Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Inhaled glycopyrrolate for the treatment of chronic obstructive pulmonary disease
Authors Tashkin DP, Gross NJ
Received 16 January 2018
Accepted for publication 19 April 2018
Published 12 June 2018 Volume 2018:13 Pages 1873—1888
DOI https://doi.org/10.2147/COPD.S162646
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Donald P Tashkin,1 Nicholas J Gross2
1Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 2Department of Medicine, University Medical Research LLC, Saint Francis Hospital and Medical Center, Hartford, CT, USA
Abstract: Long-acting muscarinic antagonists (LAMAs), along with long-acting β2-agonists (LABAs), are the mainstay for treatment of patients with COPD. Glycopyrrolate, or glycopyrronium bromide, like other LAMAs, inhibits parasympathetic nerve impulses by selectively blocking the binding of acetylcholine to muscarinic receptors. Glycopyrrolate is unusual in that it preferentially binds to M3 over M2 muscarinic receptors, thereby specifically targeting the primary muscarinic receptor responsible for bronchoconstriction occurring in COPD. Inhaled glycopyrrolate is slowly absorbed from the lungs and rapidly eliminated from the bloodstream, most likely by renal excretion in its unmetabolized form, limiting the potential for systemic adverse events. Inhaled glycopyrrolate is a fast-acting, efficacious treatment option for patients with moderate–severe COPD. It improves lung function, reduces the risk of exacerbations, and alleviates the symptoms of breathlessness, which in turn may explain the improvement seen in patients’ quality of life. Inhaled formulations containing glycopyrrolate are well tolerated, and despite being an anticholinergic, few cardiovascular-related events have been reported. Inhaled glycopyrrolate is thus of value as both monotherapy and in combination with other classes of medication for maintenance treatment of COPD. This review covers the mechanism of action of inhaled glycopyrrolate, including its pharmacokinetic, pharmacodynamic, and safety profiles, and effects on mucus secretion. It also discusses the use of inhaled glycopyrrolate in the treatment of COPD, as monotherapy and in fixed-dose combinations with LABAs and inhaled corticosteroid–LABAs, including a triple therapy recently approved in Europe.
Keywords: glycopyrronium bromide, long-acting muscarinicantagonist, anticholinergic, bronchodilator
Plain language summary
Patients with COPD have narrowed airways and cannot fully empty their lungs, which together can make breathing uncomfortable. Doctors often prescribe an inhaler containing drugs that widen the airways or reduce inflammation in the lungs, making it easier for patients with COPD to breathe. Patients who do not show enough benefit from treatment with one drug alone may be given two or more drugs, which can be combined into one inhaler. One drug used to widen the airways in patients with COPD is glycopyrrolate (also referred to as glycopyrronium bromide). Glycopyrrolate can be used to treat COPD on its own, as well as in combination with other drugs. In this article, we discuss the evidence for how glycopyrrolate works in the body, how glycopyrrolate enters and leaves the body, and the effectiveness and side effects of glycopyrrolate when used to treat patients with COPD in clinical trials alone and combined in one inhaler with another airway-widening drug with or without a drug used to reduce inflammation in the lungs.
Introduction
Long-acting muscarinic antagonists (LAMAs) or long-acting β2-agonists (LABAs), alone or in combination, are the mainstay for the maintenance treatment of patients with COPD.1,2 In the 1980s, inhaled glycopyrrolate, also known as glycopyrronium bromide, was found to be a long-acting bronchodilator3,4 and improved pulmonary function after exercise in patients with asthma,5 although inhaled glycopyrrolate is not currently licensed for use in asthma.6 Inhaled glycopyrrolate, a rapid-onset LAMA, is now US Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved for maintenance treatment of patients with COPD.1,6,7 This review covers the mechanism of action of inhaled glycopyrrolate, its pharmacokinetic (PK) and pharmacodynamic (PD) profiles, safety profile, effects on mucus secretion, and use in the treatment of COPD as monotherapy and in fixed-dose combinations (FDCs) with LABAs and inhaled corticosteroid (ICS)–LABAs.
Pharmacokinetics and pharmacodynamics of inhaled glycopyrrolate
Preganglionic parasympathetic nerves innervate the airways via the vagus nerve (Figure 1).8 At parasympathetic ganglia, preganglionic nerves synapse with postganglionic nerves.8 Acetylcholine (ACh) is a neurotransmitter that is released during parasympathetic nerve impulses and acts by binding to and activating muscarinic receptors and nicotinic receptors.8,9 There are five muscarinic receptor subtypes, referred to as M1–M5.9
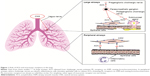  | Figure 1 Role of ACh and muscarinic receptors in the lung. |
M1 receptors are highly expressed in the peripheral airways, whereas M2 and M3 receptors predominate in the larger airways.8 M3 receptors are the muscarinic receptors primarily responsible for ACh-induced bronchoconstriction,8 as they activate phospholipase C, which produces inositol 1,4,5-triphosphate and diacylglycerol, leading to intracellular calcium release.9 Anticholinergics block parasympathetic nerve impulses by selectively preventing ACh from binding to muscarinic receptors.8,10 These drugs inhibit bronchoconstriction in peripheral airways by antagonizing the effects on airway smooth muscle cells of ACh released by epithelial cells; this release is stimulated by inflammatory cells.11
The anticholinergic effects of inhaled glycopyrrolate are primarily limited to the airways, thereby reducing the likelihood of systemic adverse events (AEs).12 Inhaled glycopyrrolate has a bioavailability of 57%, with 53% absorbed via the lungs.12 Based on its ability to inhibit methacholine-induced calcium release (half-life [t½] 6.1±2.1 minutes), inhaled glycopyrrolate has a rapid onset of action.13 Inhaled glycopyrrolate is long lasting in the body, with a terminal elimination-phase t½ of 52.5 hours following inhalation (vs t½ of 6.2 hours following intravenous administration).12 Population PK modeling has shown that inhaled glycopyrrolate is absorbed slowly, predominantly unchanged, from the lungs.12 It has a slow-phase absorption t½ of 3.5 days, accounting for 79% of drug absorption.12 Furthermore, inhaled glycopyrrolate is eliminated rapidly from the bloodstream.12 Intravenous glycopyrrolate is excreted, mainly in its unmetabolized form, by the kidney.14,15 Metabolism is less important for the elimination of this drug from the body.14,15 Renal excretion is also likely to be important for the elimination of glycopyrrolate systematically absorbed from the lung.15 However, no inhaled glycopyrrolate dose adjustments are required for patients with mild–moderate renal impairment, and those with severe renal impairment may be given inhaled glycopyrrolate if the benefits are judged to outweigh the risks.6
Pharmacokinetic and pharmacodynamic profiles of inhaled glycopyrrolate vs other LAMAs
Available anticholinergic drugs bind to all muscarinic ACh receptors present in the airways (ie, M1–M3).8,16 Importantly, M3 receptors are the primary therapeutic target for bronchodilation, with antagonism at M2 autoreceptors tending to attenuate bronchodilator effects.17 The PK and PD profiles of glycopyrrolate have been compared with other LAMAs in various preclinical studies, although not all of these studies tested the inhaled delivery of glycopyrrolate (Table 1).13,16,18,19 Glycopyrrolate is the only anticholinergic to date to show higher relative affinity for M3 than M2 receptors, although its absolute binding affinity for these receptors is lower than that of aclidinium and tiotropium.13,16 Glycopyrrolate, aclidinium, tiotropium, and ipratropium (a short-acting muscarinic antagonist) all dissociate more rapidly from M2 than M3 receptors, with all four drugs showing similar ratios of dissociation t½.13,16 In addition, glycopyrrolate has a shorter absolute dissociation time at both M2 and M3 receptors than at aclidinium and tiotropium.16 An ex vivo study examining muscarinic receptor binding in the rat lung showed receptor binding lasted 24 hours for glycopyrrolate and tiotropium, whereas ipratropium binding was observed at 2 hours, but not at 12 hours.18
In an in vivo study on guinea pigs, glycopyrrolate, ipratropium, and aclidinium had a similar onset of action, which was more rapid than tiotropium.16 However, in isolated human airways, glycopyrrolate had significantly more rapid onset of action than both aclidinium and tiotropium.19 Glycopyrrolate had a similar duration of action at M3 receptors to that of aclidinium and tiotropium in vitro, but had a shorter duration of action than tiotropium and aclidinium in vivo in guinea pig studies.16 Sykes et al proposed that drug molecules, once dissociated from their target receptors, may not be able to diffuse away from the receptor environment and thus are likely to rebind to localized receptors.13 The authors suggested that this may explain why some LAMAs have a long duration of action, despite rapid dissociation rates.13 In an in vitro study, aclidinium was shown to have lower stability than tiotropium, which in turn had lower stability than glycopyrrolate in rat, guinea pig, and human plasma.16 This indicates that glycopyrrolate undergoes a slower rate of hydrolysis, which may result in a propensity to cause anticholinergic AEs in patients with COPD.16 These findings are in agreement with a previous study that compared the plasma stability of aclidinium with tiotropium and ipratropium, with aclidinium found to be the least stable of the three bronchodilators evaluated.20 Furthermore, a lower dose of glycopyrrolate and tiotropium than aclidinium is required to inhibit salivation effectively in rats, possibly because of the slower rate of hydrolysis in the blood.16
Inhaled glycopyrrolate: effects on mucus secretion and mucociliary clearance in COPD
Mucus is secreted by submucosal mucus glands and goblet cells in the bronchi, and excessive mucus secretion is a feature of chronic bronchitis and COPD.21 M1 and M3 receptors are expressed at a 1:2 ratio in submucosal glands.22 The M3 receptor is involved primarily in mediating mucus secretion, whereas water and electrolyte secretion are likely to be regulated by M3 and M1 receptors in combination.22 LABAs are known to enhance mucociliary clearance in patients with COPD,23 probably via an effect on ciliary beat frequency,24 and there are numerous theories regarding the alteration of mucus production by LAMAs.25 However, the results from clinical trials testing these theories are conflicting.25 In an open-label, non-placebo-controlled trial in 22 patients with COPD, tiotropium reduced cough symptoms and nasal clearance times.26 The authors concluded that this effect may result from inhibition of mucus hypersecretion and an increase in mucociliary clearance in the airways.26 However, this supposition is not supported by other studies: in a randomized, double-blind trial, Hasani et al failed to find an effect of tiotropium on mucociliary clearance in 34 patients with COPD,27 whereas Meyer et al reported that tiotropium treatment slowed mucociliary clearance in their randomized, open-label, crossover study in 24 patients.28 Furthermore, in a double-blind, crossover study, ipratropium was actually found to decrease cough clearance of secretions in patients with COPD.29
Oral glycopyrrolate is known to reduce drooling in children, and glycopyrrolate injections can reduce preoperative salivation, as well as respiratory secretions during end-of-life care.30,31 However, on review of published literature cited in PubMed, no studies could be found that examined the role of inhaled glycopyrrolate in mucus secretion. There also appear to be limited published data examining the effect of glycopyrrolate on mucociliary clearance,25 and hence, there is a need for further study in this area.
The effect of inhaled glycopyrrolate on cognition
Glycopyrrolate is a water-soluble, highly polar quaternary ammonium compound, which limits its passage across lipid membranes such as the blood–brain barrier; therefore, glycopyrrolate does not exhibit any central nervous system activity.32 Inhaled glycopyrrolate is thus unlikely to have a significant effect on cognition in patients with COPD, although there is a lack of evidence to support this.
Clinical evidence for the use of inhaled glycopyrrolate in patients with COPD
In its inhaled form, glycopyrrolate and other approved LAMAs are used for the management of COPD.1,6,33–37 Glycopyrrolate is available as a monotherapy via a dry-powder inhaler (DPI)6 and was approved by the FDA in 2017 as a nebulized monotherapy (Table 2).38 Inhaled glycopyrrolate is also available in an FDC with formoterol, delivered using co-suspension delivery technology in a pressurized metered dose inhaler (pMDI),34 and in a DPI FDC with indacaterol.35,36 Inhaled glycopyrrolate has been approved in Europe as a triple FDC with formoterol and beclomethasone, delivered via a pMDI.39
  | Table 2 Available glycopyrrolate and other LAMA formulations |
Efficacy of inhaled glycopyrrolate monotherapy
Onset of action
In a randomized, double-blind study to determine the most appropriate dose of inhaled glycopyrrolate monotherapy (delivered via a DPI) for patients with moderate–severe COPD, glycopyrrolate 50 μg once daily (QD) provided significant bronchodilation over 24 hours.40 However, the efficacy of glycopyrrolate 50 μg QD was not significantly different from the same total daily dose administered twice daily (BID).40 The randomized GLOW trials were conducted to evaluate the use of 50 μg QD of inhaled glycopyrrolate for treating patients with moderate–severe COPD.41–46 The GLOW1 study showed that glycopyrrolate 50 μg QD had a rapid onset of bronchodilation in patients with COPD, with a significant improvement from baseline in lung function vs placebo as early as 5 minutes after treatment (P<0.001).41 Supporting the preclinical data, inhaled glycopyrrolate has been shown to have faster onset of action than tiotropium in several clinical trials. In the randomized GLOW2 study, glycopyrrolate 50 μg QD resulted in significantly more rapid bronchodilation than tiotropium 18 μg QD treatment at all time points from 5 minutes to 4 hours after the first dose on day 1 (P<0.01).42 Similarly, in the randomized GLOW5 study, glycopyrrolate 50 μg QD resulted in significantly more rapid improvement in lung function than tiotropium 18 μg QD treatment at 5 and 15 minutes after first dose.45 Additionally, in a post hoc analysis of the randomized SPRING study in patients with moderate–severe COPD, glycopyrrolate 50 μg QD resulted in a significantly greater improvement in lung function than tiotropium 18 μg QD at 5 minutes, 15 minutes, and 1 hour after the first dose on day 1 (P=0.015, P=0.026, and P=0.014, respectively).47 In a further study in patients with moderate–severe COPD, single doses of both glycopyrrolate (50 μg) and aclidinium (400 μg) had more rapid onset of action than tiotropium (18 μg), resulting in greater levels of bronchodilation 90 minutes after treatment, although the authors concluded that faster onset of action seen in the clinic may not be relevant for patients who are undergoing long-term treatment for chronic disease.19
Lung function and other efficacy end points
The efficacy of inhaled glycopyrrolate monotherapy (via a DPI) has been investigated in patients with COPD in numerous clinical studies, including the GLOW study series and the GEM studies (Table 3).38,41–52 Two network meta-analyses have also been conducted to compare the efficacy of glycopyrrolate with other LAMAs.53,54 The first (including 21 trials) compared glycopyrrolate 50 μg QD with aclidinium 400 μg BID, tiotropium 18 μg QD (HandiHaler), and tiotropium 5 μg QD (Respimat) in patients with moderate–severe COPD.53 After 24 weeks, glycopyrrolate 50 μg treatment resulted in a similar improvement in lung function compared with aclidinium 400 μg, tiotropium 18 μg, and tiotropium 5 μg.53 At the same time point, glycopyrrolate 50 μg treatment resulted in similar improvements in St George’s Respiratory Questionnaire (SGRQ) scores from baseline compared with aclidinium 400 μg and tiotropium 18 μg, and a greater improvement compared with tiotropium 5 μg.53 Improvements in breathlessness symptoms were similar for all treatments.53
The second network meta-analysis (including 24 trials) compared the efficacy of glycopyrrolate 50 μg QD, tiotropium 18 μg QD, aclidinium 400 μg BID, and umeclidinium 62.5 μg QD in patients with COPD.54 At weeks 12 and 24, all LAMA treatments evaluated resulted in clinically relevant (>100 mL) improvements in trough forced expiratory volume in 1 second (FEV1) compared with placebo.54 Glycopyrrolate 50 μg treatment resulted in the greatest improvement over placebo in lung function at week 24 (FEV1 difference 135.8 mL).54 For all LAMAs vs placebo, improvements were seen from baseline in 24-week SGRQ and Transition Dyspnea Index (TDI) scores.54 Glycopyrrolate 50 μg QD treatment did not reach the minimal clinically important difference (MCID) in SGRQ score of 4 units compared with placebo, but did reach the MCID of ≥1 for TDI focal score.54 However, aclidinium 400 μg and umeclidinium 62.5 μg treatments reached MCID for both SGRQ and TDI focal scores compared with placebo, whereas tiotropium did not reach MCID for either of the two measures.54
For patients with moderate–severe COPD, clinical trial data for treatment with inhaled glycopyrrolate as a monotherapy indicate that glycopyrrolate 50 μg QD (the EMA-approved dose) improves lung function and health-related quality of life (HRQoL), decreases the severity of breathlessness and the risk of exacerbations, and improves morning symptoms.41,42,45,47,51 Furthermore, glycopyrrolate 50 μg QD was found to be noninferior to tiotropium in its ability to increase airflow to the lungs.45 In a short-term, crossover trial (3-week treatment periods), glycopyrrolate also significantly improved patients’ abilities to exercise vs placebo.43 In Phase III trials of glycopyrrolate 15.6 μg BID (the FDA-approved dose) in patients with moderate–severe COPD, there were also significant improvements in FEV1 scores from baseline, TDI focal scores, and SGRQ scores vs placebo.48,49 In two Phase III trials of the nebulized form of glycopyrrolate, 50 μg BID treatment resulted in significant and clinically important increases from baseline in lung function and SGRQ scores vs placebo.38 Finally, in two studies in patients with moderate–severe COPD, glycopyrrolate was delivered by pMDI using innovative co-suspension delivery technology, which allows aerosol delivery of micronized drug suspended with microsized, phospholipid-based porous particles.52,55–57 In these studies, patients treated with glycopyrrolate (doses ≥2.4 μg BID) showed clinically relevant, significant improvements in lung function from baseline vs placebo.52,57 Furthermore, the highest glycopyrrolate dose tested in the first study (18 μg BID) was found to be noninferior to tiotropium 18 μg QD for improving lung function.52 In the second study, all glycopyrrolate doses (delivered as glycopyrronium 28.8, 14.4, 7.2, and 3.6 μg BID) were found to be noninferior to ipratropium 34 μg 4 times daily.57 Based on these studies, 18 μg (equivalent to 14.4 μg glycopyrronium) was selected as the optimal glycopyrrolate dose for the glycopyrrolate–formoterol combination studies.52,57
Efficacy of inhaled glycopyrrolate combinations
Combining a LAMA with a LABA can increase efficacy, as they have distinctly different mechanisms of action (ie, target different receptors). In addition, LABAs modify the release of ACh, leading to amplification of bronchial smooth muscle relaxation induced by the LAMA.58 Moreover, LABAs act on presynaptic β2-receptors in the efferent cholinergic pathway, resulting in the inhibition of cholinergic transmission.59
In 2016, glycopyrrolate–formoterol pMDI 18/9.6 μg BID was licensed by the FDA “for the long-term maintenance treatment of airflow obstruction in patients with COPD”.34 Several randomized controlled trials have examined the efficacy of glycopyrrolate–formoterol 18/9.6 μg BID (Table 4).60,61 Five studies (PINNACLE-1, PINNACLE-2, PT003011, PT003012, and PINNACLE-3) showed that (as expected) glycopyrrolate–formoterol 18/9.6 μg BID significantly improved lung function compared with individual components and placebo.60–62 In addition, glycopyrrolate–formoterol 18/9.6 μg BID was at least as efficacious as open-label tiotropium 18 μg QD.61,62 Furthermore, the efficacy of glycopyrrolate–formoterol 18/9.6 μg BID was maintained over the 1-year treatment period.60,61 A post hoc analysis of PINNACLE-1 and −2 indicated that glycopyrrolate–formoterol improved FEV1 independently of baseline symptom severity, although high baseline symptom severity was significantly correlated with greater improvement in health outcomes after treatment.63
Inhaled glycopyrrolate has also been combined with indacaterol for maintenance therapy in patients with COPD. Glycopyrrolate–indacaterol DPI was approved for use by the FDA in 2015 (at 15.6/27.5 μg BID)36 and by the EMA in 2013 (at 50/110 μg QD).35 Glycopyrrolate–indacaterol 50/110 μg QD significantly improved lung function compared with individual components (P<0.001)64 and tiotropium 18 μg QD (P=0.0017).65 The glycopyrrolate–indacaterol combination was also preferred to tiotropium by both patients (P=0.00004) and physicians (P<0.0001).65 Furthermore, glycopyrrolate–indacaterol 50/110 μg QD significantly increased lung function, and a significantly greater proportion of patients achieved a clinically relevant improvement in dyspnea compared with tiotropium 18 μg QD plus formoterol 12 μg BID.66 In two clinical trials, glycopyrrolate–indacaterol 50/110 μg QD was more effective at reducing COPD exacerbations than the ICS–LABA combination fluticasone propionate–salmeterol 500/50 μg BID.67,68 Additionally, glycopyrrolate–indacaterol 50/110 μg QD was found to be noninferior to tiotropium 18 μg QD plus formoterol 12 μg BID in improving HRQoL.66
The 15.6/27.5 μg BID formulation of glycopyrrolate–indacaterol DPI has been shown to perform favorably in improving lung function when compared with its individual components69,70 and provide clinically meaningful improvements in breathlessness and HRQoL.69 Two crossover studies of glycopyrrolate–indacaterol 15.6/27.5 μg BID vs umeclidinium–vilanterol 62.5/25 μg QD did not meet their primary efficacy end point of noninferiority of change from baseline in FEV1 area under the curve from 0 to 24 hours at week 12. The authors concluded that although glycopyrrolate–indacaterol was not non-inferior to umeclidinium–vilanterol, the differences in improvement in lung function between the combinations were not clinically relevant.71 Several clinical trials have indicated that glycopyrrolate–indacaterol is effective at improving lung function in patients with moderate–severe COPD,68,69,71–73 and patients with COPD and a high risk of exacerbations.67 One of these trials also demonstrated that glycopyrrolate–indacaterol decreases hyperinflation and improves patients’ ability to undertake daily physical activity.73
Glycopyrrolate also showed potential as a COPD treatment when added to the ICS–LABA combination fluticasone propionate–salmeterol.74 After 12 weeks’ treatment, glycopyrrolate 50 μg QD plus fluticasone propionate–salmeterol 500/50 μg BID significantly improved lung function when compared with placebo plus fluticasone propionate–salmeterol, and was noninferior to tiotropium (18 μg QD) plus fluticasone propionate–salmeterol.74
An inhaled triple FDC, glycopyrrolate–beclomethasone–formoterol (12.5/100/6 μg BID pMDI) was approved for use by the EMA in 2017 for the maintenance treatment of patients with COPD.39 Two long-term trials have demonstrated that this triple FDC is efficacious in this patient group.75,76 In the first trial, the triple FDC was significantly more effective at improving lung function vs beclomethasone–formoterol at week 26.75 The triple FDC also decreased the risk of exacerbations from baseline to a greater extent than beclomethasone–formoterol by week 52.75 The second trial provided evidence that this triple FDC was significantly superior to tiotropium in reducing the exacerbation rate and improving lung function from baseline.76
Safety of inhaled glycopyrrolate monotherapy
Several trials have shown that glycopyrrolate 50 μg QD DPI is well tolerated,41–47 with a similar overall incidence of AEs to tiotropium 18 μg QD.42,44,45,47 In a study comparing glycopyrrolate 50 μg QD with open-label tiotropium 18 μg QD, the incidences of AEs and serious AEs were similar between groups; AEs with incidence >10% were worsening COPD (24% [glycopyrrolate] vs 33% [tiotropium]) and nasopharyngitis (31% vs 33%, respectively).44 Glycopyrrolate also had an acceptable safety profile at 15.6 μg BID,48–50 with AE incidence after 52 weeks’ treatment similar to that seen after treatment with indacaterol 75 μg QD.50 Furthermore, nebulized glycopyrrolate (50 μg BID) was also shown to have an acceptable safety profile over 48 weeks’ treatment compared with tiotropium 18 μg QD.51 Treatment discontinuation due to treatment-emergent AEs was higher for nebulized glycopyrrolate than for tiotropium (10% vs 3%, respectively); the authors suggested several reasons for this, including the open-label nature of the trial.51 In another study, nebulized glycopyrrolate was well tolerated at 25 and 50 μg BID, as measured by the incidences of AEs and cardiovascular (CV) events.38 Finally, in two chronic-dosing trials of glycopyrrolate pMDI delivered using co-suspension delivery technology, glycopyrrolate had an acceptable safety profile at all doses evaluated, with no unexpected safety findings reported.52,57
Newly prescribed LAMAs and LABAs have been associated with a greater risk of CV events compared with nonuse in patients with COPD (adjusted OR 1.14 [95% CI 1.01–1.28, P=0.03] and 1.31 [95% CI 1.12–1.52, P<0.001] for LAMAs and LABAs, respectively).77 However, the studies discussed in this review provide no evidence for an increased risk of CV events with glycopyrrolate monotherapy vs tiotropium or indacaterol monotherapy.45,50 Furthermore, in one of the trials investigating nebulized glycopyrrolate, fewer patients treated with glycopyrrolate experienced major CV AEs than those treated with tiotropium (0.5% vs 1.7%).51
Safety of inhaled glycopyrrolate combinations
The FDC glycopyrrolate–formoterol is designed to minimize the risk of AEs associated with high doses of LAMA or LABA monotherapies. In its Phase III clinical development program, glycopyrrolate–formoterol pMDI 18/9.6 μg BID showed a safety profile consistent with that of the individual components (PINNACLE-1 and −2), placebo (PINNACLE-1 and −2, PT003011, and PT003012), and open-label tiotropium 18 μg QD (PINNACLE-1 and PT003011).60–62
Furthermore, several clinical trials have indicated that the glycopyrrolate–indacaterol DPI combination at both EMA- and FDA-approved doses (50/110 μg QD and 15.6/27.5 μg QD, respectively) has good tolerability and an acceptable safety profile in patients with moderate–severe COPD.65,67–69,71–73 A trial by Ferguson et al showed that the risk of CV events was similar with the glycopyrrolate–indacaterol FDC to with indacaterol alone.70
Safety end points have also been assessed in triple FDC therapy regimens that incorporate glycopyrrolate. For example, in a study comparing glycopyrrolate plus fluticasone propionate–salmeterol with the ICS–LABA combination administered with either tiotropium 18 μg QD or placebo, the authors reported no significant differences between the number of AEs or severe AEs in any of the treatment groups evaluated.74 In a study comparing the glycopyrrolate–beclomethasone–formoterol FDC with both tiotropium and beclomethasone–formoterol plus tiotropium, AE incidence was similar among treatment groups.76 A further study comparing glycopyrrolate–beclomethasone–formoterol with beclomethasone–formoterol also reported similar rates of AEs between treatment groups, although one serious treatment-emergent AE (atrial fibrillation) occurred in a patient from the triple-FDC group.75
Conclusion
Inhaled glycopyrrolate is a fast-acting, efficacious treatment option for patients with moderate–severe COPD and is available in a variety of doses. It improves lung function, reduces the risk of exacerbations, and alleviates the symptoms of breathlessness, which in turn may explain the improvement seen in patients’ QoL. Glycopyrrolate has comparable effects on lung function to tiotropium, and glycopyrrolate and aclidinium showed more rapid onset of action than tiotropium. Formulations containing inhaled glycopyrrolate are well tolerated and, despite being an anticholinergic, few CV-related events have been reported, with glycopyrrolate showing a similar safety profile to tiotropium. Inhaled glycopyrrolate is thus of value both as monotherapy and for optimizing bronchodilation when used in FDCs with LABAs and ICS–LABAs for maintenance treatment of COPD.
Acknowledgments
We thank Johan Karlberg, MD, PhD, of the Clinical Trial Magnifier Newsletter for some of the information in this manuscript. Medical writing support was provided by Carly Hayes, PhD, of Core (London, UK) and editorial support was provided by Maryam Vahdat, PGDip of Core (London, UK), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca LP (Wilmington, DE, USA). AstraZeneca LP reviewed the manuscript for medical accuracy prior to submission.
Author contributions
DPT and NJG made substantial contributions to the conception of this article, drafted, and critically revised it for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
DPT serves on advisory boards for AstraZeneca, Sunovion, Mylan, and Theravance/Innoviva, and as a speaker for AstraZeneca, Boehringer-Ingelheim, and Sunovion. NJG has no conflicts of interest.
References
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Bethesda, MD: GOLD; 2017. | ||
Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. | ||
Schroeckenstein DC, Bush RK, Chervinsky P, Busse WW. Twelve-hour bronchodilation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolate. J Allergy Clin Immunol. 1988;82(1):115–119. | ||
Walker FB, Kaiser DL, Kowal MB, Suratt PM. Prolonged effect of inhaled glycopyrrolate in asthma. Chest. 1987;91(1):49–51. | ||
Johnson BE, Suratt PM, Gal TJ, Wilhoit SC. Effect of inhaled glycopyrrolate and atropine in asthma: precipitated by exercise and cold air inhalation. Chest. 1984;85(3):325–328. | ||
US Food and Drug Administration. Seebri Neohaler [prescribing Information]. 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207923lbl.pdf. Accessed March 20, 2018. | ||
European Medicines Agency. European public assessment report: Seebri Breezhaler. 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002430/WC500133771.pdf. Accessed March 20, 2018. | ||
Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. | ||
Racké K, Matthiesen S. The airway cholinergic system: physiology and pharmacology. Pulm Pharmacol Ther. 2004;17(4):181–198. | ||
Franko BV, Lunsford CD. Derivatives of 3-pyrrolidinols – III: the chemistry, pharmacology, and toxicology of some N-substituted-3-pyrrolidyl α-substituted phenylacetates. J Med Pharm Chem. 1960;2:523–540. | ||
Barnes PJ. Anticholinergics. In: Celli BR, editor. Pharmacotherapy in Chronic Obstructive Pulmonary Disease. New York: Marcel Dekker; 2004:201–216. | ||
Bartels C, Looby M, Sechaud R, Kaiser G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br J Clin Pharmacol. 2013;76(6):868–879. | ||
Sykes DA, Dowling MR, Leighton-Davies J, et al. The influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012;343(2):520–528. | ||
Kaltiala E, Penttilä A, Vapaatalo H, Larmi T. The fate of intravenous (3H)glycopyrrolate in man. J Pharm Pharmacol. 1974;26(5):352–354. | ||
Sechaud R, Renard D, Zhang-Auberson L, de la Motte S, Drollmann A, Kaiser G. Pharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD). Int J Clin Pharmacol Ther. 2012;50(2):118–128. | ||
Gavaldà A, Ramos I, Carcasona C, et al. The in vitro and in vivo profile of aclidinium bromide in comparison with glycopyrronium bromide. Pulm Pharmacol Ther. 2014;28(2):114–121. | ||
Hansel TT, Barnes PJ. Tiotropium bromide: a novel once-daily anticholinergic bronchodilator for the treatment of COPD. Drugs Today (Barc). 2002;38(9):585–600. | ||
Ogoda M, Niiya R, Koshika T, Yamada S. Comparative characterization of lung muscarinic receptor binding after intratracheal administration of tiotropium, ipratropium, and glycopyrrolate. J Pharmacol Sci. 2011;115(3):374–382. | ||
Rogliani P, Calzetta L, Ora J, et al. Pharmacological assessment of the onset of action of aclidinium and glycopyrronium versus tiotropium in COPD patients and human isolated bronchi. Eur J Pharmacol. 2015;761:383–390. | ||
Sentellas S, Ramos I, Alberti J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39(5):283–290. | ||
Ramos FL, Krahnke JS, Kim V. Clinical issues of mucus accumulation in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:139–150. | ||
Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. | ||
Melloni B, Germouty J. [The influence of a new β-agonist: formoterol on mucociliary function]. Rev Mal Respir. 1992;9(5):503–507. French. | ||
Lindberg S, Khan R, Runer T. The effects of formoterol, a long-acting β2-adrenoceptor agonist, on mucociliary activity. Eur J Pharmacol. 1995;285(3):275–280. | ||
Restrepo RD. Inhaled adrenergics and anticholinergics in obstructive lung disease: do they enhance mucociliary clearance? Respir Care. 2007;52(9):1159–1173. | ||
Tagaya E, Yagi O, Sato A, et al. Effect of tiotropium on mucus hypersecretion and airway clearance in patients with COPD. Pulm Pharmacol Ther. 2016;39:81–84. | ||
Hasani A, Toms N, Agnew JE, Sarno M, Harrison AJ, Dilworth P. The effect of inhaled tiotropium bromide on lung mucociliary clearance in patients with COPD. Chest. 2004;125(5):1726–1734. | ||
Meyer T, Reitmeir P, Brand P, et al. Effects of formoterol and tiotropium bromide on mucus clearance in patients with COPD. Respir Med. 2011;105(6):900–906. | ||
Bennett WD, Chapman WF, Mascarella JM. The acute effect of ipratropium bromide bronchodilator therapy on cough clearance in COPD. Chest. 1993;103(2):488–495. | ||
Hugel H, Ellershaw J, Gambles M. Respiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromide. J Palliat Med. 2006;9(2):279–284. | ||
US Food and Drug Administration. Medical review: glycopyrrolate. 2009. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022571Orig1s000MedR.pdf. Accessed March 20, 2018. | ||
Mirakhur RK, Dundee JW, Jones CJ. Evaluation of the anticholinergic actions of glycopyrronium bromide. Br J Clin Pharmacol. 1978;5(1):77–84. | ||
European Medicines Agency. Seebri Breezhaler [summary of product characteristics]. 2012. Available from: https://www.medicines.org.uk/emc/medicine/27138. Accessed March 20, 2018. | ||
AstraZeneca Pharmaceuticals LP. Bevespi Aerosphere [prescribing information]. 2016. Available from: https://www.azpicentral.com/bevespi/bevespi_pi.pdf. Accessed March 20, 2018. | ||
European Medicines Agency. Ultibro Breezhaler [summary of product characteristics]. 2016. Available from: https://www.medicines.org.uk/emc/medicine/29533. Accessed March 20, 2018. | ||
US Food and Drug Administration. Utibron Neohaler [prescribing information]. 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207930s000lbl.pdf. Accessed March 20, 2018. | ||
European Medicines Agency. Trimbow [summary of product characteristics]. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004257/WC500233163.pdf. Accessed March 20, 2018. | ||
Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. | ||
European Medicines Agency. Trimbow [initial authorization]. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004257/WC500228080.pdf. Accessed March 20, 2018. | ||
Arievich H, Overend T, Renard D, et al. A novel model-based approach for dose determination of glycopyrronium bromide in COPD. BMC Pulm Med. 2012;12:74. | ||
D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. | ||
Kerwin E, Hébert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–1114. | ||
Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503–513. | ||
Sekiya M, Kawayama T, Fukuchi Y, et al. Safety and efficacy of NVA237 once daily in Japanese patients: the GLOW4 trial. Eur Respir J. 2012;40 (Suppl 56):P2013. | ||
Chapman KR, Beeh KM, Beier J, et al. A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study. BMC Pulm Med. 2014;14:4. | ||
Wang C, Sun T, Huang Y, et al. Efficacy and safety of once-daily glycopyrronium in predominantly Chinese patients with moderate-to-severe chronic obstructive pulmonary disease: the GLOW7 study. Int J Chron Obstruct Pulmon Dis. 2015;10:57–68. | ||
Marin JM, Beeh KM, Clemens A, et al. Early bronchodilator action of glycopyrronium versus tiotropium in moderate-to-severe COPD patients: a cross-over blinded randomized study (Symptoms and Pulmonary Function in the Morning). Int J Chron Obstruct Pulmon Dis. 2016;11:1425–1434. | ||
LaForce C, Feldman G, Spangenthal S, et al. Efficacy and safety of twice-daily glycopyrrolate in patients with stable, symptomatic COPD with moderate-to-severe airflow limitation: the GEM1 study. Int J Chron Obstruct Pulmon Dis. 2016;11:1233–1243. | ||
Kerwin E, Siler TM, Korenblat P, et al. Efficacy and safety of twice-daily glycopyrrolate versus placebo in patients with COPD: the GEM2 study. Chronic Obstr Pulm Dis. 2016;3(2):549–559. | ||
Mahler DA, Gifford AH, Satti A, et al. Long-term safety of glycopyrrolate: a randomized study in patients with moderate-to-severe COPD (GEM3). Respir Med. 2016;115:39–45. | ||
Ferguson GT, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260. | ||
Fabbri LM, Kerwin EM, Spangenthal S, et al. Dose-response to inhaled glycopyrrolate delivered with a novel Co-Suspension Delivery Technology metered dose inhaler (MDI) in patients with moderate-to-severe COPD. Respir Res. 2016;17(1):109. | ||
Karabis A, Lindner L, Mocarski M, Huisman E, Greening A. Comparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:405–423. | ||
Ismaila AS, Huisman EL, Punekar YS, Karabis A. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:2495–2517. | ||
Lechuga-Ballesteros D, Noga B, Vehring R, Cummings RH, Dwivedi SK. Novel cosuspension metered-dose inhalers for the combination therapy of chronic obstructive pulmonary disease and asthma. Future Med Chem. 2011;3(13):1703–1718. | ||
Vehring R, Lechuga-Ballesteros D, Joshi V, Noga B, Dwivedi SK. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28(42):15015–15023. | ||
Kerwin EM, Spangenthal S, Kollar C, St Rose E, Reisner C. A phase IIB randomized, chronic-dosing, incomplete block, cross-over study of glycopyrronium, delivered via metered dose inhaler, compared with a placebo and an active control in patients with moderate-to-severe COPD. Respir Res. 2018;19(1):38. | ||
Cazzola M, Matera MG. The effective treatment of COPD: anticholinergics and what else? Drug Discov Today Ther Strateg. 2006;3(3):277–286. | ||
Barisione G, Baroffio M, Crimi E, Brusasco V. Beta-adrenergic agonists. Pharmaceuticals (Basel). 2010;3(4):1016–1044. | ||
Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using Co-Suspension Delivery Technology in patients with COPD. Chest. 2017;151(2):340–357. | ||
Hanania NA, Tashkin DP, Kerwin EM, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension Delivery Technology in patients with chronic obstructive pulmonary disease. Respir Med. 2017;126:105–115. | ||
Reisner C, Gottschlich G, Fakih F, et al. 24-h bronchodilation and inspiratory capacity improvements with glycopyrrolate/formoterol fumarate via co-suspension delivery technology in COPD. Respir Res. 2017;18:157. | ||
Martinez FJ, Fabbri LM, Ferguson GT, et al. Baseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPD. Chest. 2017;152(6):1169–1178. | ||
Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. | ||
Kardos P, Hagedorn-Peinz I. The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study: the FAVOR study. Int J Chron Obstruct Pulmon Dis. 2018;13:69–77. | ||
Buhl R, Gessner C, Schuermann W, et al. Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study. Thorax. 2015;70(4):311–319. | ||
Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. | ||
Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026. | ||
Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–1079. | ||
Ferguson GT, Taylor AF, Thach C, et al. Long-term maintenance bronchodilation with indacaterol/glycopyrrolate versus indacaterol in moderate-to-severe COPD patients: the FLIGHT 3 study. Chronic Obstr Pulm Dis. 2016;3(4):716–728. | ||
Kerwin E, Ferguson GT, Sanjar S, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung. 2017;195(6):739–747. | ||
Vogelmeier C, Zhong N, Humphries MJ, et al. Indacaterol/glycopyrronium in symptomatic patients with COPD (GOLD B and GOLD D) versus salmeterol/fluticasone: ILLUMINATE/LANTERN pooled analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3189–3197. | ||
Watz H, Mailänder C, Baier M, Kirsten A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (the MOVE study). BMC Pulm Med. 2016;16(1):95. | ||
Frith PA, Thompson PJ, Ratnavadivel R, et al. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trial. Thorax. 2015;70(6):519–527. | ||
Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. | ||
Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. | ||
Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175–1185. | ||
US Food and Drug Administration. Lonhala Magnair [prescribing information]. 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208437lbl.pdf. Accessed March 20, 2018. | ||
Boehringer Ingelheim. Spiriva HandiHaler [prescribing information]. 2016; Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva/Spiriva.pdf. Accessed March 20, 2018. | ||
Boehringer Ingelheim. Spiriva Respimat [prescribing information]. 2017. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf. Accessed March 20, 2018. | ||
Boehringer Ingelheim. Stiolto Respimat [prescribing information]. 2016. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Stiolto%20Respimat/stiolto.pdf. Accessed March 20, 2018. | ||
European Medicines Agency. Spiolto Respimat [summary of product characteristics]. 2015. Available from: https://www.medicines.org.uk/emc/medicine/30495. Accessed March 20, 2018. | ||
European Medicines Agency. Incruse [summary of product characteristics]. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002809/WC500167430.pdf. Accessed March 20, 2018. | ||
GlaxoSmithKline. Incruse Ellipta [prescribing information]. 2017. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Incruse_Ellipta/pdf/INCRUSE-ELLIPTA-PI-PIL.PDF. Accessed March 20, 2018. | ||
European Medicines Agency. Anoro [summary of product characteristics]. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002751/WC500168424.pdf. Accessed March 20, 2018. | ||
GlaxoSmithKline. Anoro Ellipta [prescribing information]. 2013. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203975s000lbl.pdf. Accessed March 20, 2018. | ||
GlaxoSmithKline. Trelegy Ellipta [prescribing information]. 2017. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Trelegy/pdf/TRELEGY-PI-MG-IFU.PDF. Accessed March 20, 2018. | ||
European Medicines Agency. Eklira Genuair [summary of product characteristics]. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002211/WC500132661.pdf. Accessed March 20, 2018. | ||
AstraZeneca Pharmaceuticals LP. Tudorza Pressair [prescribing information]. 2012. Available from: http://www.azpicentral.com/tudorza/pi_tudorza.pdf. Accessed March 20, 2018. | ||
European Medicines Agency. Bretaris Genuair [summary of product characteristics]. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002706/WC500132732.pdf. Accessed March 20, 2018. | ||
European Medicines Agency. Duaklir Genuair [summary of product characteristics]. 2017. Available from: https://ec.europa.eu/health/documents/community-register/2014/20141119130022/anx_130022_en.pdf. Accessed March 20, 2018. | ||
Theravance Biopharma. Revefenacin peak inspiratory flow rate (PIFR) study in COPD. Available from: https://clinicaltrials.gov/ct2/show/NCT03095456. NLM identifier: NCT03095456. Accessed March 20, 2018. | ||
Theravance BioPharma. Theravance Biopharma and Mylan submit new drug application to FDA for revefenacin (TD-4208) in adults with chronic obstructive pulmonary disease. 2017. Available from: http://investor.theravance.com/news-releases/news-release-details/theravance-biopharma-and-mylan-submit-new-drug-application-fda. Accessed March 20, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



