Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Infantile Hemangioma Treated with Oral Propranolol: Case Presentation
Received 27 June 2021
Accepted for publication 16 August 2021
Published 25 August 2021 Volume 2021:14 Pages 1053—1055
DOI https://doi.org/10.2147/CCID.S326794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Nahid Raufi,1,2 Arash Nemat3,4
1Department of Dermatology, Maiwand Hospital, Kabul University of Medical Sciences, Kabul, Afghanistan; 2Department of Dermatology, Guangdong Provincial Dermatology Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 3Department of Microbiology, Kabul University of Medical Sciences, Kabul, Afghanistan; 4Department of Cardiology, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China
Correspondence: Arash Nemat
Department of Microbiology, Kabul University of Medical Sciences, 3rd District, Jamal Mina, Kabul, 1001, Afghanistan
Tel +93 706 717 987
Email [email protected]
Abstract: Infantile hemangiomas (IHs) are the most common vascular tumors in childhood. We report the use of propranolol to treat the growth phase of IHs. Propranolol was given to a 6-month-old infant who presented with a 4-cm sharply demarcated hemangioma on his left gluteal region. After relevant evaluation, propranolol was prescribed with a starting dose of 1 mg/kg/day, given in 3 divided doses. Vital signs were monitored during the first 6 hours of treatment. In the absence of side effects, treatment was continued at home with 2 mg/kg/day for six months, and the child was reevaluated after 7 days of treatment and then monthly. After one month, effects on color and growth were noted. Complete healing occurred in less than 6 months. Side effects and relapse were not reported. Propranolol administered orally at 2 mg/kg/day was successful, leading to near resolution of the patient’s hemangioma.
Keywords: hemangioma, infantile hemangioma, propranolol, vascular tumor, beta-blocker
Introduction
Infantile hemangiomas (IHs) are benign vascular tumors that commonly affect children with a 5% prevalence rate.1 The tumors are structured by endothelial cells, fibroblasts, mast cells and pericytes.2 Most IHs are small, innocuous, and self-resolving and require no treatment. However, because of their size, location, and ulceration, a significant minority of IHs are potentially problematic.3 In many parts of the world, beta-blockers have been successfully used for the treatment of IHs since 2008,4 but in Afghanistan, they have not yet become standard of care. The majority of clinicians in Afghanistan still do not use propranolol for treatment. Therefore, we want to share our experience to encourage other healthcare providers in Afghanistan to adopt this standard of care practice. Herein, we report our experience on the efficacy of propranolol for the treatment of a child in Afghanistan who presented with a hemangioma in the gluteal region.
Case Presentation
A 6-month-old term male infant presented to our outpatient dermatology clinic with a 4 cm × 2.5 cm sharply demarcated erythematous plaque with a small central ulceration in his left gluteal region (Figure 1). His mother reported that the lesion was barely evident at birth and was more visible several days after birth. The family did not seek medical advice before six months of age. Due to the ulceration in a sensitive location, we elected to treat with oral propranolol. Cardiac and respiratory exams, blood glucose, blood pressure, and heart rate were normal. We administered an initial dose of propranolol 1 mg/kg/day divided three times and in one hour checked blood glucose, blood pressure and heart rate which were normal. We continued this treatment for 7 days. After one week, the lesion’s size had decreased slightly with less discoloration. Propranolol dose was increased to 2 mg/kg/day divided three times, and the patient followed up every month. After 8 months, the patient’s hemangioma had nearly resolved (Figure 1). We gradually tapered the propranolol over 2 months. The HI resolved with an atrophic scar. No adverse effects were reported. There was no recurrence at follow-up at 18 months of age (Figure 1).
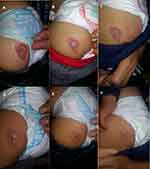 |
Figure 1 The features of hemangioma during treatment with propranolol at age 6th (A), 8th (B), 10th (C), 12th (D), 14th (E), and 15th (F) months, respectively. |
Discussion
Drug therapy is the most common treatment method for complicated infantile hemangiomas. Oral corticosteroids had long been used as first-line drugs, but since 2008, many doctors worldwide have been using propranolol to treat IHs.5 At present, propranolol is the treatment of choice for complicated IHs in many countries. However, in Afghanistan, it had not been used before 2014.6 Many clinicians continue to use oral corticosteroids with their related complications, and in some cases, surgery has been performed with adverse outcomes. Meanwhile, some dermatologists remain reluctant to use propranolol due to potential serious adverse effects in young patients.7 Studies revealed that the efficacy rate of propranolol was greater than that of corticosteroids in treating IH. Additionally, propranolol had fewer complications than steroids.5 In our dermatology department, we preferentially treat with oral propranolol now.
For the treatment of IHs, 10 mg propranolol tablets available on the market are used. We divide the tablets with a pill cutter.6 In Afghanistan, we face challenges in obtaining consent for long-term therapy, and consistent follow-up is difficult.
In this case, in addition to thorough education for the parents before initiation of treatment, they were encouraged by the rapid improvement of the IH with propranolol. Therefore, they continued the prescribed treatment and participated in regular follow-up until the IH resolved, and treatment protocol was completed.
Conclusion
Treatment of IH with oral propranolol is the first-line therapy worldwide, but still in Afghanistan, HIs are often treated with corticosteroids which are not as effective and may be associated with numerous adverse effects. This case presentation may encourage dermatologists in Afghanistan to follow standard guidelines for management of this disease.
Abbreviation
IH, Infantile hemangioma.
Ethical Approval and Consent to Participate
The authors certify that they have obtained all appropriate patient consent forms. Written informed consent was obtained from the patient’s father for the publication of this case report and accompanying images. The study was approved by the Kabul University of Medical Sciences Ethics Committee.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131(1):99–108. doi:10.1542/peds.2012-1128
2. Schwartz R, Sidor M, Musumeci M, Lin R, Micali G. Infantile haemangiomas: a challenge in paediatric dermatology. J Eur Acad Dermatol Venereol. 2010;24:631–638. doi:10.1111/j.1468-3083.2010.03650.x
3. Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143(1):e20183475. doi:10.1542/peds.2018-3475
4. Léauté-Labrèze C, de la Roque ED, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–2651. doi:10.1056/NEJMc0708819
5. You Y, Li Y, Xiao Y, Zhang J. Propranolol vs. steroids in the treatment of infantile hemangiomas: a meta-analysis. Mol Clin Oncol. 2021;15(2):1–9. doi:10.3892/mco.2021.2318
6. McMichael J, Lawley LP. Propranolol for infantile hemangiomas in developing countries. Dermatol Online J. 2017;23(3). doi:10.5070/D3233034282
7. Bakalli I, Kola E, Lluka R, et al. Deep coma in a child treated with propranolol for infantile hemangioma. BMC Pediatr. 2019;19(1):216. doi:10.1186/s12887-019-1598-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
