Back to Journals » Open Access Surgery » Volume 8
Infrared parietal colorectal flowmetry: a new application of the pulse oximeter. Is this method useful for general surgeons in preventing anastomotic leakage after colorectal resections?
Authors Delfrate R , Bricchi M, Forti P, Franceschi C
Received 1 February 2015
Accepted for publication 31 March 2015
Published 18 June 2015 Volume 2015:8 Pages 61—65
DOI https://doi.org/10.2147/OAS.S81138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Cataldo Doria
Video abstract presented by Roberto Delfrate
Views: 266
Roberto Delfrate,1 Massimo Bricchi,1 Paolo Forti,1 Claude Franceschi2
1Surgery Unit, Figlie Di San Camillo Hospital, Cremona, Italy; 2Vascular Exploration Service, St Joseph Hospital, Paris, France
Background: Anastomotic leak is a major complication of colorectal surgery. Among the causes of dehiscence, anastomotic ischemia seems to be fundamental and consequently so is the evaluation of the parietal flow. We proposed a new application of infrared flowmeter for the evaluation of the parietal flow at the stumps after colon resection.
Objective: The aim of this study is to assess the feasibility of using an intraoperative intestinal wall flowmeter to assess arterial capillary flow in order to avoid the execution of anastomoses in poorly vascularized segments of bowel, and consequently to reduce the risk of anastomotic leakage.
Methods: Retrospective analysis of two groups of patients with different methods of evaluation of colon resection stump vascularization. Ninety-two consecutive patients (Group A) underwent surgical colorectal resection for cancer. In this group, we used a pulse-oximetry sensor to assess the parietal flow: once the magnitude of the colon resection was established according to surgical and oncological criteria, the exact location of the resection was adjusted according to the parietal flowmetry curve. This method was compared with 139 consecutive colorectal resections (Group B) in which vascularization was assessed by checking the pulsatility of the mesenteric arteries, macroscopic wall resection stump appearance, and bleeding of the wall stump. The main outcome measure was the reduction in anastomotic dehiscence.
Results: In Group A no anastomotic leakage occurred (0/92). Conversely, in Group B six anastomotic leaks occurred (6/139). The statistical analysis of the two groups thanks to the Fisher's exact test shows that P<0.05, which is statistically significant.
Conclusion: We tested a new application of the pulse oximeter: the evaluation of the colon parietal flow (infrared parietal flowmeter). The infrared parietal flowmetry appears to be a feasible, simple, and low-cost method, able to detect the vascularization of the large bowel stump; for this reason this procedure appears to be useful in order to avoid a colon anastomosis of two poorly vascularized bowel stumps, thus reducing the risk of anastomotic leakage. Despite the positive results of our experience in the assessment of the intestine vascularization with the intraoperative infrared stump flowmeter, the possibility of reducing the number of anastomotic leaks through this method requires additional and more extensive trials.
Keywords: anastomotic leak, colon resection, flowmetry
Introduction
Anastomotic dehiscence is the most severe surgical complication after a large bowel resection. Despite the increased use of staples, the incidence of anastomotic leaks is approximately 4.25%–16%1–4 while the mortality rate resulting from anastomotic dehiscence and related clinical sequelae is between 10% and 39%.5,6 The elevated range of mortality is determined by the multiple etiologies and risk factors reported in the literature. Among the general factors, we must consider body mass index, advanced age, malnutrition, pneumological and cardiovascular disease, steroid and radiation treatments, while the more specific factors with more severe prognoses are sepsis, emergency surgery on a colon carcinoma at the site of the anastomosis,7,8 extended intraoperative hypotension and operative time.9 What all of these different pathological conditions might have in common is a lack of tissue perfusion causing a critical ischemia of the stumps after the resection. Indeed, the anastomotic dehiscence and necrosis of the sutured tissues could be the consequence of an impaired arterial vascularization that affects the metabolic state of the tissues due to an inadequate intake of nutrients10 and reduction of the venous capillary transmural pressure with impairment of the venous drainage at the stump.11 Therefore, considering proper vascularization as a key element in the prevention of anastomotic dehiscence, we wanted to find a simple and reliable method for checking arterial capillary perfusion. The purpose of this study is to evaluate the potential interest of intraoperative infrared flowmetry of the intestinal wall in order to determine a suitable site for the anastomosis.
Methods
Between January 2007 and September 2014, 231 consecutive colorectal resections for cancer were performed by the same surgical team, without changes in the surgical technique between the two groups of patients operated on, and without introducing new techniques or technologies; thus nothing changed in the two groups in suture technique whether performed by hand or mechanically. Anastomoses were performed either sewn by hand or with mechanical staplers: manual anastomosis was generally performed after a right hemicolectomy, or a segmental resection of the transverse colon or splenic flexure. The hand-sewn anastomoses were performed with separate stitches using a slow-absorption suture material. ASA 4 (The American Society of Anesthesiologists physical status classification system) patients were excluded, and also bowel obstruction surgery or emergency surgery for perforated colon patients. The average age was similar in the two groups: 71.5 years in Group B, 71 years in Group A. Fifty percent was male and 50% female in Group B. Fifty-three point two percent was male and 46.7% female in Group A. None of the patients had radiotherapy or chemotherapy before surgery. The only difference between the two groups of patients is represented by the evaluation of the parietal stump flow thanks to a pulse oximeter. Between January 2007 and January 2011, 139 colon and rectal resections for cancer patients were performed (Group B). Before performing the anastomosis, the vascularization of the stumps was assessed by detecting the pulse of the mesentery arteries close to the stump of the resection using the fingers, sometimes hampered by thick mesenteric fat, and also by the macroscopic appearance of the colonic wall, especially the color and the bleeding of the bowel wall at the stump site. Since February 2011 parietal stump flow was checked in 92 consecutive procedures (Group A), using an infrared pulse-oximeter to evaluate capillary flow at the stump site. The flowmetry was first performed in a segment of small bowel used as a healthy track basis and was then performed, after ligation or simple clamping of the vessels feeding the segment of colon to be resected on the basis of the need for a correct resection, at the resection site. After resection, we did a further flowmetry control of both the stumps. Flowmetry was performed by using a disposable pulse-oximetry sensor with a disposable laparoscopic camera cover (Figure 1) with the aim of recording the parietal flow curve (Covidien Nelcor “OxiMax™ Max-Fast®” Forehead Sensor). First, the presence of a curve on the flow diagram was assessed; second, the slope angle of the flow curve, a direct consequence of the speed of the flow, and lastly, the amplitude of the curve, expression of the pressure. The anastomosis was performed only after the evaluation of a correct flow curve. If necessary we changed the resection site until we detected the presence of a suitable flowmetry curve.
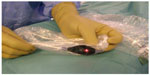  | Figure 1 Pulse-oximetry sensor (Covidien Nelcor “OxiMax™ Max-Fast®” Forehead Sensor) in a disposable laparoscopic camera cover. |
Results
Among the 139 operations performed in Group B, six clinically evident anastomotic leaks occurred (4.3%), three after a left hemicolectomy, two after a right hemicolectomy, and one after an anterior resection of the rectum. The remaining 133 patients were discharged between 8 and 10 days after surgery without any complications. In Group A, no clinically evident anastomotic leakages occurred (0/92) (Figure 2). In these series of 92 consecutive colon resections, in four cases of left hemicolectomy and two of right hemi colectomy (6.5%) the site of the stump was modified (5–10 cm), despite the appearance of a trophic wall, according to flowmetry. In these cases the extension of the resection was increased until the curve of the parietal flowmetry showed a correct vascularization of the wall. All 92 patients were discharged 8–10 postoperative days later without any complications.
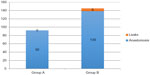  | Figure 2 Group A: flowmetry evaluation; Group B: no flowmetry evaluation. |
Statistical analysis
A statistical evaluation of the outcomes of the two groups of patients has been done comparing the number of anastomotic leaks. The Fisher’s exact test was used to compare results. The statistical analysis shows that P<0.05, which is statistically significant.
Discussion
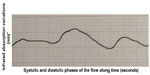
It is known that the blood supply of the stumps is a key element in reducing the incidence of leakage. The arterial circulatory system provides vital oxygen and nutrients to the tissues. The blood pressure must be high enough to allow the systolic wave to reach and feed the tissues. That flow can be assessed by detecting the blood systolic and diastolic volume at the capillary level, as evoked by infrared plethysmography. The pulse oximeter gives us two pieces of information. The first is the oxygenated hemoglobin (HbO²) rate provided by its selective absorbance compared with the reduced hemoglobin (infrared spectrometry). The other is the curve that represents capillary flow. That curve results from the variation of the oxygenated and reduced hemoglobin amount, ie, the blood volume: infrared plethysmography (infrared plethysmography represents the infrared spectrometry variations over time).12,13 Therefore, this curve is the expression of arterial capillary flow and the amplitude of the curve is the expression of the blood volume variations and, consequently, of the pressure in the parietal vessels. Information about the hemodynamic condition (pressure and speed) in the capillary flow is the main difference between the flowmetry parietal evaluation we propose and the fluorescence angiography with indocyanine green.14,15 The pulse oximeter is one of the most sensitive noninvasive monitors of oxygenation and tissue perfusion. For that reason, over the years, various oximeters have been created, suitable for monitoring different anatomical sites, from the finger to the skin of the forehead.16–18 For this reason we started using pulse oximetry, because in our opinion it could also be valuable for the study of capillary perfusion of the colon wall. We registered different types of curves: a normal one that shows a good blood supply (Figure 3), a curve with reduced amplitude that shows an arterial vascularization that is still sufficient (Figure 4), and a third type which shows a clearly insufficient blood supply, at risk for ischemia (Figure 5), and even cases of the absence of variation of blood volume between the systolic and diastolic phases which imply a very critical loss of perfusion.
  | Figure 3 Perfect flowmetry curve. |
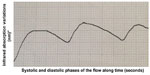  | Figure 4 Still sufficient parietal flow. |
Conclusion
The use of a flowmeter with sterile transparent cover is a valuable, economic, and quick method for assessing the presence and quality of vascularization of the stumps. We found no contraindications and no problems arising from the use of an infrared flowmeter. Regarding the cases of damped infrared curve, flowmetry has proved to be an extremely sensitive method capable of detecting any parietal flow variation. Even if used in a heterogeneous group of patients, this study has shown the feasibility, and potential utility of using an intraoperative flowmeter to identify the safest location for the anastomosis, thereby reducing the risk of dehiscence, without any increase in costs or significant loss of time. The statistical analysis (Fisher’s exact test) shows a need for P<0.05, which is statistically significant. At the moment, the limitations of the method seem to be the absence of a pulse oximeter for laparoscopic procedures. Despite the favorable outcome of this study, the infrared-flowmetry method must be checked in further randomized trials.
Disclosure
The authors have no conflicts of interest to disclose.
References
Erdas E, Zedda A, Pitzalis A, et al. La deiscenza anastomotica in chirurgia colorettale Incidenza, fattori di rischio e trattamento. [Anastomotic leak following colorectal surgery: incidence, risk factors and treatment]. Chir Ital. 2009;61(4):407–441. Italian. | |
Di Matteo G, Cancrini A Jr. Principi e tecniche nella chirurgia per cancro del retto sottoperitoneale. Incidenti, complicazioni, sequele ed esiti: descrizione e trattamento. [Principles and techniques of surgery for subperitoneal rectal cancer: Complications of surgery]. Relazione Biennale. Collana monografica della Società Italiana di Chirurgia. 1995;3:333–350. Italian. | |
Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148(2):177–182. | |
Köckerling F, Rose J, Schneider C, et al. Laparoscopic colorectal anastomosis: risk of postoperative leakage. Results of a multicenter study. Laparoscopic Colorectal Surgery Study Group (LCSSG). Surg Endosc. 1999;13(7):639–644. | |
Efron EJ, Vernava III AM. Reoperative surgery for acute colorectal anastomotic dehiscence and persistent abdominalsepsi. In: Longo WE, Northover JM, editors. Reoperative Colon and Rectal Surgery. London: Martin Duniz Ltd; 2003:1–26. | |
den Dulk M, Noter SL, Hendriks ER, et al. Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol. 2009;35(4):420–426. | |
Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large bowel cancer: a multicentre study. Br Med J. 1980;281(6237):411–414. | |
Basilico V, Griffa B, Castiglione N, Giacci F, Zanardo M, Griffa A. Le fistole anastomotiche dopo resezioni coliche e rettali per neoplasia. Incidenza e trattamento nella nostra esperienza recente [Anastomotic fistulas after colorectal resection for carcinoma: incidence and treatment in our recent experience]. Minerva Chir. 2006;61(5):373–380. Italian. | |
Manilich E, Vogel JD, Kiran RP, Church JM, Seyidova-Khoshknabi D, Remzi FH. Key factors associated with postoperative complications in patients undergoing colorectal surgery. Dis Colon Rectum. 2013; 56(1):64–71. | |
Frasson M, Braga M, Vignali A, et al. Laparoscopic-assisted versus open surgery for colorectal cancer: postoperative morbidity in a single center randomized trial. Minerva Chir. 2006;61(4):283–292. | |
Delfrate R. A new diagnostic approach to varicose veins: haemodynamic evaluation and treatment. Lorena Dioni Publisher; 2014:31–33. | |
Barker SJ, Tremper KK. Pulse oximetry: applications and limitations. Int Anesthesiol Clin. 1987;25(3):155–175. | |
Wukitsch MW, Petterson MT, Tobler DR, Pologe JA. Pulse oximetry: analysis of theory, technology and practice. J Clin Monit. 1988;4(4):290–301. | |
Kudszus S, Roesel C, Schachtrupp A, Höer JJ. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg. 2010;395(8):1025–1030. | |
Hellan M, Spinoglio G, pigazzi A, Lagares-Garcia JA. The influence of fluorescence imagin on the location of bowel transection during robotic left-side colorectal surgery. Surg Endosc. 2014;28(5):1695–1702. | |
Cheng EY, Hopwood MB, Kay J. Forehead pulse oximetry compared with finger pulse oximetry and arterial blood gas measurement. J Clin Monit. 1988;4(3):223–226. | |
Cortinez L, MacLeod D, Wright D, Cameron D, Moretti E. Assessment of vasoactivity at different sites using oximeter’s plethismograph. Anesthesiology. 2003;99:A-560. | |
Branson R, Davis B, Davis K, et al. Comparison of forehead and finger oximetry in mechanically ventilated patients with poor perfusion. Respir Care. 2003;48:1086. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.