Back to Journals » Patient Preference and Adherence » Volume 13
Inhaler technique errors in Romanian patients with asthma – a multicenter study
Authors Munteanu LA, Fildan AP , Tudorache E, Fira-Mladinescu O , Frandes M , Timar B, Oancea C, Tofolean DE
Received 22 March 2019
Accepted for publication 7 July 2019
Published 19 August 2019 Volume 2019:13 Pages 1401—1414
DOI https://doi.org/10.2147/PPA.S209717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Laura Adela Munteanu,1 Ariadna Petronela Fildan,2 Emanuela Tudorache,1 Ovidiu Fira-Mladinescu,1 Mirela Frandes,3 Bogdan Timar,3 Cristian Oancea,1 Doina Ecaterina Tofolean2
1Department of Pulmonology, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania; 2Faculty of Medicine, Internal Medicine Discipline, Medical Clinical Disciplines I, “Ovidius” University of Constanta, Constanta, Romania; 3Department of Biostatistics and Medical Informatics, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
Correspondence: Ariadna Petronela Fildan
Faculty of Medicine, Internal Medicine Discipline, Medical Clinical Disciplines I, “Ovidius” University of Constanta. Aleea Universitatii nr.1, Constanta 900470, Romania
Tel +40 74 115 7526
Email [email protected]
Background: Non-adherence to treatment is associated with poor asthma control, increased exacerbations, decline in lung function, and decreased quality of life. M-health applications have become increasingly in the last years, but little research regarding the efficiency of the instructional videos for correct inhaler use exist. The aim of this study is to assess and improve the inhalator technique and to establish which types of errors were made more often with the help of a mobile health application.
Materials and methods: Seventy-five patients with partially controlled or uncontrolled asthma, using any of turbuhaler, diskus, pressurized metered dose inhaler (pMDI) or soft mist inhaler (SMI), were included in the study. When they first entered the study, the patient’s inhaler technique was assessed by a trained medical professional and the technique errors were categorized in handling, respectively inhalation errors. After the first evaluation, the patients downloaded an application on their Smartphone and were encouraged to use the application as much as needed to remind them the correct inhalation technique. The patients were re-called every three months for evaluation, treatment, and assessment of inhalation technique.
Results: We analyzed both handling and inhalation errors for each of the four considered inhalers. We observed a significantly reduced number of inhalation technique errors after using the mobile phone application. Turbuhaler median errors were 6.00, and after six months we did not observe errors. Diskus median error was 6.00, and after six months we observed a maximum of one error. pMDI median errors were 7.00, and after six months we observed just one error. Similarly, SMI median error was 7.00, and after six months we observed just one error.
Conclusion: Although technique inhalation errors are very common among asthma patients, video instructions provided through specific mobile phone applications could improve the inhaler technique in order to achieve a better control of the disease.
Keywords: inhaler device, mobile phone application, adherence
Introduction
Asthma is a chronic respiratory disease that is highly prevalent in the overall population affecting over 334 million people worldwide.1 Although it has a high prevalence, asthma is still under-diagnosed and under-treated, with over 50% of the patients in European countries not achieving the control of the disease.2
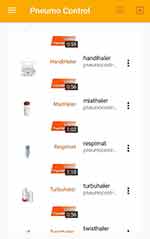 |
Figure 2 List of devices. |
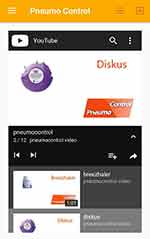 |
Figure 3 Device selection. |
Guidelines for asthma recommend multiple medication strategies which include the use of inhaler devices such as pressurized metered dose inhalers (pMDI), soft mist inhalers (SMI), and dry powder inhalers (DPI).1 Although there is evidence of the effectiveness of current asthma treatments, non-adherence to inhaled corticosteroids therapy remains a significant problem,3,4 associated with poor asthma control, increased exacerbations, decline in lung function , and decreased quality of life.5 The reasons for non-adherence to asthma medication are complex and various, but a common factor relates to patient behavior.5,6
Successful management of asthma patients includes controlling the symptoms and reducing the risk of exacerbations. There are many reasons for poor asthma control, one of the most important is the incorrect utilization of inhalers devices.7,8 Many studies have shown that 50–80% of the investigated patients use the inhaler device incorrectly thus substantially reducing the delivery of the administrated substance and consequently the effectiveness of the medication.9,10 Predictors of a poor inhaler technique like inadequate knowledge of the disease, increasing age, female gender, and low education have been previously reported.11
The main types of errors found in the inhaler technique are represented by the “critical or essential” error, which significantly impairs the delivery of adequate medication to the lung and the “non-critical” error, which results in a reduced amount of drug reaching the lungs.12
In the recent documents, the Global Initiative for Asthma (GINA) highlighted the importance of the assessment and correction of the poor inhalation technique before escalating drug therapy.1 Price et al, emphasized the association between the incorrect inhaler technique and the poor clinical outcomes and increased health care costs.12 The estimated prevalence of asthma is 4–6% which accounts for 800 thousand to 1 million Inhabitants.13
Since the early 80`s, Self et al, observed that patients who received personal instructions or videotape had a better inhalation technique than the patients who only read the package instructions.14 Video education has become increasingly in the last years but little research regarding the efficiency of the instructional videos for correct inhaler use exist.
Given these facts, the aim of this study is to assess and improve the inhalation technique and to establish which types of errors were made more often with the help of a mobile health application.
Materials and methods
Study design and patients
This was an observational multicenter study that was performed over a period of one year. The study design and contract forms were approved by the Ethics Committee of the “Victor Babes” Clinical Hospital of Infectious Diseases and Pneumophtisiology, Timisoara, and of the Clinical Pneumophtisiology Hospital of Constanta (no 261/10.01.2018 and no 87/08.01.2018, respectively). No invasive procedures have been applied. All the subjects have been informed upon the research, and informed consent was obtained before the beginning of the study. This research is respecting the Declaration of Helsinki ethical principles for research regarding the safety of human subjects.
Patients were included in the analysis if the following criteria were met: age over 18 years old; diagnosed with asthma and met the standards of the GINA guidelines ;1 received previous inhaled medication but still did not manage to control the disease; partially controlled disease or uncontrolled disease according to the physicians evaluation and GINA guidelines.1
In order to evaluate the recorded inhaler technique errors, we adopted a within-subject design. One of the main advantages of the present study design is that only fewer participants are required and the chance of discovering differences between the repeated measurements increases. Patients were both randomly selected and randomly assigned to evaluate an inhaler device. Although we included patients that received previous inhaled medication, none of them had experience with a mobile phone application for medical purpose.
Patients were excluded if they were younger than 18 years, illiteracy, unable to read the instructions, no available Smartphone, or no knowledge how to properly use a Smartphone.
Inhaler technique errors assessment
We chose to analyze the following devices: turbuhaler, diskus, pMDI, and SMI, because these are the most prescribed inhalers in the Romanian population with asthma.
When the patients first entered the study, the inhaler technique was assessed by a trained medical professional. The correct use was assessed by following a pre-defined checklist for each inhaler type based on instruction package from the manufacturers. We considered a checklist used by Gregiano et al.15,16 and critical errors were noted according to van der Palen J, et al.17
The technique was split in handling errors and inhalation errors. A critical error was considered if it prevented the subject from inhaling any of the medications contained in the inhaler. A non-critical error was when it caused a suboptimal administration of the drug, although the subject could still inhale part of the dose. The steps were marked with “1” if the technique was correct and “0” if incorrect. The same physician evaluated the technique at every visit.
After the first evaluation, the patients downloaded an application on their Smartphone and were encouraged to use the application as much as needed to remind them the correct inhalation technique. The patients were re-called every three months for evaluation and treatment. The inhalation technique was assessed at every evaluation.
Device application description
Pneumocontrol (Figures 1–4) is an application developed by a group of medical physicians together with an IT specialist. The application was developed for the self-management of the patients with asthma and chronic obstructive pulmonary disease (COPD) and it can also be tracked on www.pneumocontrol.ro
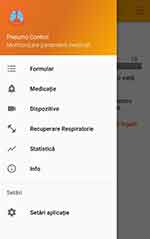 |
Figure 1 Application menu. |
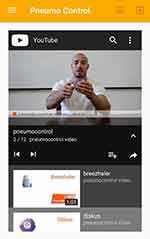 |
Figure 4 Instructional video. |
Depending on their diagnosis (asthma or COPD), patients will complete either Asthma control test (ACT), either COPD assessment test. In this situation, patients completed the ACT questionnaire. Depending on the score obtained from the questionnaire a presumptive patient classification and will display messages of: normal evolution (continue treatment), alarming evolution (need for medical consultation), and immediate medical emergencies. Medical values are automatically processed, and a graphical score will indicate patient classification according to medical standards and disease control level (scores obtained from the ACT). The mobile application allows the identification of the patient through a user account. From the first login, the patient will introduce the identification number of the current physician (which is also logged in the application) and the physician will be able to monitor the patient’s evolution. Furthermore, it will save a history of the connections made, the number of completed ACT forms, and the statistical evolution of the score (ACT questionnaire total score).
The application has three specific areas: self-management plan with specific questionnaires, inhalation devices techniques with video presentation, and pulmonary rehabilitation exercises with video explanation and subtitles.
Statistical analysis
Collected data were presented as mean (standard deviation) for numerical continuous variables with Gaussian distribution, median (percentile 25%–percentile 75%). for numerical variables without Gaussian distribution, or absolute frequency (percentage) for categorical variables. Continuous variable distributions were tested for normality using the Shapiro–Wilk’s test and for equality of variances by using Levene’s test.
To assess the significance of the differences between groups, the Student’s t-test (means, Gaussian populations), Mann–Whitney U test (medians, non-Gaussian populations) or Kruskal–Wallis test (medians, non-Gaussian populations), and Pearson chi-square or Fisher’s exact test (proportions) were used.
To evaluate the change within-subjects, we conducted a one-way repeated measures ANOVA. The condition of sphericity was assessed by the Mauchly’s test. When the sphericity condition was violated, we applied a Greenhouse-Geisser correction. Post-hoc analysis with a Bonferroni adjustment was applied for testing all possible pairwise combinations of levels of the within-subjects factor.
Data were analyzed using the SPSS v.17 software (SPSS Inc., Chicago, IL, USA). The graphical representations were generated using GraphPad Prism v8.0.2. A p-value of 0.05 was considered as the threshold for statistical significance, and a confidence level of 0.95 was considered for estimating intervals.
Results
We analyzed both handling and inhalation errors for each of the four considered inhalers, namely, Turbuhaler, Diskus, pMDI, and SMI.
In order to evaluate the errors recorded by the Turbuhaler device, we considered a sample of 25 individuals, 12 men and 13 women, aged between 22 and 64 years, mean age 39.44 (±12.59), 95% CI (34.24; 44.64). The total number of errors decreased from the first evaluation to the evaluations after 3 months and 6 months (Table 1).
 |
Table 1 Description of collected errors using Turbuhaler (N=25) at the three evaluation moments |
At the first evaluation, a total number of 7.00 errors, median 6.00 (5.00–6.00) errors were noted, and after three months, a maximum number of 3.00 errors, median 2.00 (2.00–2.00) errors were recorded, while after six months, no errors were observed. When considering only the critical errors, at the first evaluation, we noticed a maximum number of 4.00 errors, median 4.00 (3.00–4.00), while after three and six months, the number decreased to 2 errors, median 0.00 (0.00–1.00) errors, and zero errors, respectively (Figure 5).
 |
Figure 5 Representation of both total errors (left) and critical errors (right) recorded by the Turbuhaler device at the three evaluation moments when patients were grouped by gender. |
One-way repeated measures ANOVA conducted in order to determine if the number of total errors was statistically significant over the course of the six-month evaluation period revealed that the data were not normally distributed, but the sphericity condition was met, Mauchly’s test of sphericity, χ2(2) =2.891, p=0.236. There were not outliers, as indicated by the box-plots. The number of total errors statistically significant changed over time, F(2,48) =49.607, p<0.001, partial η2=0.954. Post-hoc analysis with a Bonferroni adjustment revealed that the total number of errors has significantly decreased from initial evaluation to three months (4.72 (95% CI, 4.15–5.28) errors, p<0.001), from the initial evaluation to six months (5.64 (95% CI, 5.15–6.13) errors, p<0.001), and from three months to six months (0.92 (95% CI, −1.33 to 1.32) errors, p<0.001).
In addition, when comparing the number of critical errors recorded over the six-month evaluation period, we observed that the assumption of sphericity was met, as assessed by Mauchly’s test of sphericity, χ2(2) =1.465, p=0.481. The number of critical errors significantly changed over time, F(2,48) =42.995, p<0.001, partial η2=0.947. Post-hoc analysis with a Bonferroni adjustment revealed that the number of critical errors has significantly decreased from initial evaluation to three months (3.12 (95% CI, 2.47 to 3.49) errors, p<0.001), from the initial evaluation to six months (3.52 (95% CI, 3.21 to 3.82) errors, p<0.001), and from three months to six months (0.4 (95% CI, 0.12 to 0.92) errors, p=0.015).
No statistically significant difference between the number of errors at the handling the device operation “No upright posture before inhalation”, or other handling and inhalation errors, in case of men vs women patients, 53.3% vs 46.7%, Chi-square test, X2(1) =0.214, p=0.775, were noted. On the contrary, when considering the age groups, patients aged between 22 and 40 years presented statistically significant lower number of errors at the handling the device operation “No upright posture before inhalation” than the patients aged between 42 and 64 years, Chi-square test, X2(1) =6.55, p=0.018.
Regarding the evaluation of the errors recorded by the Diskus inhaler, a sample of 14 individuals, nine men and five women, aged between 22 and 61 years, mean age 39.21 (±12.11) years, 95% CI (32.22; 46.21) was considered. The total number of errors decreased from the first evaluation (10.00 errors, median 6.00 (6.00–7.00) errors) to the evaluation after three months (maximum number of 5.00 errors, median 2.00 (2.00–2.00) errors) and six months (one error) (Table 2).
 |
Table 2 Description of collected errors using inhaler Diskus (N=14) at the three evaluation moments |
When considering only the critical errors, we noticed a maximum number of 6.00 errors, median 4.00 (4.00–4.00), at the first evaluation, while after three months and six months, the number decreased to 3 errors, median 1.00 (1.00–2.00) errors, and only one error, respectively. The number of total errors statistically significant changed over time, F(2,26) =50.738, p<0.001, partial η2=0.956. At the same time, the number of critical errors statistically significant changed over time, F(2,26) =31.509, p<0.001, partial η2=0.956. The total number of errors has significantly decreased from initial evaluation to three months (4.357 (95% CI, 3.676 to 5.039) errors, p<0.001), and from the initial evaluation to six months (6.429 (95% CI, 5.486 to 7.371) errors, p<0.001), and from three months to six months (2.071 (95% CI, 1.68 to 2.463) errors, p<0.001).
Additionally, we did not find significant differences between the number of errors in case of men patients vs women patients, when considering the handling and inhalation operations (Figure 6). At the same time, we did not observe significant differences between the number of errors in case of patients from different age groups.
 |
Figure 6 Representation of both total number of errors (left) and critical errors (right) recorded by the Diskus inhaler at the three evaluation moments when patients were grouped by gender. |
For testing the errors recorded by the pMDI inhaler, we considered a sample of 26 individuals, 13 men and 13 women, aged between 20 and 62 years, mean age 37.81 (±11.12) years, 95% CI (33.31; 42.30). We observed that the number of errors decreased from the first evaluation to the evaluations after three months and six months (Table 3).
 |
Table 3 Description of collected errors using inhaler pMDI (N=26) at the three evaluation moments |
At the first evaluation, we found a total number of 9.00 errors, median 7.00 (6.00–8.00) errors. After three months, we observed a total number of 3.00 errors, median 2.00 (1.00–2.00) errors, while after six months, we observed just one error. When considering only the critical errors, we noticed a number of 5.00 errors, median 3.50 (3.00–4.00) at the first evaluation, while after three months and six months, the number decreased to only two errors, median 1.00 (0.00–1.00) errors, and zero errors, respectively. The number of total errors statistically significant changed over time, F(2,50) =49.391, p<0.001, partial η2=0.963. At the same time, the number of critical errors statistically significant changed over time, F(2,50) =31.601, p<0.001, partial η2=0.903. The total number of errors has significantly decreased from initial evaluation to three months (5.077 (95% CI, 4.565– 5.589) errors, p<0.001), and from the initial evaluation to six months (6.846 (95% CI, 6.265–7.428) errors, p<0.001), and from three months to six months (1.769 (95% CI, 0.98–2.143) errors, p<0.001).
When comparing the number of errors recorded in case of patients grouped by gender, we observed that men did significantly more errors compared to women at the handling the device operation “Mouthpiece not enclose tightly with the lips”, 76.9% vs 23.1%, Chi-square test, X2(1) =7.54, p=0.017. However, we did not observe significant differences between the number of errors in case of men patients vs women patients when considering the other handling and inhalation errors (Figure 7). On the contrary, when we compared the number of errors recorded in case of patients at the age group of 20–40 years vs age group of 41–62 years, we observed statistically significant differences between the number of errors at the handling the device operation “Not removing/opening the cap”, 16.7.% vs 83.3%, Chi-square test, X2(1) =6.82, p=0.017. When considering the other handling and inhalation errors, we did not observe significant differences between the number of errors for the two age groups.
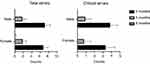 |
Figure 7 Representation of the total number of errors (left) and the critical errors (right) recorded by the pMDI inhaler at the three evaluation moments when patients were grouped by gender. |
When evaluating the inhaler technique errors recorded by the SMI, we considered a sample of 10 individuals, 6 men and 4 women, aged between 24 and 57 years, mean age 40.10 (±10.6) years, 90% CI (32.52; 47.68). We observed that the number of errors decreased from the first evaluation to the evaluations after three months and six months (Table 4).
 |
Table 4 Description of collected errors using inhaler SMI (N=10) at the three evaluation moments |
We found a total number of 11.00 errors, median 7.00 (5.00–8.00) errors at the first evaluation. After three months, we observed a total number of 7.00 errors, median 3.00 (2.00–4.00) errors, while after six months, we observed just one error, median 0.50 (2.00–4.00) errors. When considering only the critical errors, we noticed a number of 6.00 errors, median 5.00 (3.00–5.00) at the first evaluation, while after three months and six months, the number decreased to 3 errors, median 2.00 (1.00–2.00) errors, and just one error, respectively.
We observed that the number of the total recorded errors at the three evaluation moments has significantly decreased from the first evaluation to the third evaluation, F(2,18) =61.127, p<0.001, partial η2=0.872. At the same time, we observed that differences between the number of critical errors at the three evaluation moments were statistically significant, F(2,18) =85.172, p<0.001, partial η2=0.904. The total number of errors has significantly decreased from initial evaluation to three months (2.800 (95% CI, 1.660–3.940) errors, p<0.001), and from the initial evaluation to six months (4.300 (95% CI, 3.224 to 5.376) errors, p<0.001), and from three months to six months (1.500 (95% CI, 1.098–2.002) errors, p<0.001).
In addition, no significant differences between the number of errors recorded in case of men vs women, when considering both the handling and inhalation errors were observed (Figure 8). Moreover, no significant differences between the number of errors recorded by the inhaler when considering patients at different age groups were found.
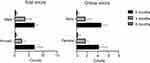 |
Figure 8 Representation of both total number of errors (left) and critical errors (right) of the SMI inhaler at the three evaluation moments when patients were grouped by gender. |
The comparative representation of both total number of errors and number of critical errors recorded by the considered inhalers is shown in Figure 9. The total number of errors decreased from the first evaluation to the evaluations after both three and six months when considering all the inhalers.
 |
Figure 9 Representation of both total errors (left) and critical errors (right) recorded by the considered inhalers at the three evaluation moments. |
Discussion
It is known that for the past 40 years, the type and the frequency of inhaler errors did not significantly change, and treatment outcomes are often reduced due to deficient inhaler technique.18
In this study, we analyzed the inhaler technique and the error evolution after using a specific M-health application that contains demonstrative inhaler technique videos. We compared our findings with the literature results and presented it as a valuable option in order to reduce the inhaler technique errors.
We found major inhaler technique errors in all the analyzed devices, every single one having at least four critical errors.
The CRITIKAL study, which is one of the largest studies that investigated the inhaler technique in a multinational population, the authors have observed that inspiratory effort errors were frequent in all studied device cohorts. Patients that used DPI devices inhaled insufficiently fast and forceful: 32.1% of the patients that used Turbuhaler, 38.4% of the patients using Diskus, and 47.2% in patients that used MDI devices.19
The most frequent errors remain the generic ones, such as dose preparation for DPIs, coordination problems with MDIs, and other current reported errors consisting in not exhaling, not holding breath and insufficient speed of inhalation.19
Compared to the above-mentioned studies, in the present study, 84% of the patients that used Turbuhaler device, and all the patients that used Diskus device did not inhale sufficiently fast and forceful. One explanation for our high rate of errors could be that we analyzed a significantly reduced number of patients compared to the other studies. Regardless of the number of patients, it is clear that this type of critical error remains high.
The CRITIKAL study also highlighted “exhaling into the device” as a critical MDI error.19 Although the literature has found patients that exhaled into the device, in our study not a single patient made this error.
Sanchis et al, analyzed a large number of studies during 2000 and 2013 and found a wide variability between the subjects making errors across different studies. The most commonly made errors in pMDI across studies were failing to actuate the inhaler while breathing in slowly and failing to hold breath after inhalation. The proportion of former errors ranged between 24% and 77%, respectively 10% to 68% across different studies.20
Plaza et al, found that inhalation techniques remain poor in Spain, the most common error reported for the pMDI being: failing to breath out prior to inhalation, poor coordination between inhalation and actuation of the device, forgetting to shake the inhaler, and failing to hold breath after medication delivery.21
Compared to the result of these studies, 96% of our patients failed to actuate the inhaler while breathing in slowly, while 57% of the patients failed to hold their breath for about 5 s after inhalation.
Other authors found that the most common mistakes in DPI inhalers are failing to breathe out and away from the inhaler, and failing to hold breath. Furthermore, 30% of the subjects that used the Turbuhaler device made an error related to the inhaler position.22
Other studies reported that the steps with the highest error rate in DPIs were critical errors: 34% of the patients did not shake the inhaler well, 46.2% did not exhale completely away from the inhaler, and 40.9% of the studied patients did not hold the breath for 10 s.23
In this study, older patients operated the inhalers with multiple technique errors compared to the younger ones. Reduced dexterity and cognitive function can be some of the factors that limit their ability to correctly use the inhalers. Statistically significant differences between the age groups 22–40 and 41–64 years were observed in the Turbuhaler group regarding the handling error “no upright posture before inhalation”. When we analyzed the pMDI group we found significant differences between the same group ages but this time the handling error was “not removing/opening the cap” (16.7% vs 83.3%). No handling errors were observed between the two group ages for the SMI.
A study that used video instructions to promote correct technique for DPI observed that non-verbal videos were an inadequate way of providing inhaler education for first time users of inhalers.24
Compared to their study, our instructional videos were verbal and had clear subtitle, this could be one reason why our subjects had an improved inhaler technique. Instead of learning how to use inhalers by reading the instructions only, visual demonstrations with the help of M-health applications can reduce the critical errors during inhaler use.
Other studies had also proved that the use of video technology can improve the patient knowledge and understanding regarding inhaler technique.25
It has been observed that the inhaler technique is deteriorating over time, sometimes as little as 2–3 months.26 Thus, it is important to have repeated training in order to obtain and maintain a correct technique. Providing inhaler education via M-health applications could be suitable for this situation instead of recalling the patient to verify the technique. This option is also supported by Wilson et al, who emphasized that the use of instructional videos can promote recall on inhaler use in asthma patients.27
We evaluated the patients over a period of six months with a constant reminder to use the phone application when needed and thus we obtained significant result regarding the inhaler technique. After six months, all types of errors (critical and non-critical) had been significantly reduced to almost no error. For the future, it would be interesting to observe if no further reminder from the physician and no use of an M-health application can maintain the inhaler technique for a longer period of time.
One limitation of this study could be that we did not include the education level of the subjects. Education level could affect the ability to understand instructions, memorize the technique steps, and operate the inhaler.
Conclusion
Incorrect inhaler technique remains frequent among patients with asthma, and it did not improve significantly over the past years. This problem could be an important obstacle in achieving good asthma control. Careful instructions, demonstration, and repeated tuition are possible strategies that could enhance the proper inhaler technique, but new approaches should be explored. The results of this study suggest that video instructions provided through specific mobile phone applications could improve the inhaler technique in patients with asthma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bateman SS, Hurd PJ, Barnes J, et al. Zar European Respiratory Journal. 2008;31:143–178. doi:10.1183/09031936.00138707
2. Demoly P, Paggiaro P, Plaza V, Bolge SC, Kannan H, Sohier B. Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Rev. 2009;112(18):105–112. doi:10.1183/09059180.00001209
3. Miller L, Schüz B, Walters J, Walters EH. Mobile technology interventions for asthma self-management: systematic review and meta-analysis. JMIR mHealth uHealth. 2017;5(5):e57. doi:10.2196/mhealth.7168
4. Rascu A, Elena Popa D, Arghir O, Otelea M. Effects of corticosteroid treatment on respiratory muscles function in patients with severe obstructive lung disease. Farmacia. 2016;64(6):819–822.
5. Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe. 2015;11(1):15–24. doi:10.1183/20734735.008914
6. Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MCJM, Verhamme KMC. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396LP–407. doi:10.1183/09031936.00075614
7. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. doi:10.1016/j.rmed.2011.01.005
8. Mihailov C, Jimborean G, Rascu A, Arghir OC. Impact of tobacco smoke exposure on asthma COPD-like patients. Journal of Environmental Protection and Ecology. 2016;17(4):1523–1533.
9. Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360LP–1375. http://rc.rcjournal.com/content/50/10/1360.abstract.
10. Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16(3):249–254. doi:10.1089/089426803769017613
11. Rootmensen GN, van Keimpema ARJ, Jansen HM, de Haan RJ. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23(5):323–328. doi:10.1089/jamp.2009.0785
12. Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46. doi:10.1016/j.rmed.2012.09.017
13. Institutul Național de Statistică. Rezultate definitive ale Recensământului Populaţiei şi al Locuinţelor – 2011 (caracteristici demografice ale populaţiei) [Final results of the Household and Population Census - 2011 (population demographic characteristics)]; 2011:1–14. http://www.recensamantromania.ro/rezultate-2/.
14. Self TH, Brooks JB, Ryan MR. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128(2):129–131.
15. Cvetkovski B, Azzi EA, Tan R, Bosnic-Anticevich SZ, Srour P, Kritikos V. Identifying critical errors: addressing inhaler technique in the context of asthma management. Pulm Ther. 2018;4(1):1–12. doi:10.1007/s41030-018-0051-0
16. Gregoriano C, Dieterle T, Breitenstein AL, et al. Use and inhalation technique of inhaled medication in patients with asthma and COPD: data from a randomized controlled trial. Respir Res. 2018;19(1):237. doi:10.1186/s12931-018-0936-3
17. van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079. doi:10.1038/npjpcrm.2016.79
18. Sanchis J, Gich I, Pedersen S. Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394–406. doi:10.1016/j.chest.2016.03.041
19. Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–1081.e9. doi:10.1016/j.jaip.2017.01.004
20. Sanchis J, Corrigan C, Levy ML, Viejo JL. Inhaler devices – from theory to practice. Respir Med. 2013;107(4):495–502. doi:10.1016/j.rmed.2012.12.007
21. Plaza V, Sanchis J, Roura P, et al. Physicians’ knowledge of inhaler devices and inhalation techniques remains poor in Spain. J Aerosol Med Pulm Drug Deliv. 2012;25(1):16–22. doi:10.1089/jamp.2011.0895
22. Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the aerosol drug management improvement team. Respir Med. 2006;100(9):1479–1494. doi:10.1016/j.rmed.2006.01.008
23. Bartolo K, Balzan M, Schembri EL, et al. Predictors of correct technique in patients using pressurized metered dose inhalers. BMC Pulm Med. 2017;17(1):6–15. doi:10.1186/s12890-017-0386-6
24. von Schantz S, Katajavuori N, Juppo AM. The use of video instructions in patient education promoting correct technique for dry powder inhalers: an investigation on inhaler-naive individuals. Pharm (Basel, Switzerland). 2018;6(4). doi:10.3390/pharmacy6040106
25. Ferguson LA. Implementing a video education program to improve health literacy. J Nurse Pract. 2012;8(8):e17–e22. doi:10.1016/j.nurpra.2012.07.025
26. Ovchinikova L, Smith L, Bosnic-Anticevich S. Inhaler technique maintenance: gaining an understanding from the patient’s perspective. J Asthma. 2011;48(6):616–624. doi:10.3109/02770903.2011.580032
27. Wilson EAH, Park DC, Curtis LM, et al. Media and memory: the efficacy of video and print materials for promoting patient education about asthma. Patient Educ Couns. 2010;80:393–398. doi:10.1016/j.pec.2010.07.011
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
