Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Inflammation-Based Prognostic Scores in Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma After Liver Transplantation
Authors Ren A, Li Z , Zhang X, Deng R, Ma Y
Received 2 May 2020
Accepted for publication 21 June 2020
Published 7 July 2020 Volume 2020:7 Pages 101—106
DOI https://doi.org/10.2147/JHC.S259992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Ao Ren, Zhongqiu Li, Xuzhi Zhang, Ronghai Deng, Yi Ma
Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China
Correspondence: Yi Ma
Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China
Email [email protected]
Background: Inflammation-based prognostic scores including systemic immune-inflammation index (SII), platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) have prognostic value in various cancers. We investigated the prognostic value of SII, PLR and NLR in patients who underwent liver transplantation (LT) for HBV-related hepatocellular carcinoma (HCC).
Methods: We retrospectively analyzed the records of 189 patients who underwent LT for HBV-related HCC. The receiver operating characteristic (ROC) curve was used to determine the optimal SII, PLR and NLR cut-off value. Overall survival (OS) and recurrence-free survival (RFS) following LT were calculated. The Kaplan–Meier method and the Cox proportional hazards model were used to evaluate the prognostic value of SII, PLR and NLR.
Results: The 1-, 3-, and 5-year OS rates were significantly lower in the high SII group (74.1%, 34.2%, and 32.3%, respectively) than in the low SII group (78.5%, 66.9%, and 59.9%, respectively; p = 0.000). The 1-, 3-, and 5-year RFS rates were, respectively, 75.9%, 59.7%, and 49.4% in the high SII group and 93.3%, 80.2%, and 73.7% in the low SII group (p = 0.000). Finally, OS curves were plotted by the Kaplan–Meier method and compared using the Log rank test. High PLR and NLR scores were also associated with poor OS (p = 0.000 and p = 0.003) and poor RFS (p = 0.000 and p = 0.000). The multivariate analysis demonstrated that AFP ≥ 400 ng/mL, high MELD score, largest tumor size ≥ 5cm, SII ≥ 449.61, NLR ≥ 5.29, and PLR ≥ 98.52 were independent prognostic factors for OS.
Conclusion: High SII, PLR and NLR are significantly poor prognostic factors for overall survival and recurrence-free survival in patients with HBV-related hepatocellular carcinoma after liver transplantation.
Keywords: hepatocellular carcinoma, HBV, liver transplantation, systemic immune-inflammation index, platelet to lymphocyte ratio, neutrophil to lymphocyte ratio
Introduction
Hepatocellular carcinoma (HCC) is the most common cancers and cause of cancer-related death, and the incidence and mortality have been increasing in European and North America.1 HBV infection is the leading etiology of HCC, up to 400 million individuals are infected with HBV worldwide, and most of cases found in Asia and Africa.2 Liver transplantation (LT) is considered to be the best choice for oncologic cure. However, selection of suitable patients for liver transplantation remains controversial, especially facing global organ shortage. The efficacy of LT is limited by the risk of HCC recurrence, which negatively affects patient survival. The HCC recurrence after LT has been reported to be 30% approximately.3 The selection of LT is mainly based on the Milan criteria, which is dependent upon tumor size and number. Recent studies have shown that predictors of HCC recurrence post LT have emerged, including AFP, microvascular invasion, platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), neutrophil to lymphocyte ratio (NLR) and systemic Immune-Inflammation Index (SII).4–6
There is increasing evidence that correlates preoperative immunological statuses with not only postoperative complications but also long-term outcomes of patients with certain tumors, which is associated with poorer cancer-specific survival in patients with malignant tumors.7–9 In addition, a growing number of studies have shown that circulating immune inflammatory cells such as platelets, neutrophils and lymphocytes play an important role in promoting the proliferation, invasion and migration of cancer cells by changing the tumor microenvironment.10 Recently, several inflammation-based scores, mainly including SII, NLR and PLR, have been served as useful prognostic biomarkers for multiple solid tumors.11,12 A novel indicator known as the SII was developed recently and has been demonstrated to be an effective and powerful prognostic indicator for several types of tumors, which combines lymphocyte, neutrophil, and platelet counts.13,14 However, the relationship between preoperative SII and prognosis in patients with HBV-related HCC after LT remains unclear. The aim of this study was to investigate the prognostic value of inflammation-based prognostic scores such as SII, PLR and NLR in patients undergoing LT for HBV-related HCC.
Methods
The records of 189 patients with HBV-related HCC who received LT at The First Affiliated Hospital, Sun Yat-Sen University (Guangzhou, China) from 2010 to 2015 were retrospectively reviewed. The diagnosis was confirmed by medical imaging and pathological examination of tissue specimens. Data extracted from the medical records included the recipient age and sex, model for end-stage liver disease (MELD) score, hepatitis B virus (HBV) pre-operative laboratory results (total bilirubin, albumin, prothrombin time, international normalized ratio [INR], lymphocyte, neutrophil, platelet counts and AFP), imaging features (number and volume of tumor nodules, vascular invasion, ascites), pathologic diagnosis, and pretransplant treatments. The follow-up data extracted included death, cause of death, HCC recurrence or date of last follow-up. Patients were followed monthly for the first 6 months, peripheral blood was tested for tumor markers such as AFP and ultrasonography and enhanced CT were performed every 6 months.
All tumor patients including HCC on the waiting list were evaluated of extrahepatic metastasis by PET-CT, and cardiopulmonary function and general condition of the patients were evaluated by relevant examination. For tumor patients, after laparotomy, abdominal exploration will be performed routinely and then further operation will be performed. If the exploration finds that the condition is poor, the operation may be stopped. Patients with an expected waiting list time of over 6 months could have been treated with transarterial chemoembolization (TACE), ablation as a bridge to LT.
All data are presented as n (%) or median (IQR). Independent χ 2 tests were used to compare categorical variables. Continuous variables were compared using t-tests. Survival curves were analyzed using the Kaplan–Meier method and compared using the Log rank test. The Cox proportional hazards model was used for univariate and multivariate analyses. Receiver operating characteristic (ROC) curve analysis was performed, and the area under the ROC curve (AUC), sensitivity, and specificity were calculated to examine the predictive value of the proposed model. A cut-off value was derived from the AUC based on the highest Youden index. All statistical analyses were performed using SPSS version 19.0 statistical software (SPSS, Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
All organs came from voluntary donations from citizens; no organs from executed prisoners (even with his/her consent) were used. The study was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University and was performed in accordance with the Declaration of Istanbul. All protocols conformed to the ethical guidelines of the 1975 Helsinki Declaration. All patients signed informed consent before liver transplantation for their data to be used for research.
Results
A total of 189 consecutive adult liver transplant patients with HBV-related HCC who met the inclusion criteria were included in the analysis. Patient demographic and clinical characteristics are summarized in Table 1. The diagnosis was confirmed by medical imaging, seropositivity for hepatitis B surface antigen (HBsAg), and pathological examination of tissue specimens. Immunosuppressive therapy for all patients after LT was individualized therapy, and was mainly based on Simulect and tacrolimus, combined with other immunosuppressive agents. Drug dosages were adjusted based on drug blood concentrations. Only a subset of patients received TACE and ablation for pretransplant locoregional therapy.
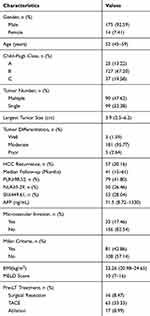 |
Table 1 Clinical and Demographic Characteristics of the Patients with HBV-Related Hepatocellular Carcinoma (HCC) |
The 189 patients in the study were 175 (92.59%) male and 14 (7.41%) female. Median age was 52 (interquartile range (IQR) 45–59) years. During a median follow-up of 41 months, 96 patients died and 57 were confirmed to have a tumor recurrence. The 1-, 3- and 5-year OS rates were 77.3%, 57.8%, and 52.2%, respectively; the 1-, 3- and 5-year RFS rates were 88.4%, 74.6%, and 67.0%, respectively.
We plotted a ROC curve to determine the optimal cut-off of SII, PLR and NLR for predicting survival after LT for HCC. Table 2 shows the AUC for survival with significant associations of inflammation cut-off scores of 449.61 for SII (AUC=0.629), 98.52 for PLR (AUC=0.636) and 5.29 for NLR (AUC=0.587).
 |
Table 2 Comparisons of the Areas Under the Curve (AUC) Values and the Cut-Off Values Among the Inflammatory Markers |
The 1-, 3-, and 5-year OS rates were significantly lower in the high SII group (74.1%, 34.2%, and 32.3%, respectively) than in the low SII group (78.5%, 66.9%, and 59.9%, respectively; p = 0.000)(Figure 1A). The 1-, 3-, and 5-year RFS rates were, respectively, 75.9%, 59.7%, and 49.4% in the high SII group and 93.3%, 80.2%, and 73.7% in the low SII group (p = 0.000)(Figure 1B). Finally, OS curves were plotted by the Kaplan–Meier method and compared using the Log rank test. High PLR and NLR scores were also associated with poor OS (p = 0.000 and p = 0.003; Figure 1C and E) and poor RFS (p = 0.000 and p = 0.000; Figure 1D and F).
Univariate analysis showed that AFP ≥400 ng/mL, SII ≥449.61, NLR ≥5.29, PLR ≥98.52, high MELD Score, largest tumor size ≥5cm, tumor number ≥2 and microvascular invasion were associated with poor OS (Table 3). To avoid multicollinearity, we conducted multivariate analysis using three models separately. Each multivariate model included only one immune-inflammatory indicator (SII, PLR, or NLR) or other significant predictor identified in univariate analysis. The multivariate analysis demonstrated that AFP ≥400 ng/mL, high MELD Score, largest tumor size ≥5cm, SII ≥449.61, NLR ≥5.29, and PLR ≥98.52 were independent prognostic factors for OS (Table 4).
 |
Table 3 Univariate Analysis of Factors Associated with Mortality by Cox Proportional Hazards Model |
 |
Table 4 Multivariate Analysis of Factors Associated with Mortality by Cox Proportional Hazards Model |
Discussion
HCC is a highly angiogenic tumor which is in the setting of chronic inflammation and cirrhosis. LT is an ideal option for well-selected HCC, especially the Milan criteria was introduced.15 Since then, several expanded criteria were introduced in clinical practice.16,17 Most of these criteria are based on tumor number, size, and macro-vascular invasion. However, the tumor biological behavior such as histological differentiation and micro-vascular invasion cannot be evaluated preoperatively, which are strongly related to tumor recurrence after LT.18,19 So we need to identify other predictors of HCC recurrence after LT.
Recently, systemic inflammation has been recognised to play an important role in the tumorigenesis of HCC, most cases developing as a consequence of chronic liver disease progressing to fibrosis and ultimately malignancy.20 Moreover, numerous inflammation-related features have been identified in the peripheral blood of HCC patients, which include thrombocytosis, leukocytosis, hypoproteinemia and relative lymphopaenia.21–23 A growing number of studies support the use of a combination of various acute phase proteins to develop composite, inflammation‐based prognostic scores, which include the systemic Immune-Inflammation Index (SII), neutrophil‐to‐lymphocyte ratio (NLR), the platelet‐to‐lymphocyte ratio (PLR). Systemic inflammatory response as measured by SII, NLR and PLR have been shown to be good predictors of HCC prognosis. The exact mechanism is still unclear, but several hypotheses have been put forward. Platelets can be activated by tumor cells, and then form tumor bolus with tumor cells through the adhesion molecules on their surface to protect tumor cells from the killing effect of the immune system and promote tumor cell metastasis.24,25 And both basic and clinical studies have confirmed that antiplatelet therapy can promote tumor cell apoptosis and inhibit tumor metastasis, thereby reducing the risk of tumor recurrence and improving the prognosis of patients.26 Neutrophils can induce tumor proliferation and angiogenesis, as well as enhance the migration and metastasis of cancer cells. In addition, HCC cells induce neutrophils to release hepatocyte growth factor, which makes cancer cells become more aggressive.27 Lymphocytes play an important role in anti-tumor immunity, which can directly kill tumor cells and secrete a series of cytokines to activate anti-tumor immunity, thus inhibiting the proliferation and migration of tumor cells and play an anti-tumor role. The decrease of lymphocytes in peripheral blood can weaken the body’s anti-cancer defense ability and lead to tumor recurrence and progression.28
Several studies have reported that SII, PLR and NLR are good predictors of risk of post-LT recurrence. A meta-analysis showed that high SII was correlated with poor OS and RFS in HCC (p < 0.001).29 This conclusion was also confirmed in another meta-analysis.30 Wang et al31 identified that high NLR (≥2.92) and high PLR (≥128.1) are useful prognostic factors in predicting outcomes in patients with HCC who underwent liver resection. This may be due to their reflection of parameter values for tumour growth and invasiveness.32 Our data are consistent with previous studies suggesting that high SII, PLR and NLR predict poor OS and RFS in HBV-related HCC patients receiving LT. The multivariate analysis demonstrated that AFP ≥400 ng/mL, high MELD Score, largest tumor size ≥5cm, SII ≥449.61, NLR ≥5.29, and PLR ≥98.52 were independent prognostic factors for OS.
Nevertheless, this study has several limitations. First, this was a retrospective, single-center analysis, which could lead to biases. Second, this study mainly focused on HBV-related HCC, whereas chronic HCV infection is the major cause for the development of HCC in Western countries.
In conclusion, our study identified that elevated pre-transplant SII, PLR and NLR were associated with poor OS and RFS, which could be used as prognostic factors for patients with HBV-related hepatocellular carcinoma after liver transplantation.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China, China (81873591, and 81670591), Guangdong Natural Science Foundation, China (2016A030311028), The Science and Technology Planning Project of Guangdong Province, China (2018A050506030), and Science and Technology Program of Guangzhou, China (201704020073).
Disclosure
The authors declare no conflicts of interest.
References
1. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi:10.1053/j.gastro.2018.08.065
2. Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68(5):916–927. doi:10.1136/gutjnl-2018-316510
3. Al-Ameri AAM, Wei X, Lin L, et al. Preoperative risk stratification for early recurrence of HBV-related hepatocellular carcinoma after deceased donor liver transplantation: a five-eight model development and validation. BMC Cancer. 2019;19(1):1136. doi:10.1186/s12885-019-6343-4
4. Yoon YI, Lee SG. Living donor liver transplantation for hepatocellular carcinoma: an Asian perspective. Dig Dis Sci. 2019;64(4):993–1000. doi:10.1007/s10620-019-05551-4
5. Ismael MN, Forde J, Milla E, Khan W, Cabrera R. Utility of inflammatory markers in predicting hepatocellular carcinoma survival after liver transplantation. Biomed Res Int. 2019;2019:7284040. doi:10.1155/2019/7284040
6. Fu H, Zheng J, Cai J, et al. Systemic Immune-Inflammation Index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within Hangzhou criteria. Cell Physiol Biochem. 2018;47(1):293–301. doi:10.1159/000489807
7. Harimoto N, Yoshizumi T, Shimagaki T, et al. Inflammation-based prognostic score in patients with living donor liver transplantation for hepatocellular carcinoma. Anticancer Res. 2016;36(10):5537–5542. doi:10.21873/anticanres.11137
8. Yamamoto M, Kobayashi T, Kuroda S, et al. Verification of inflammation-based prognostic marker as a prognostic indicator in hepatocellular carcinoma. Ann Gastroenterol Surg. 2019;3(6):667–675. doi:10.1002/ags3.12286
9. Wang C, He W, Yuan Y, et al. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020;40(1):229–239. doi:10.1111/liv.14281
10. Kim WJ, Lim TW, Park PJ, et al. Prognostic markers affecting the early recurrence of hepatocellular carcinoma with liver cirrhosis after curative resection. Int J Biol Markers. 2019;34(2):123–131. doi:10.1177/1724600819834306
11. Mowbray NG, Griffith D, Hammoda M, et al. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB (Oxford). 2018;20(5):379–384. doi:10.1016/j.hpb.2017.12.009
12. Sellers CM, Uhlig J, Ludwig JM, et al. Inflammatory markers in intrahepatic cholangiocarcinoma: effects of advanced liver disease. Cancer Med. 2019;8(13):5916–5929. doi:10.1002/cam4.2373
13. Chrom P, Zolnierek J, Bodnar L, et al. External validation of the systemic immune-inflammation index as a prognostic factor in metastatic renal cell carcinoma and its implementation within the international metastatic renal cell carcinoma database consortium model. Int J Clin Oncol. 2019;24(5):526–532. doi:10.1007/s10147-018-01390-x
14. De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25(13):3839–3846. doi:10.1158/1078-0432.CCR-18-3661
15. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi:10.1056/NEJM199603143341104
16. Prasad KR, Young RS, Burra P, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl. 2011;17(Suppl 2):S81–S89. doi:10.1002/lt.22380
17. Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85(12):1726–1732. doi:10.1097/TP.0b013e31816b67e4
18. Ciccarelli O, Lai Q, Goffette P, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int. 2012;25(8):867–875. doi:10.1111/j.1432-2277.2012.01512.x
19. Li WX, Li Z, Gao PJ, Gao J, Zhu JY. Histological differentiation predicts post-liver transplantation survival time. Clin Res Hepatol Gastroenterol. 2014;38(2):201–208. doi:10.1016/j.clinre.2013.11.002
20. Kocabayoglu P, Friedman SL. Cellular basis of hepatic fibrosis and its role in inflammation and cancer. Front Biosci (Schol Ed). 2013;5:217–230. doi:10.2741/S368
21. Hwang SJ, Luo JC, Li CP, et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10(17):2472–2477. doi:10.3748/wjg.v10.i17.2472
22. Aino H, Sumie S, Niizeki T, et al. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol Clin Oncol. 2014;2(3):393–398. doi:10.3892/mco.2014.259
23. Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi:10.1002/hep.26057
24. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi:10.1016/j.ccr.2011.09.009
25. Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30(1):95–108.
26. Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874–883. doi:10.1245/s10434-016-5520-9
27. Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. 2015;2015:983698. doi:10.1155/2015/983698
28. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017
29. Yang R, Chang Q, Meng X, et al. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi:10.7150/jca.25691
30. Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2020;99(1):e18571. doi:10.1097/MD.0000000000018571
31. Wang D, Bai N, Hu X, et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. doi:10.7717/peerj.7132
32. Suner A, Carr BI, Akkiz H, et al. Inflammatory markers C-reactive protein and PLR in relation to HCC characteristics. J Transl Sci. 2019;5(3):10.doi:10.15761/JTS.1000260
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

