenesis have recently been conducted. The active compounds of natural plants may contain active ingredients with anti-keloid activity.9 Physalis angulata has been used as a medicinal herb for the treatment of skin diseases. Antiproliferative compound has been isolated from P. angulata leaves such as quercetin and kaempferol.10,11 An in vitro study of quercetin on earlobe keloid fibroblast showed inhibition of cellular proliferation and collagen synthesis.12 Kaempferol attenuated collagen synthesis, proliferation, and activation of fibroblasts in vitro and in vivo studies. Western blot analysis revealed that kaempferol significantly downregulated SMAD2 and SMAD3 phosphorylation which was induced by the selective binding of kaempferol to TGF-β receptor type I.13 P. angulata extracts significantly augmented the expression of TIMP-1, TIMP-2, PAI-1, and PAI-2 in human oral squamous carcinoma (HSC-3) cell-line cultures.14
The objective of this study was to observe P. angulata on fibroblast viability and collagen type I, TIMP-1, and PAI-1 levels in human keloid fibroblasts.
Materials and Methods
Materials
The culture medium consisted of Dulbecco’s Modified Eagle’s medium (DMEM) with GlutaMAXTM-I Gibco (10567–014) and an antibiotic-antimycotic solution Gibco (15240–062). P. angulata simplicia was obtained from the Center for Medicinal Plants (Materia Medika, Batu, Indonesia). TIMP-1, PAI-1, and collagen type I levels were measured using an Elabscience ELISA kit (E-EL-H0184), RayBio Human PAI-1 ELISA Kit (ELH-PAII), and MyBiosource Human Collagen Type I ELISA kit (MBS2506379), respectively. Fibroblast viability was measured using the MTT assay (Nacalai Tesque (23547) MTT assay).
Cell Culture
The primary keloid dermal fibroblast culture skin explant method was used in this study. Dermal fibroblasts were obtained from a female patient who had undergone keloid excision. This study was conducted with the signing of an informed consent form and in compliance with the Declaration of Helsinki. This study was approved by the Health Study Ethics Committee of the Dr Saiful Anwar General Hospital, Malang. Keloid tissue was directly inserted into a 50 mL centrifugal tube containing sterile phosphate-buffered saline (PBS). The tissue was washed by shaking the tube slowly for 10 min and repeated three times. Cell culture procedures were performed within 6 h after the excision procedure. The edge of the keloid tissue was cut, placed on a petri dish, and dissected to separate the dermis and epidermis. Dermal tissue was washed in a tube containing sterile PBS and used as a keloid fibroblast culture cell source. Dermal tissue was cut into 5–10 thin slices, each of 2-3 mm2, and placed in a tissue culture dish covered with a glass coverslip of 22 mm. Several drops of culture medium at 4°C were slowly added under the edge of the coverslip and slowly 1–2 mL more were added. The tissue culture dish was put in 37°C a 5% CO2 incubator. Fibroblast growth was observed every 3–4 days with a change in medium. Subculture process was performed when fibroblast growth reached 80–90% of tissue culture dish.
Fibroblast Viability
Fibroblast viability was measured in cell culture after 24 h incubation with 3, 5, and 10 µg/mL P. angulata and media culture in the control group. MTT assay was performed by added 15 µL solution of MTT from Nacalai Tesque (23547) in 0.5 mg/mL PBS incubated in CO2 5% incubator for 2 h. Next, 100 µL dimethyl sulfoxide (the) was added as a stopper solution. The colour absorbance of purple formazan was measured using a 570 nm spectrophotometer.
Collagen Type I, TIMP-1, and PAI-1 Measurement
Extracellular TIMP-1, PAI-1, and collagen type I levels were measured in the supernatant of the cell culture by ELISA. ELISA was performed using the Elabscience ELISA kit (E-EL-H0184) for TIMP-1, RayBio Human PAI-1 ELISA Kit (ELH-PAII) for PAI-1, and MyBiosource Human Collagen Type I ELISA kit (MBS2506379) for collagen type I. The color absorbance was measured by ELISA reader wavelength 450 nm using an ELISA reader.
Physalis angulata Extraction
We used the Soxhletation method to obtain P. angulata extract. The dry powder form of P. angulata simplicia, weighing 200 mg, was wrapped in filter paper and inserted in an extraction funnel for immersion in ethanol for 24 h. The obtained solution was collected and precipitated in 250 mL Soxhlet. The solution was separated from the precipitated product and dried using a rotator evaporator at 70–80°C to obtain the extract. The extract was heated in an oven at 70°C to remove ethanol residue. The extract was dissolved in the culture medium at multiple concentrations.
Physalis angulata Extract Incubation in Cell Culture
Third-passage keloid fibroblast subcultures were used as the study samples. Keloid fibroblast cells were divided into a well culture plate as a control group and P. angulata group. Keloid fibroblast cultures in the control group were supplemented with culture medium only, whereas P. angulata group was added to the culture medium containing P. angulata. The P. angulata concentrations used to observe the effects on fibroblast viability were 3, 5, and 10 µg/mL. The P. angulata concentration used to observe effects on collagen type I, TIMP-1, and PAI-1 levels were 10%, 20%, 30%, and 40% of inhibitory concentration 50 (IC50). We performed four replications for each group. All the groups were incubated for 24 h.
Statistical Analysis
Mean comparisons between multiple treatment groups were analyzed with one-way ANOVA followed by post hoc analysis if the distribution of data was normal or with the Mann–Whitney U-test if the distribution of data was not normal. The data distribution was analyzed using the Shapiro–Wilk test. All statistical analyses were performed using IBM SPSS Statistics for Windows version 27.
Results
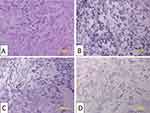
Keloid tissue was excised from the abdomen of 22 years old woman by excision procedure. Keloid dermal fibroblasts were grown on culture medium and subjected to a subculture process for three passages. The morphology of keloid dermal fibroblast on three passages sub-culture was spindle, adherent, and growing from the edge (Figure 1).
 |
Figure 1 Keloid fibroblast culture. (A) One day after culture procedure. (B) First passage. (C) Second passage. (D) Third passage. Bar: 200 μm. |
Physalis angulata Extract Inhibition Effect on Keloid Fibroblast Viability
Fibroblast viability was measured using the MTT assay after incubation with P. angulata for 24 h. The 10 µg/mL P. angulata group showed the lowest absorbance of formazan purple (Figure 2). Fibroblast viability data were normally distributed and homogenous. ANOVA showed a significant inhibitory effect of P. angulata extract on fibroblast viability (p<0.05). Tukey’s post hoc analysis showed a significant inhibition of fibroblast viability in the 10 µg/mL P. angulata group (p<0.05) (Figure 3). The inhibition concentration 50 (IC50) was determined to be 6.3 µg/mL. The P. angulata concentrations used to observe effects on collagen type I, TIMP-1, and PAI-1 levels were 10%, 20%, 30%, and 40% of IC50 which were equal to 0.63, 1.25, 1.88, and 2.51 µg/mL.
Physalis angulata Extract Inhibition Effect on Collagen Type I
Collagen type I levels were measured by ELISA in the supernatant of third passage culture of keloid fibroblasts. Collagen type I data are normally distributed and homogeneous. ANOVA showed a significant inhibitory effect of P. angulata extract on collagen type I levels (p<0.05). Tukey’s post hoc analysis showed significant collagen type I inhibition in 0.63 µg/mL (10% IC50) P. angulata group (p=0.004) (Figure 4).
Physalis angulata Extract Inhibition Effect on TIMP-1
TIMP-1 levels were measured by ELISA on the supernatant of keloid fibroblast third passage culture. The Shapiro–Wilk test showed that TIMP-1 data were not normally distributed. The Kruskal–Wallis test showed a significant inhibitory effect of P. angulata extract on TIMP-1 levels (p=0.015). Mann–Whitney analysis showed significant TIMP-1 inhibition in 2.51 µg/mL (40% IC50) P. angulata group (p=0.043) (Figure 5).
Physalis angulata Extract Effect on PAI-1
The level of PAI-1 in the supernatant of the third passage culture of keloid fibroblasts was measured using ELISA. The Shapiro–Wilk test showed that the PAI-1 level data were not normally distributed. The mean difference between the groups, as analyzed by the Kruskal–Wallis test did not show any significant difference (Figure 6).
Discussion
The objective of this study was to determine the effect of P. angulata leaf ethanol extract on keloid dermal fibroblast primary cultures. Fibroblast viability was observed after incubation with cytotoxic concentrations of P. angulata extract. The levels of collagen type I, TIMP-1, and PAI-1 were observed after incubation with P. angulata extract at concentrations below the IC50, indicating that keloid fibroblasts were still viable. The etiology of keloids remains unclear and requires further investigation. The major limitation of keloid therapeutic development is the lack of animal models for investigating keloid pathology. Keloid primary dermal fibroblast culture as in vitro model is essential not only for a better understanding of disease biology but also for identifying and evaluating novel drug targets.15 In vitro study on keloid fibroblast could observe the effect of developing drug on fibroblast viability by MTT assay.12 In vitro study on keloid fibroblast could also observe secreted protein in extracellulars, such as collagen type I and TIMP-1, which are measured from cell culture medium.16,17
Keloid fibroblasts have a higher proliferation rate than that of normal fibroblasts. Keloid fibroblasts also had faster collagen type I synthesis than normal fibroblasts.4 Keloid fibroblasts are primary effector cells in keloid formation by inducing a persistent inflammatory response and excessive ECM deposition.18,19 Keloid fibroblasts have stronger anti-apoptosis, collagen synthesis, and migration abilities which some studies have shown is caused by many factors such as overexpression of TGF-β, microRNA-21, and hypoxia-inducible factor-1α (HIF-1α).5,20 Changes in keloid fibroblast phenotype are developed as target therapy.21 One study obtained an inhibition effect on keloid fibroblast proliferation, such as the study of ginsenoside Rg3 on inhibition of keloid fibroblast proliferation and migration.22 Another study showed vitamin D and quercetin inhibition on keloid fibroblast viability and collagen synthesis.12
This study demonstrated the effect of P. angulata leaf ethanol extract on keloid dermal fibroblast viability. A significant inhibition of fibroblast viability was observed in the 10 µg/mL P. angulata group. The P. angulata inhibition concentration 50 (IC50) on keloid fibroblast viability was 6.3 µg/mL. Studies on P. angulata extract in keloids are lacking. A study on the effect of P. angulata on cancer cell viability showed an inhibitory effect. The extract of P. angulata inhibited the viability of human oral squamous cell carcinoma cell line (HSC-3). Inhibition of viability was observed at 10 µg/mL concentration.14 The active compounds of P. angulata are known to have antiproliferative and anticancer effects. P. angulata leaf contains phenolic compounds such as quercetin and kaempferol, which have antiproliferative effect.11 Quercetin had inhibition the proliferation and viability of primary keloid fibroblast culture in vitro.12 Kaempferol also has an inhibitory effect on keloid fibroblast proliferation in vitro from primary cell culture.13 Several studies have been conducted on the effect of P. angulata on cancer cells. The ethanol extract of P. angulata leaves has pro-apoptotic and antiproliferative effects on retinoblastoma cell culture.23 Another study suggested that P. angulata leaf ethanol extract has antiproliferative and inhibitory activities on human blood and ovarian cancer cell lines.24
This study also observed the effect of P. angulata at concentrations below the IC50 to determine the effects on collagen type I, TIMP-1, and PAI-1 when keloid fibroblasts were still viable. This study showed inhibition effect of P. angulata extract on collagen type I level although significant collagen type I inhibition only at 0.63 µg/mL (10% IC50) P. angulata group. The collagen inhibition effect observed in this study could be facilitated by mechanisms other than inhibition of fibroblast viability. A study on physalin D isolated from Physalis species showed the inhibition of TGF-β1 induced expression of collagen type I in mouse liver fibrosis. This study also showed the inhibition of phosphorylated SMAD2/3 which had an important role in TGF-β/Smad signalling pathway.25 A study on withagulatin A isolated from P. angulata L showed inhibition of TGF-β stimulated type I procollagen gene expression in primary hepatic stellate cells isolated from normal livers of male Sprague Dawley rats, which was attributable to the suppression of TGF-β stimulated SMAD2 and SMAD3 phosphorylation.26 The expression of keloid procollagen 1 mRNA increased 35 times compared to preputium fibroblast.6 Excessive collagen formation in keloid is the target therapy for keloid. The sustained TGF-β/Smad signalling pathway can inhibit excessive collagen formation.27
This study demonstrated the inhibitory effect of P. angulata extract on TIMP-1 levels, although significant inhibition only at 2.51 µg/mL (40% IC50) P. angulata group. The study showed that the expression of TMP-1 in keloid tissue was higher than that in regular scar tissue.7 Some studies have shown a TIMP-1 inhibition effect on keloid fibroblasts as a target therapy. The study of small interfering RNAs targeting TIMP-1, which transduces human keloid-derived fibroblasts (KFs), leads to the degradation of collagen type 1.28 Study papain, a protease cysteine enzyme in the collagen degradation process, on Rattus norvegicus as an experimental animal showed a reduction in TIMP-1 levels. This study also showed a direct relationship between papain dose and TIMP-1.29 A study of P. angulata on TIMP-1 in keloid fibroblasts is still lacking. A study of P. angulata extract on human oral squamous cell carcinoma cell line (HSC-3) TIMP-1 level showed contrary results. This study showed that P. angulata extract upregulated TIMP-1 levels. The discrepancy in this study was caused by the different P. angulata concentration used. This study used sub-cytotoxic concentration (5–15 µg/mL), whereas our study used lower concentration below IC50 (below 6.3 µg/mL).14 Another study of P. angulata extract on gingival tissue of periodontitis-induced mice showed no significant effect on TIMP-1 level.30
This study showed no significant difference in PAI-1 levels between P. angulata and control groups. Study showed that the expression of PAI-1 on keloid fibroblast is higher than on normal fibroblast.4 Epidemiology study on 241 keloid patients compared to 207 normal healthy individuals without keloid diagnosis showed significantly higher plasma PAI-1 level on the keloid than the control group.31 Another study showed that high levels of PAI-1 expression were significantly associated with an increased risk for keloid.32 A study showed that silencing of PAI-1 decreased protein level of collagen type I and collagen type III also inhibited growth and induced keloid shrinkage.33 The study of P. angulata effect on PAI-1 of keloid fibroblast is still lacking. In contrast, the effect of P. angulata extract on PAI-1 levels was observed in a study on a human oral squamous cell carcinoma cell line (HSC-3). This study showed that subcytotoxic concentration (5–15 µg/mL) of P. angulata extract increased PAI-1 expression.14
Conclusion
This study showed the inhibitory effect of 10 µg/mL P. angulata extract on keloid fibroblast viability, with an IC50 of 6.3 µg/mL. This study also showed P. angulata had a concentration below the IC50 inhibitory effect on collagen type-1 and TIMP-1 in keloid fibroblasts. There were no significant effects on keloid fibroblast PAI-1. Future studies will be conducted to observe the effect of P. angulata extract on intracellular signalling of keloid fibroblasts to determine the mechanism of collagen type 1 and TIMP-1 inhibition by noncytotoxic concentrations of P. angulata.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Young WG, Worsham MJ, Joseph CLM, Divine GW, Jones LRD. Incidence of keloid and risk factors following head and neck surgery. JAMA Facial Plast Surg. 2014;16(5):379–380.
2. Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12(2):28–31.
3. Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: the paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix Biol. 2016;51:37–46.
4. Ashcroft KJ, Syed F, Bayat A. Site-specific keloid fibroblasts alter the behaviour of normal skin and normal scar fibroblasts through paracrine signalling. PLoS One. 2013;8(11):e75600.
5. Liu Y, Li Y, Li N, et al. TGF-β1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci Reports. 2016;6:32231.
6. Wulandari E, Jusman SRIWA, Moenadjat Y, Jusuf AA, Sadikin M. Expressions of collagen I and III in hypoxic keloid tissue. Kobe J Med Sci. 2016;62(3):E58–69.
7. Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg. 2010;63:1015–1021.
8. Ogawa R, Akita S, Akaishi S, et al. Diagnosis and Treatment of Keloids and Hypertrophic Scars—Japan Scar Workshop Consensus Document 2018. Burns Trauma. 2019;7:1–40.
9. Unahabhokha T, Sucontphunt A, Nimmannit U, Chanvorachote P, Yongsanguanchai N, Pongrakhananon V. Molecular signalings in keloid disease and current therapeutic approaches from natural based compounds. Pharm Biol. 2015;53(3):457–463.
10. Ahmadu AA, Omonigho U. Flavonoids from the leaves of Physalis angulata Linn. African J Pharm Res Dev. 2013;5(1):40–43.
11. Cobaleda-Velasco M, Alanis-Bañuelos RE, Almaraz-Abarca N, et al. Phenolic profiles and antioxidant properties of Physalis angulata L. as quality indicators. J Pharm Pharmacogn Res. 2017;5(2):114–128.
12. Ramakrishnan KM, Babu M, Madhavi MSL. Response of keloid fibroblasts to vitamin D3 and quercetin treatment - In vitro study. Ann Burns Fire Disasters. 2015;28(3):187–191.
13. Li H, Yang L, Zhang Y, Gao Z. Kaempferol inhibits fibroblast collagen synthesis, proliferation and activation in hypertrophic scar via targeting TGF-b receptor type I. Biomed Pharmacother. 2016;83:967–974.
14. Hseu YC, Wu CR, Chang HW, et al. Inhibitory effects of Physalis angulata on tumor metastasis and angiogenesis. J Ethnopharmacol. 2011;135(3):762–771.
15. Sharma JR, Lebeko M, Kidzeru EB, Khumalo NP, Bayat A. In vitro and ex vivo models for functional testing of therapeutic anti-scarring drug targets in keloids. Adv Wound Care. 2019;8(12):655–670.
16. Kurniawati Y, Suwarsa O, Agung A, Adi S. The Comparison of the Levels of Leukotriene B4, TGF-β1, Collagen in Keloid Fibroblasts and Normal Skin Fibroblast. Open Access Libr. 2016;3:e2801.
17. Dohi T, Miyake K, Aoki M, et al. Tissue inhibitor of metalloproteinase-2 suppresses collagen synthesis in cultured keloid fibroblasts. Plast Reconstr Surg - Glob Open. 2015;3(9):1–11.
18. Luo L, Li J, Liu H, et al. Adiponectin is involved in connective tissue growth factor-induced proliferation, migration and overproduction of the extracellular matrix in keloid fibroblasts. Int J Mol Sci. 2017;18(5):1–21.
19. Suarez E, Syed F, Alonso-Rasgado T, Bayat A. Identification of biomarkers involved in differential profiling of hypertrophic and keloid scars versus normal skin. Arch Dermatol Res. 2015;307(2):115–133.
20. Zhang Z, Nie F, Kang C, et al. Increased periostin expression affects the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxic conditions. Int J Mol Med. 2014;34(1):253–261.
21. Feng F, Liu M, Pan L, et al. Biomechanical regulatory factors and therapeutic targets in keloid fibrosis. Front Pharmacol. 2022;13(May):1–12.
22. Tang M, Bian W, Cheng L, et al. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGF-β/Smad and ERK signaling pathways. Int J Mol Med. 2018;41:1487–1499.
23. Chairissy MD, Wulandari LR, Sujuti H. Pro-apoptotic and anti-proliferative effects of Physalis angulata leaf extract on retinoblastoma cells. Int J Ophthalmol. 2019;12(9):1402–1407.
24. Hidayat T, Priyandoko D, Perdana FS, Insan AM. Cytotoxicity effects of leaf extracts of Ciplukan (Physalis angulata; Solanaceae) on human blood and ovary cancer cell lines. J Phys: Conf Ser. 2019;1280(2):022009.
25. Xiang D, Zou J, Zhu X, et al. Physalin D attenuates hepatic stellate cell activation and liver fibrosis by blocking TGF-β/Smad and YAP signaling. Phytomedicine. 2020;78:153294.
26. Liu Q, Chen J, Wang X, Yu L, Hu LH, Shen X. Withagulatin A inhibits hepatic stellate cell viability and procollagen I production through Akt and Smad signaling pathways. Acta Pharmacol Sin. 2010;31(8):944–952.
27. Zhang T, Wang XF, Wang ZC, et al. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129(June):110287.
28. Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi S, Hyakusoku H. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. J Invest Dermatol. 2014;134(3):818–826.
29. Wihastyoko HYL, Soeharto S, Widjajanto E, Handono K, Pardjianto B. Does papain enzyme improve collagen degradation? Syst Rev Pharm. 2021;12(3):676–684.
30. Vieceli PS, Juiz PJL, Lauria PSS, et al. Physalis angulata reduces the progression of chronic experimental periodontitis by immunomodulatory mechanisms. J Ethnopharmacol. 2021;273:113986.
31. Wang Y, Long J, Wang X, Sun Y. Association of the plasminogen activator inhibitor-1 (PAI-1) Gene −675 4G/5G and −844 A/G promoter polymorphism with risk of keloid in a Chinese han population. Med Sci Monit. 2014;20:2069–2073.
32. Gong ZH, Ji JF, Yang J, et al. Association of plasminogen activator inhibitor-1 and vitamin D receptor expression with the risk of keloid disease in a Chinese population. Kaohsiung J Med Sci. 2017;33(1):24–29.
33. Syed F, Bagabir RA, Paus R, Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab Invest. 2013;93(8):946–960.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.