Back to Journals » Cancer Management and Research » Volume 12
Influence of Programmed Death Ligand-1-Gene Polymorphism rs822336 on the Prognosis and Safety of Postoperative Patients with NSCLC Who Received Platinum-Based Adjuvant Chemotherapy
Authors Zhao M, Zhang J, Chen S , Wang Y, Tian Q
Received 23 March 2020
Accepted for publication 26 June 2020
Published 4 August 2020 Volume 2020:12 Pages 6755—6766
DOI https://doi.org/10.2147/CMAR.S255072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Ming Zhao,1,* Jing Zhang,1,* Siyu Chen,2,* Yuqi Wang,1 Qing Tian1
1Department of Thoracic Surgery, The General Hospital of the People’s Liberation Army, Beijing, 100853, People’s Republic of China; 2Department of Thoracic Surgery, The Sixth Medical Center of PLA General Hospital, Beijing, 100048, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuqi Wang
Department of Thoracic Surgery, The General Hospital of the People’s Liberation Army, 28 Fuxing Road, Haidian District, Beijing, People’s Republic of China
Tel +86 136-0127-9155
Email [email protected]
Qing Tian Email [email protected]
Purpose: This study was done to investigate the influence of PDL1-gene polymorphism on the prognosis and safety of postoperative patients with non–small cell lung cancer (NSCLC) who had received platinum-based adjuvant chemotherapy.
Methods: A total of 289 postoperative patients with NSCLC who had received platinum-based adjuvant chemotherapy from January 2012 to June 2019 participated in this study. Recurrence status and adverse reactions were documented during adjuvant chemotherapy. Overall survival (OS) data were obtained through telephone follow-up. DNA extracted from hematologic specimens was genotyped for PDL1-gene polymorphism. Associations between genotype status and prognosis were assessed using Kaplan–Meier survival analysis, and multivariate adjustment was performed using Cox regression analysis.
Results: Median disease-free survival of the 289 patients with NSCLC was 3.3 years and median OS 4.9 years. With regard to the PDL1 gene polymorphism, only rs822336 was of clinical significance in the subsequent analysis. The minor-allele frequency of rs822336 was 0.21, and distribution of the three genotypes was in accordance with the Hardy–Weinberg equilibrium (P=0.807). Survival analysis according to genotype status suggested that median disease-free survival of patients with GG and GC/CC genotypes was 2.8 and 4.1 years, respectively (P=0.01). Median OS of patients with GG and GC/CC genotypes was 4.1 and 5.4 years, respectively (P=0.008). However, the safety analysis failed to find a significant association between the polymorphism and adverse reactions. Interestingly, expression analysis of RNA extracted from peripheral blood mononuclear cells indicated that PDL1-mRNA expression of patients with the GG genotype was significantly higher than for the GC/CC genotype (P< 0.001).
Conclusion: The prognosis of postoperative patients with NSCLC who have received platinum-based adjuvant chemotherapy may be influenced by the rs822336 polymorphism through mediation of the mRNA expression of PDL1.
Keywords: non–small cell lung cancer, adjuvant chemotherapy, PDL1, polymorphism, prognosis
Background
Lung cancer is the most common malignancy and the leading cause of cancer-related death worldwide, accounting for approximately 2.1 million new cases and 1.77 million new deaths globally.1 Currently, there are about 0.733 million new cases and 0.61 million new deaths from lung cancer in China annually.2 Non–small cell lung cancer (NSCLC) is the most common category in lung cancer — 80–85%.3 Recent years have witnessed great progress in the treatment of NSCLC with molecular and targeted drugs, rendering advanced NSCLC the most successfully treated type of cancer in precision medicine.4 Drugs targeting EGFR, ALK, ROS1, and BRAF and other positive driver genes have provided significant survival benefits for the patients.5 Studies have suggested that approximately 80% of patients with NSCLC were diagnosed with advanced or metastatic disease.6 In consequence, only 20% had the opportunity to receive surgical resection.7 Therefore, platinum-based regimens became the standard of care for stage II and III NSCLC patientsas postoperative adjuvant treatment, which has proven to improve the 5-year survival rate by 5.3%.8
Initially, a regimen of cisplatin combined with vinorelbine was widely used as adjuvant therapy in NSCLC. However, a considerable number patients experienced adverse hematologic reactions, resulting in poor compliance and relatively worse completion cycles for adjuvant chemotherapy.9 Platinum combinations with pemetrexed and docetaxel regimens have proven to be safe, and have subsequently been widely used as adjuvant chemotherapy.10,11 Five-year overall survival (OS) rates ranged 36%–66% for stage IB and IIIA NSCLC patients with when receiving platinum-based adjuvant chemotherapy.12 In addition to pathological staging, which serves as the most important prognostic indicator, several factors are of clinical significance for the evaluation of efficacy of platinum-based adjuvant chemotherapy and prognosis, including gene polymorphisms.13 Furthermore, the licensing of nivolumab and pembrolizumab in mainland Chinabrought new options for patients with advanced NSCLC.14PDL1 is one of the most important biomarkers for PD1/PDL1 inhibitors, and indications are that the higher the expression level of PDL1, the better the efficacy of PD1/PDL1 inhibitors.15 To our knowledge, the PDL1 protein is mainly expressed on surfaces of tumor cells and binds to PD1 on the surfaces of activated T cells. In this process, T-cell activity is inhibited and T-cell apoptosis induced, thus contributing to the immuno escape of tumor cells.16 Although patients with high PDL1 expression could benefit from the of PD1/PDL1-inhibitor therapy, the clinical significance of PDL1-expression status in the prognosis of patients with NSCLC who have received platinum-based adjuvant chemotherapy remains unknown.
The PDL1 gene is located on chromosome 9p24.1, contains eight exons, and shows large ethnic differences.17 As a member of the PDL family, PDL1 is not conservative in the Chinese population, and its expression of PDL1 demonstrates great individual differences.18 Xie et al explored the clinical significance of polymorphism 901A>G in PDL1 in patients with hepatocellular carcinoma.19 The results suggested that 901A>G was significantly correlated with susceptibility to and prognosis of hepatocellular carcinoma. Furthermore, recent research by Krawczyk et al investigated the PDL1-promoter polymorphism and PDL1 expression in tumor cells. The results demonstrated that rs822335 might be a predictor of PDL1-protein expression in tumor cells.20 However, there has been no comprehensive research focusing on the association between PDL1-gene polymorphisms and prognosis of patients with NSCLC who have received platinum-based adjuvant chemotherapy in the Chinese population.
Therefore, the present study was done to investigate the influence of PDL1-gene polymorphisms on the prognosis and safety of postoperative patients with NSCLC who have received platinum-based adjuvant chemotherapy in the real world. Additionally, the influence of polymorphisms on PDL1-mRNA expression in peripheral blood mononuclear cell (PBMC) specimens was also performed to gain insight on detailed mechanisms.
Methods
Patients
Given that a considerable number of patients with NSCLC are treated with platinum-based adjuvant treatment clinically, this study was designed as a single-center retrospective analysis. Patients with NSCLC who had received surgical resection in the Department of Thoracic Surgery of the General Hospital of the People’s Liberation Army from January 2012 to June 2019 were included continuously in this study. Patients who fulfilled the following eligibility criteria were enrolled: age 18–80 years; Eastern Cooperative Oncology Group (ECOG) performance score 0–2; normal cardiac function (left ventricular ejection fraction >50%); normal renal and bone-marrow function before adjuvant chemotherapy; histological diagnosis of NSCLC; surgical resection having been performed (from January 2012 to December 2017, the seventh edition of the AJCC Cancer Staging Manual was used; from January 2018 to June 2019, the eighth edition was used for TNM classification); and platinum-based adjuvant treatment having been administered. Exclusion criteria were with other tumors or serious diseases; diagnosed with SCLC; had not received surgical resection or platinum-based adjuvant chemotherapy; and adjuvant radiotherapy or adjuvant chemoradiotherapy after surgical resection having been administered. Smoking status of the patients was categorized into never-smokers (no) and ever-smokers (yes). A never-smoker was defined as someone who had smoked fewer than 100 cigarettes in their life. Ever-smokers comprised current (smoking within past 6 months) and former (cessation at least 6 months prior to study) smokers.21
A flowchart for this retrospective study is given in Figure 1. Finally, a total of 289 patients with NSCLC were included in this study. The primary end point was disease-free survival (DFS), and the secondary end point was OS, with analysis of safety and PDL1 polymorphisms. This study was approved by the ethics committee of the General Hospital of the People’s Liberation Army. Informed consent was signed by each enrolled patient or their family members in accordance with the recommendations of the Dclaration of Helsinki. Only in circumstances that patients had passed away was written informed consent obtained from family members.
 |
Figure 1 Flowchart of this retrospective study of 289 postoperative patients with NSCLC who had received platinum-based adjuvant chemotherapy. |
All the 289 patients with NSCLC had been administered platinum-based adjuvant chemotherapy 4–6 weeks after surgery according to their physical status. Patients had been given cisplatin 75 mg/m2 or carboplatin AUC5 combined with pemetrexed 500 mg/m2 (non–squamous cell carcinoma), docetaxel 60–75 mg/m2, or vinorelbine 25–30 mg/m2 on the first day and every 21 days as one course. Adjuvant chemotherapy was given for four courses or depended on the actual situation of the patient. Adverse reactions during treatment were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) 4.03 to document hematologic and nonhematologic reactions that might be drug-related.22 Grade ≥2 adverse reactions of incidence ≥10% were recorded and analyzed.
Follow-Up
Each patient was followed up from the administration of adjuvant chemotherapy. The initial follow-up was performed while the patient was receiving adjuvant chemotherapy in the hospital, where baseline characteristics, adverse reactions, and recurrence status could be clearly obtained through the electronic medical record system. The subsequent follow-up was performed mainly using telephone. Patients were followed up every 3 months for date of recurrence, treatment after recurrence, and death status. For quality control, when the disease status of the patient was recurrence or death, it had to be be confirmed by at least two research colleagues before it could be confirmed as such. A total of 16 patients were lost to follow-up during the study (5.54%).
Collection of Peripheral Blood Specimens and Genotyping for PDL1-Gene Polymorphisms
Peripheral blood specimens (approximately 4 mL) from each enrolled patient were collected before adjuvant chemotherapy and genomic DNA extracted using phenol chloroform according to the standard operating procedure and stored at −20°C. With regard to the PDL1 gene–polymorphism analysis, single-nucleotide polymorphisms included in this study (in the NCBI database) were those with a minor-allele frequency (MAF) >10% in the Chinese population: rs2297136, rs822336, and rs822337. As shown in Table 1, of the three polymorphisms analyzed, only rs822336 was significantly associated with DFS. Therefore, subsequent analysis of this study was focused on this polymorphism. rs822336 was genotyped using restriction fragment–length polymorphism polymerase chain reaction (PCR). The PCR product of this polymorphism was amplified, with forward primer 5ʹ-CTGTTTTTCAATCTCCGGGTA−3ʹ and reverse primer 5ʹ-TGAAAGCAGTGTTCAGGGTCT−3ʹ. The product of PCR was 228 bp, and 2 μL PCR products was digested using the restriction enzyme ApaI (Thermo Fisher Scientific, Waltham, MA, USA). The genotype status of the polymorphism was determined by the size of the PCR bands: GG genotype — one 228 bp band; GC genotype — one 228 bp band, one 163 bp band, and one 65 bp band; and CC genotype — one 163 bp band and one 65 bp band.
 |
Table 1 Preliminary analysis of genotype status of the three polymorphisms and DFS |
Collection of Peripheral Blood Mononuclear Cells and Analysis of PDL1-mRNA Expression
PBMC specimens were collected from 105 randomly selected samples of the NSCLC patients initially. However, nine PBMC specimens were not available and ten patients failed for RNA extraction. Finally, 86 patient samples were available for further analysis and preserved in liquid nitrogen. Total RNA samples were extracted using Trizol reagents (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions and stored at −80°C for mRNA-expression analysis. A total of 500 ng RNA extracted from the PBMC specimens was used as the templates for reverse-transcription PCR to prepare the first stand of cDNA with a PrimeScript RT reagent kit (Bao Biological Engineering). Relative quantitative analysis of PDL1-mRNA expression was carried out using a LightCycler 480 (Roche, Shanghai, China) using an SYBR Premix EX Taq system (Bao Biological Engineering). The forward primer of PDL1 was 5ʹ-TTCAATGTGACCAGCACACTGAG-3ʹ, and the reverse primer was 5ʹ-TTTTCACATCCATCATTCTCCCT-3ʹ. The amplification system was comprised of a 20 µL containing 10 µL SYBR Premix EX Taq, 0.2 µL of each primer (20 µM), 7.6 µL double-distilled water and 2 µL cDNA. PDL1-mRNA expression was indicated by comparative Ct (2–ΔΔCt) with GAPDH-mRNA expression as an endogenous control.
Statistical Analysis
All data in this study were analyzed using SPSS version 25.0. With regard to genotype status of polymorphisms, the Hardy–Weinberg equilibrium test was used for the rs822336 genotypes using χ2. The significance of proportion variables was also tested using χ2. Analysis between continuous variables and rs822336-genotype status was assessed with the Mann–Whitney U nonparametric test (between the two groups). Kaplan–Meier curves were drawn using Stata 14.0 to compare differences in DFS and OS according to genotype status, and survival differences were compared using log-rank testing. DFS was defined as the period from the initiation of adjuvant chemotherapy treatment to the date of recurrence or death from any cause, whichever occurred first. OS was defined as the interval between the initiation of adjuvant chemotherapy and death from any cause. For those without recurrence or death by the end of follow-up, survival end points were censored at the date of last follow-up.23 A Cox proportional-hazard model was constructed for OS in multivariate analysis. Backward selection was used to adjust potential confounding covariate. P<0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients with NSCLC and Genotype Results of rs822336 Polymorphism
Baseline characteristics of the 289 patients with NSCLC are shown in Table 2. The median age was 61 years old (range: 28–80 years old). A total of 205 patients were male (70.93%). An ECOG 0 score was observed in 191 patients (66.09%). Ever-smokers numbered 194 patients, (67.13%), adenocarcinoma was reported in 153 (52.94%), squamous-cell carcinoma observed in 81 (28.03%), large-cell carcinoma in 32 patients (11.07%), and other histological types in 23 (7.96%). Regarding pathological staging, stages II and IIIA were confirmed in 179 and 110 patients, respectively. Positive gene mutations were noted in 109 cases (37.72%), negative gene mutations in 96 (33.22%), and 84 patients (29.06%) had not had gene-mutation status recorded . In terms of surgical resection type, lobectomy was observed in 248 cases (85.81%) and pneumonectomy in 41 (14.19%). Cisplatin-based adjuvant chemotherapy was reported in 205 patients (70.93%) and carboplatin-based adjuvant chemotherapy in 84 (29.07%). Patients on pemetrexed, docetaxel, and vinorelbine regimens numbered 156, 75, and 58, respectively. With regard to adjuvant chemotherapy, the median duration was three courses (range one to five).
 |
Table 2 Baseline characteristics of the 289 patients with NSCLC according to rs822336-genotype status |
Of the PDL1 polymorphisms analyzed, only rs822336 was of clinical significance. The prevalence of rs822336 among patients with NSCLC was: GG genotype, 179 cases (61.94%); GC genotype, 96 cases (33.22%); and CC genotype, 14 cases (4.84%). The MAF was 0.21, and the distribution of the three genotypes was in accordance with the Hardy–Weinberg equilibrium (P=0.807). GC and CC genotypes were merged in subsequent analysis. As shown in Table 2, baseline characteristics of the patients according to GG and GC/CC genotypes were comparable and well balanced.
Prognosis of the 289 Patients with NSCLC Who Received Platinum-Based Adjuvant Chemotherapy and Analysis of PDL1-Gene Polymorphism
This study was designed as retrospective research. A total of 289 patients with NSCLC were continuously included and analyzed. The last follow-up was in December 2019. Median follow-up total follow-up was 4.5 years (range 0.3–6.5 years). All of the 289 patients were available for prognosis evaluation. As illustrated in Figure 2, median DFS was 3.3 years (95% CI 2.85–3.75 years). With regard to polymorphism analysis, as shown in Figure 3, median DFS of those with GG and GC/CC rs822336 genotypes was 2.8 and 4.1 years, respectively, which was statistically significant (χ2=5.52, P=0.01).
 |
Figure 2 Disease-free survival and overall survival of the 289 patients with NSCLC who had received platinum-based adjuvant chemotherapy. |
 |
Figure 3 Disease-free survival of the 289 patients with NSCLC who had received platinum-based adjuvant chemotherapy according to PDL1rs822336-genotype status. |
Additionally, given that the follow-up period was long enough, OS was evaluated as well. Median OS was 4.9 years (95% CI 4.33–5.47 years), as illustrated in Figure 2. Regarding polymorphism analysis, as shown in Figure 4, median OS of patients with GG and GC/CC rs822336 genotypes was 4.1 and 5.4 years, respectively, which was statistically significant (χ2=6.86, P=0.008). Furthermore, in order to adjust for confounding factors, a multivariable Cox regression model was constructed including baseline characteristics that might have impactd OS and DFS. Multivariate-analysis results are shown in Table 3. After other baseline-characteristic adjustment, a statistically significant difference was observed for the influence of PDL1rs822336 on OS and DFS, which suggested that this polymorphism was an independent factor for OS (OR 0.76, P=0.015) and DFS (OR 0.81, P=0.035). Furthermore, as illustrated in Table 3, after adjusted in the Cox regression model, age (OR 1.44, P=0.011), sex (OR 0.76, P=0.027), ECOG score (OR 1.76, P=0.008), pathological staging (OR 2.25, P=0.004), and courses of adjuvant chemotherapy (OR 0.68, P=0.021) were also independent factors for OS. Additionally, age (OR 1.31, P=0.023), ECOG score (OR 1.55, P=0.014), pathological staging (OR 2.20, P=0.008), and courses of adjuvant chemotherapy (OR 0.56, P=0.011) were also independent factors for DFS.
 |
Table 3 Multivariate Cox regression analysis for OS and DFS according to baseline characteristics and rs822336-polymorphism status |
 |
Figure 4 The overall survival of the 289 patients with NSCLC who received platinum-based adjuvant chemotherapy according to PDL1rs822336 genotype status. |
Association Analysis Between PDL1−1813G>C Polymorphism and Grade ≥2 Adverse Reactions of Incidence ≥10%
Adverse reactions were evaluated in this study as well. Grade ≥2 adverse reactions of incidence ≥10% were recorded in the analysis. As illustrated in Table 4, the incidence of neutropenia, thrombocytopenia, and anemia in adverse hematologic reactions in the 289 patients with NSCLC who received platinum-based adjuvant chemotherapy after surgery were 34.26%, 23.18%, and 19.03%, respectively. Incidence of nausea and vomiting, fatigue, AST/ALT elevation, constipation, and diarrhea with nonhematologic reactions in the was 25.61%, 19.72%, 14.19%, 12.46%, and and 10.38%, respectively. On PDL1rs822336 analysis, as shown in Table 4, no statistically significant difference was observed in incidence of the grade ≥2 adverse reactions according to rs822336-genotype status (P>0.05).
 |
Table 4 Association analysis Between PDL1rs822336 polymorphism and grade ≥2 adverse reactions of incidence ≥10% |
Correlation Between rs822336 Polymorphism and PDL1-mRNA Expression
PBMC specimens were collected from 105 randomly selected and matched subjects initially. Prevalence of rs822336 polymorphism among the 86 PBMC specimens was: GG genotype, 53 cases (61.63%); GC genotype, 29 cases (33.72%); andCC genotype,four cases (4.65%), with MAF of 0.22. Distribution of the three genotypes was in accordance with the Hardy–Weinberg equilibrium (P=0.989) and comparable to the 289 patients with NSCLC. Similarly, CC and GC genotypes were merged. As illustrated in Figure 5, the GG genotype showed higher relative expression of PDL1 mRNA in PBMC specimens compared with the GC/CC genotype (3.705±0.613 vs 2.650±0.701), which was statistically significant (P<0.001).
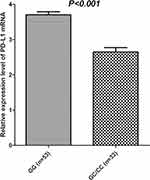 |
Figure 5 Relative expression level of PDL1 mRNA according to PDL1rs822336genotype status. |
Discussion
Our retrospective study provided real-world evidence on the prognosis and safety of platinum-based adjuvant chemotherapy in treatment of patients with NSCLC who had undergone surgical resection. Polymorphism analysis indicated that patients with the GG genotype had worse DFS and OS. Additionally, mRNA-expression analysis suggested mRNA-expression levels of PDL1 were significantly different according to rs822336-genotype status. The prognosis of patients with NSCLC who received platinum-based adjuvant chemotherapy might be influenced by PDL1 rs822336 polymorphism through mediating the PDL1-mRNA expression.
Although our study was designed as a retrospective analysis, prognosis of patients was evaluated as well. Median DFS of the 289 NSCLC patients was 3.3 years, and median OS was 4.9 years. From an objective view, the median DFS in our study was inferior to the cisplatin-based adjuvant-chemotherapy Phase III clinical trial initiated by the international lung cancer assistive trials team among resectable NSCLC patients, which found median DFS of 3.8 years.24 The reason underlying this might be the retrospective design of our study: management of patients was insufficient compared with the phase III clinical trial, which was proved in accordance with another retrospective study.25 Furthermore, patients with ECOG 2 scores were also included in our study, in contrast to the phase III clinical trial, where these patients were excluded. To the best of our knowledge, the influence of ECOG score on the prognosis of patients was confirmed in a previous study, which suggested that the higher the score, the worse the prognosis.26 Results of the Cox regression analysis in our study indicated that patients with ECOG 1–2 scores were associated with worse DFS. On the other hand, in view of the poor adherence to drug administration among patients with NSCLC in the real world, the median course of adjuvant chemotherapy in our study was three (range one to five), and the insufficient adjuvant chemotherapy failed to reduce the recurrence risk of patients with NSCLC, in accordance with proved a previous study.27 Interestingly, median OS in our study was relatively longer than that of the phase III clinical trial (4.9 years vs 4.1 years). We speculated that the reason might be the recent approval of immunotherapy and targeted drugs. Patients have more opportunities to receive these regimens when relapsed, thus prolonging OS significantly.28 Additionally, the influence of age, sex, ECOG score, and pathological stage on OS were statistically significant and consistent with a previous study.29
NSCLC heterogeneous malignant tumors show the highest morbidity and mortality. Recent years have witnessed the in-depth progress of targeted therapies and immunotherapy, making advanced NSCLC enter the chemotherapy-free stage.30 However, platinum-based adjuvant chemotherapy still plays an important therapeutic role in early-stage NSCLC. Although bevacizumab and EGFR tyrosine-kinase inhibitors have been explored in adjuvant therapy, the results have been negative. Trials of adjuvant therapyfor NSCLC with immunocheckpoint inhibitors are ongoing and the results not yet published.31 Therefore, the exploration of biomarkers for the prognosis of platinum-based adjuvant chemotherapy is necessary. Recently, several pharmacogenomic studies on patients with NSCLC who have received platinum-based treatment were reported: the impact of GLUT3-gene polymorphism on survival of patients with NSCLC after surgical resection was reported in 2019.32 ERCC5-gene polymorphism and susceptibility to lung cancer and its effect on the prognosis of patients who have received platinum-based chemotherapy has been investigated.33 Also, a study reported in 2018 was on the polymorphism and expression of ATP7B transporter gene relating to efficacy in patients with NSCLC who had received platinum-based chemotherapy.34 These results suggest that gene polymorphisms are independent factors in the prognosis of patients with NSCLC treated with platinum-based therapy.
To our knowledge, our study iss the first to focus on the association between prognosis of patients with NSCLC who have received platinum-based adjuvant chemotherapy and the PDL1-gene polymorphism in a Chinese population. The MAF of the rs822336 polymorphism was 0.21, consistent with the mutation frequency among the Chinese population in the NCBI database. However, MAF results in a Caucasian population have demonstrated that genotype-distribution frequency of the polymorphism showed large racial difference and that CC genotype was the main allele in a Caucasian population: MAF of the polymorphism was close to 0.5.20 Regarding prognosis analysis, our study was similar to previous research initiated by Wu et al.35 A total of 728 patients with gastric cancer who had undergone surgical resection were included and PD1 and PDL1 polymorphisms analyzed. The results indicated that rs822336 and rs2297136 polymorphisms in PDL1 were of clinical significance and that the CC genotype of rs822336 was correlated with superior prognosis, consistent with the results in our study.35 Furthermore, aYeo et al included cancer-tissue samples from 147 patients with NSCLC who had undergone surgical resection.36 The results indicated that higher PDL1 expression was associated with worse prognosis, consistent with our study to some extent. Interestingly Lee et al included 379 patients with NSCLC treated with first-line paclitaxel–cisplatin chemotherapy and investigated whether polymorphism of genes involved in PDL1 could predict the clinical outcomes of the patients.37 The results demonstrated that rs2297136 and rs4143815 were significantly associated with clinical outcomes after chemotherapy. The design of the research was consistent with that of our study. However, the polymorphism rs2297136 in our study showed no significant association with clinical outcomes, which might be attributed to the heterogeneity of the patients enrolled. Additionally, Takashi Nomizo et al explored the clinical impact of polymorphisms in PDL1 inn response to nivolumab in 50 patients with advanced NSCLC.38 Their esults suggested that rs2282055 and rs4143815 polymorphisms of PDL1 might be biomarkers for nivolumab efficacy. Both these studies demonstrated that polymorphisms of PDL1 might be of clinical significance for patients with NSCLC who have been treated with chemotherapy or immuno checkpoint inhibitors.
PDL1 is a hot-spot gene in immunotherapy at present, and numerous clinical trials have show that the expression level of PDL1 can predict the efficacy of PD1/PDL1 inhibitors.39 However, the relationship between the expression level of PDL1 and prognosis of patients with NSCLC who have received conventional platinum-based chemotherapy is still controversial.40 Our study preliminarily indicated that high PDL1-mRNA expression might confer a worse prognosis when patients have received platinum-based adjuvant chemotherapy, in line with the previous studies.41,42 We speculated that PDL1 not only participated in the regulation of tumor immunity, but also played a role in tumorigenesis, growth, and metastasis.43 A previous study had shown that high PDL1 expression in skin accelerated inflammation-associated carcinogenesis in a methylcholanthrene-induced model of squamous-cell carcinoma and induced epithelial–mesenchymal transition features in tumors, indicating that PDL1 facilitate tumorigenesis and epithelial–mesenchymal transition. In consequence, high expression of PDL1 might be associated with worse prognosis.44 However, a study has also indicated that patients with high expression of PDL1 demonstrated a better prognosis.45 These results might be related to the heterogeneity of the patients enrolled. Briefly, further in-depth clinical trials are needed.
From an objective view, limitations inevitably existed in our study. Firstly, this was a retrospective analysis, and some bias could not be avoided. Secondly, wefailed to investigate more detailed mechanisms in PDL1-protein expression,and the sample size was small for a real-world study. However, the prognosis significance of the polymorphism was fully evaluated and PDL1-mRNA expression performed in our study to try to interpret in-depth mechanisms. Additionally, PDL1 genotyping might be a low-cost auxiliary-chemotherapy prognostic method for patients with NSCLC, as a previous study suggested that polymorphism genotyping was a low-cost lung adenocarcinoma–screening tool in developing countries.46 Therefore, we believe our study is of clinical significance in prognoses of patients with NSCLC who have been treated with platinum-based adjuvant chemotherapy.
Abbreviations
NSCLC, non–small cell lung cancer; PDL1, programmed death–ligand 1; PBMCs, peripheral blood mononuclear cells; ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; CTCAE, Common Terminology Criteria for Adverse Events; CI, confidence interval; df, degrees of freedom; AST, aspartate transaminase; ALT, alanine aminotransferase.
Funding
This research was supported through grants from the Medical Big Data Research and Development Project of PLA General Hospital (2017MBD-017).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338
3. Zhi XY, Zou XN, Hu M, et al. Increased lung cancer mortality rates in the Chinese population from 1973–1975 to 2004–2005: an adverse health effect from exposure to smoking. Cancer. 2015;121(Suppl 17):3107–3112. doi: 10.1002/cncr.29603
4. Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin. 2020;13(1):17–33. doi: 10.1016/j.path.2019.11.002
5. Dong J, Li B, Lin D, et al. Advances in targeted therapy and immunotherapy for non-small cell lung cancer based on accurate molecular typing. Front Pharmacol. 2019;10:230. doi: 10.3389/fphar.2019.00230
6. Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18(1):63–70. doi: 10.1080/14737140.2018.1409624
7. Katsurada N, Tachihara M, Hatakeyama Y, et al. Feasibility study of adjuvant chemotherapy with carboplatin and nab-paclitaxel for completely resected NSCLC. Cancer Manag Res. 2020;12:777–782. doi: 10.2147/cmar.s239647
8. Pirker R, Filipits M. Adjuvant therapy in patients with completely resected non-small-cell lung cancer: current status and perspectives. Clin Lung Cancer. 2019;20(1):1–6. doi: 10.1016/j.cllc.2018.09.016
9. Kreuter M, Vansteenkiste J, Fischer JR, et al. Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Ann Oncol. 2013;24(4):986–992. doi: 10.1093/annonc/mds578
10. Yamamoto N, Kenmotsu H, Yamanaka T, et al. Randomized phase III study of cisplatin with pemetrexed and cisplatin with vinorelbine for completely resected nonsquamous non-small-cell lung cancer: the JIPANG study protocol. Clin Lung Cancer. 2018;19(1):e1–e3. doi: 10.1016/j.cllc.2017.05.020
11. Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev. 2015;3:Cd011430. doi: 10.1002/14651858.cd011430
12. Okamoto T, Yano T, Shimokawa M, et al. A Phase II randomized trial of adjuvant chemotherapy with S-1 versus S-1 plus cisplatin for completely resected pathological stage II/IIIA non-small cell lung cancer. Lung Cancer. 2018;124:255–259. doi: 10.1016/j.lungcan.2018.08.015
13. Chen K, Liu H, Liu Z, et al. Genetic variants in RUNX3, AMD1 and MSRA in the methionine metabolic pathway and survival in nonsmall cell lung cancer patients. Int J Cancer. 2019;145(3):621–631. doi: 10.1002/ijc.32128
14. Reck M, Brahmer J, Bennett B, et al. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30. doi: 10.1016/j.ejca.2018.05.005
15. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865
16. Wu Y, Chen W, Xu ZP, et al. PD-L1 distribution and perspective for cancer immunotherapy-blockade, knockdown, or inhibition. Front Immunol. 2019;10:2022. doi: 10.3389/fimmu.2019.02022
17. Ma LJ, Feng FL, Dong LQ, et al. Clinical significance of PD-1/PD-Ls gene amplification and overexpression in patients with hepatocellular carcinoma. Theranostics. 2018;8(20):5690–5702. doi: 10.7150/thno.28742
18. Han K, Zhang Y. mRNA expression of programmed cell death ligand 1 and components of the phosphatidylinositol 3-kinase/AKT/phosphatase and tensin homolog pathway in epidermal growth factor receptor mutation-positive lung adenocarcinoma. J Cancer Res Ther. 2019;15(4):914–920. doi: 10.4103/jcrt.JCRT_636_18
19. Xie Q, Chen Z, Xia L, et al. Correlations of PD-L1 gene polymorphisms with susceptibility and prognosis in hepatocellular carcinoma in a Chinese Han population. Gene. 2018;674:188–194. doi: 10.1016/j.gene.2018.06.069
20. Krawczyk P, Grenda A, Wojas-Krawczyk K, et al. PD-L1 gene copy number and promoter polymorphisms regulate PD-L1 expression in tumor cells of non-small cell lung cancer patients. Cancer Genet. 2019;237:10–18. doi: 10.1016/j.cancergen.2019.06.001
21. Cheng TD, Darke AK, Redman MW, et al. Smoking, sex, and non-small cell lung cancer: steroid hormone receptors in tumor tissue (S0424). J Natl Cancer Inst. 2018;110(7):734–742. doi: 10.1093/jnci/djx260
22. Bennett AV, Dueck AC, Mitchell SA, et al. Mode equivalence and acceptability of tablet computer-, interactive voice response system-, and paper-based administration of the U.S. National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Health Qual Life Outcomes. 2016;14:24. doi: 10.1186/s12955-016-0426-6
23. Ozaki Y, Muto S, Takagi H, et al. Tumor mutation burden and immunological, genomic, and clinicopathological factors as biomarkers for checkpoint inhibitor treatment of patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2020;69(1):127–134. doi: 10.1007/s00262-019-02446-1
24. Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644
25. Li S, Zhu L, Cheng X, et al. The significance of CO2 combining power in predicting prognosis of patients with stage II and III colorectal cancer. Biomark Med. 2019;13(13):1071–1080. doi: 10.2217/bmm-2018-0321
26. Sun DC, Shi Y, Wang YR, et al. KRAS mutation and primary tumor location do not affect efficacy of bevacizumab-containing chemotherapy in stagae IV colorectal cancer patients. Sci Rep. 2017;7(1):14368. doi: 10.1038/s41598-017-14669-2
27. Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage iii colon cancer. N Engl J Med. 2018;378(13):1177–1188. doi: 10.1056/NEJMoa1713709
28. Chen Y, Zhou Y, Tang L, et al. Immune-checkpoint inhibitors as the first line treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer. 2019;10(25):6261–6268. doi: 10.7150/jca.34677
29. Soh J, Toyooka S, Okumura N, et al. Impact of pathological stage and histological subtype on clinical outcome of adjuvant chemotherapy of paclitaxel plus carboplatin versus oral uracil-tegafur for non-small cell lung cancer: subanalysis of SLCG0401 trial. Int J Clin Oncol. 2019;24(11):1367–1376. doi: 10.1007/s10147-019-01508-9
30. Huang D, Zhang F, Tao H, et al. Tumor mutation burden as a potential biomarker for PD-1/PD-L1 inhibition in advanced non-small cell lung cancer. Target Oncol. 2020;15(1):93–100. doi: 10.1007/s11523-020-00703-3
31. Yi C, He Y, Xia H, et al. Review and perspective on adjuvant and neoadjuvant immunotherapies in NSCLC. Onco Targets Ther. 2019;12:7329–7336. doi: 10.2147/ott.s218321
32. Do SK, Choi SH, Lee SY, et al. Glucose transporter 3 gene variant is associated with survival outcome of patients with non-small cell lung cancer after surgical resection. Gene. 2019;703:58–64. doi: 10.1016/j.gene.2019.04.013
33. Lawania S, Singh N, Behera D, et al. XPG polymorphisms and their association with lung cancer susceptibility, overall survival and response in North Indian patients treated with platinum-based doublet chemotherapy. Future Oncol. 2019;15(2):151–165. doi: 10.2217/fon-2018-0408
34. Li YQ, Chen J, Yin JY, et al. Gene expression and single nucleotide polymorphism of ATP7B are associated with platinum-based chemotherapy response in non-small cell lung cancer patients. J Cancer. 2018;9(19):3532–3539. doi: 10.7150/jca.26286
35. Wu Y, Zhao T, Jia Z, et al. Polymorphism of the programmed death-ligand 1 gene is associated with its protein expression and prognosis in gastric cancer. J Gastroenterol Hepatol. 2019;34(7):1201–1207. doi: 10.1111/jgh.14520
36. Yeo MK, Choi SY, Seong IO, et al. Association of PD-L1 expression and PD-L1 gene polymorphism with poor prognosis in lung adenocarcinoma and squamous cell carcinoma. Hum Pathol. 2017;68:103–111. doi: 10.1016/j.humpath.2017.08.016
37. Lee SY, Jung DK, Choi JE, et al. PD-L1 polymorphism can predict clinical outcomes of non-small cell lung cancer patients treated with first-line paclitaxel-cisplatin chemotherapy. Sci Rep. 2016;6:25952. doi: 10.1038/srep25952
38. Nomizo T, Ozasa H, Tsuji T, et al. Clinical impact of single nucleotide polymorphism in PD-L1 on response to nivolumab for advanced non-small-cell lung cancer patients. Sci Rep. 2017;7:45124. doi: 10.1038/srep45124
39. Yang Q, Xu Z, Zheng L, et al. Multimodal detection of PD-L1: reasonable biomarkers for immune checkpoint inhibitor. Am J Cancer Res. 2018;8(9):1689–1696.
40. Xu Y, Wan B, Chen X, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8(4):413–428. doi: 10.21037/tlcr.2019.08.09
41. Enkhbat T, Nishi M, Takasu C, et al. Programmed cell death ligand 1 expression is an independent prognostic factor in colorectal cancer. Anticancer Res. 2018;38(6):3367–3373. doi: 10.21873/anticanres.12603
42. Zhou C, Che G, Zheng X, et al. Expression and clinical significance of PD-L1 and c-Myc in non-small cell lung cancer. J Cancer Res Clin Oncol. 2019;145(11):2663–2674. doi: 10.1007/s00432-019-03025-8
43. Shan T, Chen S, Wu T, et al. PD-L1 expression in colon cancer and its relationship with clinical prognosis. Int J Clin Exp Pathol. 2019;12(5):1764–1769.
44. Zippelius A, Schreiner J, Herzig P, et al. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015;3(3):236–244. doi: 10.1158/2326-6066.cir-14-0226
45. Berntsson J, Eberhard J, Nodin B, et al. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: relationship with sidedness and prognosis. Oncoimmunology. 2018;7(8)e1465165. doi: 10.1080/2162402x.2018.1465165
46. Cavic M, Spasic J, Krivokuca A, et al. TP53 and DNA-repair gene polymorphisms genotyping as a low-cost lung adenocarcinoma screening tool. J Clin Pathol. 2019;72(1):75–80. doi: 10.1136/jclinpath-2018-205553
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
