Back to Journals » Infection and Drug Resistance » Volume 12
Indole-core-based novel antibacterial agent targeting FtsZ
Authors Yuan W, Yu Z , Song W, Li Y, Fang Z, Zhu B, Li X, Wang H, Hong W, Sun N
Received 14 March 2019
Accepted for publication 3 July 2019
Published 24 July 2019 Volume 2019:12 Pages 2283—2296
DOI https://doi.org/10.2147/IDR.S208757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Wenchang Yuan,*,1 Zhiwu Yu,*,2 Weiqi Song,*,3 Yanan Li,4 Zhiyuan Fang,1 Baizhen Zhu,1 Xiaomei Li,1 Hao Wang,5 Wei Hong,6 Ning Sun1,7
1The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou 510700, People’s Republic of China; 2Division of Laboratory Science, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou 510095, People’s Republic of China; 3School of Public Health, Guangzhou Medical University, Guangzhou 511436, People’s Republic of China; 4Department of Pharmacy, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai 519000, People’s Republic of China; 5School of Pharmacy, Ningxia Medical University, Yinchuan 750004, People’s Republic of China; 6School of Chemistry and Chemical Engineering, North Minzu University, Yinchuan 750021, People’s Republic of China; 7State Key Laboratory of Chemical Biology and Drug Discovery, and Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, People’s Republic of China
*These authors contributed equally to this work
Background: The prevalence of drug-resistant bacterial infections urges the development of new antibacterial agents that possess a mechanism of action different from traditional antibiotics. FtsZ has been recognized as a key functional protein in bacterial cell division and it is currently believed to be a potential target for the development of novel antibacterial agents.
Purpose: The primary aim of the study is to screen out an inhibitor targeting at FtsZ and followed to investigate its antibacterial activity and mode of action.
Methods: Cell-based cell division inhibitory screening assay, antimicrobial susceptibility test, minimum bactericidal concentration assay, time-killing curve determination, FtsZ polymerization assay, GTPase activity assay, and molecular modeling were performed in the present study.
Results: The screening study from a small library consisting of benzimidazole and indole derivatives discovered a compound (CZ74) with an indole-core structure. The compound exhibited strong cell division inhibitory effect. In addition, CZ74 shows high antibacterial potency against a number of tested Gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. The minimum inhibitory concentration values obtained were within the range of 2–4 μg/mL. The results of biological study revealed that CZ74 at 2 μg/mL is able to disrupt FtsZ polymerization and inhibit GTPase activity and cell division. From molecular modeling study, CZ74 is found possibly binding into the interdomain cleft of FtsZ protein and then leads to inhibitory effects.
Conclusion: This indole-cored molecule CZ74 could be a potential lead compound and could be further developed as a new generation of antibacterial agents targeting FtsZ to combat against multidrug-resistant bacteria.
Keywords: indole-core-based derivative, antibacterial, cell division, FtsZ, antibiotic resistant
Introduction
Over the past few decades, the use of antibiotics has greatly improved public health worldwide. However, due to the improper use of antibiotics, bacteria develop drug resistance rapidly to most antibiotics. This may imply that the battle against bacterial infections is endless.1,2 Nowadays, the issue of antimicrobial resistance is critical and is a global problem. For example, two typical representative bacteria, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF), are resistant to the clinically used antibiotics such as methicillin and vancomycin.3,4 Considering the rapid emergence of drug-resistant bacteria and limited clinically used antibiotic treatment options, to develop alternative strategies to fight against bacterial infections is therefore urgently needed.
With respect to the recent progress on new drug discovery, bacterial divisome could be a new area for the development of new generation of antibiotics. Among the proteins in the divisome, FtsZ has been recognized as a key factor.5,6 During bacterial cell division, FtsZ assembled into a circumferential ring (Z-ring) at the division site. The process involves GTP binding and hydrolysis followed by stabilization and anchoring onto the inner surface of the cytoplasmic membrane via FtsZ-binding proteins. When Z-ring is ready, it recruits a series of other cell division proteins that ultimately trigger cytokinesis.7–9 The high conservation and functional importance of FtsZ make it as a potential target for the development of new antibacterial agents.10 Recently, a few FtsZ-targeting compounds have been reported to inhibit bacterial cell division through interfering FtsZ activity.10–16 However, no FtsZ-targeting small molecule is found in clinical trial stage, meaning that further advancement on the molecular scaffold design to achieve higher inhibition potency is required.
Benzimidazoles and indoles are natural compounds and their aromatic heterocyclic structures are widely used for pharmaceutical synthesis and modification. Many benzimidazole or indole core-based derivatives have good activity against various microbials, such as fungi and bacteria.17,18 Previous studies revealed that tri-substituted benzimidazole derivatives and indole derivatives (Figure 1) can inhibit effectively the growth of bacterial strains such as M. tuberculosis, S. aureus, and B. subtilis via disrupting FtsZ activity.19–22 In this study, we screened a small library of compounds containing 100 benzimidazole or indole core-based derivatives through a cell-based screening assay and successfully identified an indole core-based compound (CZ74, (E)-2-((1-(Phenylsulfonyl)-5-(4-(trifluoromethyl)phenyl)-1H-indol-3-yl)methylene) hydrazine-1-carboximidamide hydrochloride) (Figure 1). This small molecule was found possessing strong cell division inhibitory effect. In addition, we further investigated its antibacterial activity against several bacterial strains and the antibacterial mechanism.
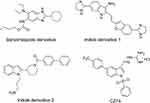 |
Figure 1 Structures of benzimidazole and indole derivatives. |
Materials and methods
Cell-based cell division inhibitory screening assay
To search for compounds possessing strong cell inhibitory effects, a screening assay as described previously was performed.23 In brief, B. subtilis 168 (ATCC 23857) cells were grown in starvation medium containing 1% hydrolyzed casein overnight. The culture was then diluted to OD600 of ~0.01 in starvation medium containing 3% hydrolyzed casein and incubated at 37°C. The stationary phase culture was diluted to OD600 of ~0.06, and 10 µL aliquots were transferred to 96-well microtiter plates containing different concentrations of the screening compounds in 100 µL volumes of medium. After incubation for approximately 5 hrs at 37°C, cell morphology was observed by phase-contrast light microscopy. Berberine, a natural compound has been reported to inhibit B. subtilis cell division at 64 μg/mL,24 was used as a positive control in the assay. Each sample was repeated three times. The compound inhibited the B. subtilis cell division at the concentration <64 μg/mL would be selected to further investigation.
Antimicrobial susceptibility test
All the strains used in this test were purchased from American Type Culture Collection (ATCC, USA). And all of them are clinically relevant pathogens except B. subtilis. The minimum inhibitory concentrations (MICs) of CZ74 against the tested strains were determined following procedures described in the Clinical and Laboratory Standards Institute (CLSI) guidelines.25 S. aureus strains were cultured in cation-adjusted Mueller Hinton broth (CA-MHB), E. faecalis and E. faecium strains were cultured in Brain Heart Infusion broth (BHI), the other strains were cultured in Mueller Hinton broth (MHB) in the assays. The MIC was defined as the lowest compound concentration at which prevents visible growth of bacterium. Methicillin and berberine were applied as a reference in this assay. Each test was repeated three times.
Minimum bactericidal concentration (MBC) assay
This assay was used to detect the MBC value of an antibacterial agent, and to further confirm whether the compound is bactericidal or not. The test performed using the protocol described above. The MBC was determined by placing 10 μL of culture volume from the MIC assay onto CA-MHB agar plate for S. aureus strains, MHB agar plate for B. subtilis, and BHI agar plate for E. faecium. Then, the plates were incubated at 37°C, and the colony formation was examined after 24 hrs. MBC was recorded as the lowest concentration at which bacterial colonies were not observed.26 Each test was repeated three times.
Time-killing curve determination
This assay was used to detect the kinetics of antibacterial activity of CZ74. An overnight culture of S. aureus ATCC 25923 and B. subtilis 168 was diluted to 106 CFU/mL approximately. Different concentrations of CZ74 were added to bacterial culture. The cultures were then incubated at 37°C 250 rpm. After that, 100 μL of the culture were taken for serial dilutions and then spread on CA-MHB or MHB agar plates at different time intervals (0, 2, 4, 6, 8, 24 hrs). The plates were incubated at 37°C for 24 hrs and then the cell counts (CFU/mL) were recorded. Each test was repeated three times.
FtsZ polymerization assay
To investigate the possible antibacterial mechanism of CZ74, S. aureus FtsZ was prepared according to the previous study.24 Some biochemical assays were also performed to confirm the effect of CZ74 on the FtsZ. Firstly, the light scattering assay and transmission electron microscopy (TEM) analysis were performed by following a protocol from the literature27 to investigate the effect of CZ74 on the FtsZ polymerization. In the light scattering assay, FtsZ (6 μM) was incubated with different concentrations of CZ74 at room temperature for 10 mins. The light scattering signal was immediately detected by a thermostatically (37°C) controlled fluorescence spectrometer after additions of 20 mM KCl, 5 mM MgCl2, 1 mM GTP. One percentage DMSO and 5 μg/mL methicillin were tested as vehicle and negative control in the assay. In the TEM analysis, 12 μM FtsZ was incubated with one percentage DMSO or 4 μg/mL of CZ74 in 50 mM buffer for 10 mins at room temperature. Then, 5 mM MgCl2, 50 mM KCl, and 1 mM GTP were added to the mixtures. The samples were then incubated in water bath at 37°C. After 15 mins, 10 μL of the sample was transferred to a Formvar carbon-coated copper grid and subjected to 0.5% phosphotungstic acid for negative staining. And the air-dried sample was observed with a transmission electron microscope.27
GTPase activity assay
The GTPase activity assay was conducted by following the previously described procedures in the investigation of CZ74 on the GTPase activity of FtsZ.16 In short, 3.5 μM FtsZ was incubated with different concentrations of CZ74 (0, 1, 2, 4, 8, and 16 μg/mL) at room temperature in 20 mM Tris buffer. After 10 mins, 5 mM of MgCl2, 200 mM of KCl, and 500 mM GTP were added. The samples were then incubated at 37°C for 30 mins. After that, the reactions were quenched by adding 100 mL of Cytophos reagent for 10 mins. Inorganic phosphate was quantified with a microplate reader at 650 nm.
Molecular modeling
Discovery Studio (DS) 2016 was used to perform the molecular modeling study. The X-ray crystal structure of SaFtsZ was downloaded from the PDB database (PDB entry: 4DXD).28 FtsZ and the ligand CZ74 were prepared for this study using the own tools in DS. And then, the CDocker protocol was used to predict the binding poses and the top-scoring poses were visually inspected.
Preparation of compound CZ74
The preparation and characterization of CZ74 can be referred to supplementary material and Figure S1.
 |
Figure S1 1H-NMR spectrum of CZ74. |
Results
Cell inhibitory effect of CZ74 against B. subtilis
The cell-based screening assay identified three potential compounds (Table S1), which inhibited the growth of B. subtilis at the concentration equal to or below 64 µg/mL. However, only CZ74 possesses bacterial cell division inhibitory effect. Compared to the control experiments, CZ74 elongated the cell length of B. subtilis obviously at the concentration of 2 µg/mL (Figure 2). The untreated B. subtilis cells have typical short rod morphology with cell lengths from 5 to 15 μm (Figure 2A). After incubation with CZ74 at 2 µg/mL, the cells were found longer than 50 μm (Figure 2B). This phenomenon was similar to the B. subtilis cells treated with 64 µg/mL berberine (Figure S2). These results are also consistent with the reported FtsZ inhibitors, such as benzamide and pyrimidine derivatives,13,29,30 and suggest that CZ74 may disrupt FtsZ function and lead to the abnormal cell division.
 |
Figure 2 Cell division inhibitory effect of CZ74. Cells of B. subtilis 168 were grown in the absence (A) and presence of 2 µg/mL of CZ74 (B). Scale bar=15 µm. |
 |
Figure S2 Cell inhibitory effect of 64 μg/mL berberine on B. subtilis cells. Scale bar=15 μm. |
Antibacterial activity of CZ74
To investigate the antibacterial activity of CZ74, the compound was examined against several bacterial strains including some drug-resistant strains. The results showed that CZ74 inhibited all the tested Gram-positive strains effectively. The MIC values are found in the range of 2–4 µg/mL. For instance, the growth of B. subtilis 168, S. aureus ATCC 25923, and S. epidermidis ATCC 12228 were inhibited at 2 µg/mL. The MIC value is comparable to that of methicillin and is much lower than that of berberine. Furthermore, the MIC values of CZ74 against the MRSA (ATCC BAA-41, 33591, 33592, and 43300) is as low as 2 µg/mL. Compared with methicillin and berberine, the antibacterial activity of CZ74 against MRSA is much more potent (Table 1). The compound was also evaluated against 50 clinical isolated MRSA. The results also show that CZ74 has a potent antibacterial activity with the MIC value in the range from 2 to 4 µg/mL (Table S2). CZ74 also inhibited the growth of E. faecalis and E. faecium effectively with the MIC value of 4 µg/mL (Table 1). Since the MICs of vancomycin against vancomycin-resistant E. faecalis and E. faecium (VREs) are higher than 96 µg/mL, CZ74 possesses higher antibacterial activity against VREs than that of vancomycin.24 However, in the antibacterial effect against Gram-negative strains such as P. aeruginosa, K. pneumoniae, and E. coli, the compound at 64 µg/mL did not show any antibacterial effect. The antibacterial property of CZ74 observed is similar to those of reported FtsZ-targeting compounds such as benzamide derivatives.30,31
 |
Table 1 Antibacterial activity of CZ74 against a panel of bacterial strains |
Bactericidal test of CZ74 against the selected bacterial strains
After obtained the antibacterial activity of CZ74, it is important to further investigate whether the antibacterial activity is bactericidal or bacteriostatic. In this study, the MBC values of CZ74 against B. subtilis 168, S. aureus ATCC 25923, MRSA (ATCC 43300), and VREF (ATCC 700221) were determined (Table 2). According to the CLSI standards, an MBC/MIC ratio of 1–2 is considered indicative of bactericidal behavior.25 The results show that all the MBC/MIC ratios obtained are equal to or lower than 2, indicating bactericidal activity of CZ74 against the tested bacterial species.
 |
Table 2 The MBC of CZ74 against drug-resistant bacterial strains (µg/mL) |
Kinetics of the bactericidal activity of CZ74
After the bactericidal profile of CZ74 was confirmed, the time-killing curve determinations were performed to investigate the kinetics of killing of CZ74 against S. aureus ATCC 25923 and B. subtilis 168. As shown in Figure 3A, S. aureus grew rapidly in the first 6 hrs without CZ74. After 6 hrs incubation, the growth of S. aureus went into the stationary phase. On the other hand, the concentration of CZ74 at 2× MIC and 4× MIC can completely inhibit the growth of S. aureus after incubation overnight. At the concentration of 1× MIC, the compound also reduces the viable counts of S. aureus below 103 CFU mL−1 after incubation for 24 hrs. The growth inhibition with different concentration gradients of CZ74 demonstrated that the rate of killing S. aureus was dose-dependent. In addition, a similar kinetic was found when B. subtilis incubated with CZ74 for 24 hrs (Figure 3B).
Effect of CZ74 on the FtsZ polymerization
In order to understand the mechanism of antibacterial activity of CZ74, the effect of CZ74 on the dynamic polymerization of FtsZ was investigated by using a light scattering assay. Figure 4A shows the time-dependent polymerization profile of FtsZ in the absence and in the presence of CZ74 at a concentration range from 1 to 4 µg/mL. The results revealed that CZ74 stimulated FtsZ polymerization in a concentration-dependent profile. The similar phenomena can also be observed in other reported FtsZ-targeting compounds.10,11 Methicillin was also tested under the same conditions as a negative control. As expected, it does not affect FtsZ polymerization. Apart from the light scattering assay, we also used TEM to visualize the effect of CZ74 on the FtsZ polymerization. The results showed that both the size of the FtsZ polymers and the bundles of the FtsZ protofilaments were dramatically increased when using 2 µg/mL CZ74 (Figure 4B and C).
Effect of CZ74 on the GTPase activity of FtsZ
The rate of GTP hydrolysis is correlated with the dynamic polymerization of FtsZ.8,9 To investigate whether the antibacterial mechanism of CZ74 is related to the GTPase inhibitory effect, the effect of this compound on the GTPase activity of the FtsZ was determined. The results revealed that the compound inhibited the GTPase activity of FtsZ in a dose-dependent profile. In the assay, 1, 2, 4, 8, and 16 μg/mL of CZ74 inhibited the GTPase activity by 15%, 30%, 45%, 60%, and 62%, respectively (Figure 5). The result indicates that the inhibitory effect of CZ74 on GTPase activity may probably lead to the disruption of FtsZ polymerization.
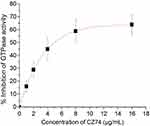 |
Figure 5 Inhibition of GTPase activity of FtsZ by different concentrations of CZ74. |
Predicted the binding mode of CZ74
Molecular modeling study was also conducted to predict the binding sites and the interaction pose of CZ74 in FtsZ. The optimal docking pose suggests that the compound probably binds to a hydrophobic and narrow cleft constituted by the T7-loop, H7-helix, and a four-stranded β-sheet (Figure 6A). The possible interactions between CZ74 and the amino acids of FtsZ were shown in a 2D ligand interaction diagram (Figure 6B). The guanidyl group of CZ74 could interact with Val203, Asn208, and Leu209 of FtsZ protein through four conventional hydrogen bonds. Several favorable hydrophobic interactions between CZ74 and the side chains of Asp199, Val203, Met226, Thr265, Val297, and Ile311 were predicted in the binding model. In addition, the trifluoromethyl group of CZ74 was predicted participating in one carbon-hydrogen bond with the side chain of Gly193 and three halogen bonds with Gly193, Gly227, and Val310. Moreover, a lot of amino acids in this pocket (such as Gly196, Val307, Thr309, etc.) could interact with CZ74 via van der waals force (data not shown).
 |
Figure 6 Predicted binding mode of CZ74 bound to FtsZ. (A) CZ74 bound to the C-terminal interdomain cleft of FtsZ; (B) predicted interaction between CZ74 and amino acids of FtsZ. |
Discussion
Indoles are a class of important heterocycles possessing diverse biological activities, such as anticancer, antibacterial, and antiviral.32–34 In this study, we found that an indole derivative CZ74 possesses strong antibacterial activity against the tested Gram-positive bacteria, including MRSA and VRE. The MIC values obtained are in the range of 2–4 µg/mL, which is similar to or slightly better than certain reported inhibitors targeting at FtsZ. In particular, berberine derivative inhibited the growth of MRSA with a MIC value of 2 µg/mL and VREF with a MIC value of 8 µg/mL.24 PC 190723 can effectively against MRSA at the concentration of 1 µg/mL.30 However, CZ74 has not any antibacterial activity against the tested Gram-negative strain at the concentration of 64 µg/mL, likely owing to the intrinsically lower permeability of the Gram-negative cell envelope.35
In the biological assays, CZ74 was found to stimulate FtsZ polymerization with GTPase inhibitory effect. The similar biochemical results were also found in some FtsZ inhibitors, such as PC190723 and quinoline derivative.30,36 FtsZ polymerization is found to be regulated by GTPase activity of FtsZ8 and the increasing of FtsZ polymers may probably contribute from the conformational change to the high-affinity state, which enables FtsZ assembly.37 In addition, same as those reported FtsZ-targeting compounds,10 the strong inhibitory effect of CZ74 on the bacterial cell division was observed. Recently, our group has reported some FtsZ-targeting compounds with strong antibacterial activity, such as berberine derivatives, pyrimidine derivatives, and quinolinium derivatives.15,16,24,27,29 Unfortunately, the low solubility of these compounds hindered their further investigation in animal study. We expected that the polar guanidyl group of CZ74 could overcome the solubility issue and make it possible for in vivo study. In addition, this hydrophilic guanidyl group was predicted to interact with FtsZ through hydrogen bonds (Figure 6).
In summary, an indole-core-based compound was demonstrated as a potential antibacterial agent targeting FtsZ. The compound exhibits good antibacterial activity against several clinically representative Gram-positive strains including some drug-resistant bacterial strains, such as MRSA and VRE. From the biochemical evaluations with the compound, the mode of action was probed. It was suggested that the compound could possibly bind into the interdomain cleft of FtsZ and take functions in disrupting the GTPase activity, stimulating FtsZ polymerization, and eventually inhibiting bacterial cell division to cause cell death. Therefore, this indole-cored small molecule is suitable for further investigation on their biological activities, particularly the antibacterial mechanism.
Acknowledgments
We acknowledge the support from the National Natural Science Foundation of China (81703333 and 81660588), the Science and Technology Program of Guangzhou Municipal Health Commission (20192A011018), and the Medical Scientific Research Foundation of Guangdong Province (A2017588).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zhang SX, Zhou YM, Tian LG, et al. Antibiotic resistance and molecular characterization of diarrheagenic Escherichia coli and non-typhoidal Salmonella strains isolated from infections in Southwest China. Infect Dis Poverty. 2018;7(1):53. doi:10.1186/s40249-018-0427-2
2. Wright GD. Antibiotics: a new hope. Chem Biol. 2012;19(1):3–10. doi:10.1016/j.chembiol.2011.10.019
3. Li Y, Yang L, Fu J, Yan M, Chen D, Zhang L. Microbial pathogenicity and virulence mediated by integrons on Gram-positive microorganisms. Microb Pathog. 2017;111:481–486. doi:10.1016/j.micpath.2017.09.035
4. Gould IM, David MZ, Esposito S, et al. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents. 2012;39(2):96–104.
5. Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7(9):642–653.
6. Lock RL, Harry EJ. Cell-division inhibitors: new insights for future antibiotics. Nat Rev Drug Discov. 2008;7(4):324–338.
7. Roseboom W, Nazir MG, Meiresonne NY, et al. Mapping the contact sites of the Escherichia coli division-initiating proteins FtsZ and ZapA by BAMG cross-linking and site-directed mutagenesis. Int J Mol Sci. 2018;19(10):2928.
8. Yang X, Lyu Z, Miguel A, McQuillen R, Huang KC, Xiao J. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science. 2017;355(6326):744–747.
9. Bisson-Filho AW, Hsu YP, Squyres GR, et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science. 2017;355(6326):739–743.
10. Hurley KA, Santos TM, Nepomuceno GM, Huynh V, Shaw JT, Weibel DB. Targeting the bacterial division protein FtsZ. J Med Chem. 2016;59(15):6975–6998. doi:10.1021/acs.jmedchem.5b01098
11. Haranahalli K, Tong S, Ojima I. Recent advances in the discovery and development of antibacterial agents targeting the cell-division protein FtsZ. Bioorg Med Chem. 2016;24(24):6354–6369. doi:10.1016/j.bmc.2016.05.003
12. Foss MH, Eun Y-J, Weibel DB. Chemical–biological studies of subcellular organization in bacteria. Biochemistry-US. 2011;50(36):7719–7734. doi:10.1021/bi200940d
13. Fang Z, Zheng S, Chan KF, et al. Design, synthesis and antibacterial evaluation of 2,4-disubstituted-6-thiophenyl-pyrimidines. Eur J Med Chem. 2019;161:141–153. doi:10.1016/j.ejmech.2018.10.039
14. Bi F, Song D, Zhang N, et al. Design, synthesis and structure-based optimization of novel isoxazole-containing benzamide derivatives as FtsZ modulators. Eur J Med Chem. 2018;159:90–103. doi:10.1016/j.ejmech.2018.09.053
15. Sun N, Du RL, Zheng YY, et al. Antibacterial activity of 3-methylbenzo[d]thiazol-methylquinolinium derivatives and study of their action mechanism. J Enzyme Inhib Med Chem. 2018;33(1):879–889. doi:10.1080/14756366.2018.1465055
16. Sun N, Du RL, Zheng YY, et al. Antibacterial activity of N-methylbenzofuro[3,2-b]quinoline and N-methylbenzoindolo[3,2-b]-quinoline derivatives and study of their mode of action. Eur J Med Chem. 2017;135:1–11. doi:10.1016/j.ejmech.2017.04.018
17. Zhao F, Liu N, Zhan P, Jiang X, Liu X. Discovery of HCV NS5B thumb site I inhibitors: core-refining from benzimidazole to indole scaffold. Eur J Med Chem. 2015;94:218–228. doi:10.1016/j.ejmech.2015.03.012
18. Zhang MZ, Mulholland N, Beattie D, et al. Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur J Med Chem. 2013;63:22–32. doi:10.1016/j.ejmech.2013.01.038
19. Kumar K, Awasthi D, Lee SY, et al. Novel trisubstituted benzimidazoles, targeting Mtb FtsZ, as a new class of antitubercular agents. J Med Chem. 2011;54(1):374–381. doi:10.1021/jm1012006
20. Margalit DN, Romberg L, Mets RB, et al. Targeting cell division: small-molecule inhibitors of FtsZ GTPase perturb cytokinetic ring assembly and induce bacterial lethality. Proc Natl Acad Sci U S A. 2004;101(32):11821–11826. doi:10.1073/pnas.0404439101
21. Jennings LD, Foreman KW, Rush TS
22. Sun N, Zheng YY, Du RL, et al. New application of tiplaxtinin as an effective FtsZ-targeting chemotype for an antimicrobial study. Medchemcomm. 2017;8(10):1909–1913. doi:10.1039/c7md00387k
23. Stokes NR, Sievers J, Barker S, et al. Novel inhibitors of bacterial cytokinesis identified by a cell-based antibiotic screening assay. J Biol Chem. 2005;280(48):39709–39715. doi:10.1074/jbc.M506741200
24. Sun N, Chan F-Y, Lu Y-J, et al. Rational design of berberine-based FtsZ inhibitors with broad-spectrum antibacterial activity. PLoS One. 2014;9(5):e97514. doi:10.1371/journal.pone.0097514
25. Wikler MA, Hindler JF, Cookerill FR, Patel JB, Bush K, Powell M. Methods for Dilution Antimicrobial Sucseptibility Tests for Bacteria That Grow Aerobically. Wayne, PA, USA: Clinical and Laboratory Standards Institute. Vol. 29; 2009:M07–A08.
26. Zheng Z, Tharmalingam N, Liu Q, et al. Synergistic efficacy of aedes aegypti antimicrobial peptide cecropin A2 and tetracycline against pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:7. doi:10.1128/AAC.00686-17
27. Sun N, Lu YJ, Chan FY, et al. A thiazole orange derivative targeting the bacterial protein FtsZ shows potent antibacterial activity. Front Microbiol. 2017;8:855. doi:10.3389/fmicb.2017.00855
28. Tan CM, Therien AG, Lu J, et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to beta-lactam antibiotics. Sci Transl Med. 2012;4(126):126ra135. doi:10.1126/scitranslmed.3003592
29. Chan KF, Sun N, Yan SC, et al. Efficient synthesis of amine-linked 2,4,6-trisubstituted pyrimidines as a new class of bacterial FtsZ inhibitors. ACS Omega. 2017;2(10):7281–7292. doi:10.1021/acsomega.7b00701
30. Haydon DJ, Stokes NR, Ure R, et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science. 2008;321(5896):1673–1675. doi:10.1126/science.1159961
31. Haydon DJ, Bennett JM, Brown D, et al. Creating an antibacterial with in vivo efficacy: synthesis and characterization of potent inhibitors of the bacterial cell division protein FtsZ with improved pharmaceutical properties. J Med Chem. 2010;53(10):3927–3936. doi:10.1021/jm9016366
32. Kaur M, Singh M, Chadha N, Silakari O. Oxindole: A chemical prism carrying plethora of therapeutic benefits. Eur J Med Chem. 2016;123:858–894. doi:10.1016/j.ejmech.2016.08.011
33. Yang YT, Zhu JF, Liao GC, Xu HJ, Yu B. The development of biologically important spirooxindoles as new antimicrobial agents. Curr Med Chem. 2018;25(19):2233–2244.
34. Zhang MZ, Chen Q, Yang GF. A review on recent developments of indole-containing antiviral agents. Eur J Med Chem. 2015;89:421–441.
35. Keffer JL, Huecas S, Hammill JT, Wipf P, Andreu JM, Bewley CA. Chrysophaentins are competitive inhibitors of FtsZ and inhibit Z-ring formation in live bacteria. Bioorg Med Chem. 2013;21(18):5673–5678.
36. Fang Z, Ban L, Li Y, et al. A quinoline-based FtsZ inhibitor for the study of antimicrobial activity and synergistic effects with beta-lactam antibiotics. J Pharmacol Sci. 2018;137(3):283–289.
37. Elsen NL, Lu J, Parthasarathy G, et al. Mechanism of action of the cell-division inhibitor PC190723: modulation of FtsZ assembly cooperativity. J Am Chem Soc. 2012;134(30):12342–12345.
Supplementary material
Part 1: Preparation and characterization of CZ74
The preparation of CZ74 was performed using a procedure as previously described (The Journal of Antibiotics. 2017;70:832–844).1
Characterization:
1H-NMR (400 MHz, DMSO): δ 12.05 (s, 1H, NH), 8.57 (s, 1H, Ar-H), 8.49 (d, J=1.5, 1H, Ar-H), 8.43 (s, 1H, CH), 8.02–8.13 (m, 3H, Ar-H), 7.95 (d, J=8.1, 2H, Ar-H), 7.70–7.85 (m, 7H, Ar-H and NH), 7.64 (t, J=7.8, 2H, Ar-H); ES-MS 486.1 (M + H)+; HRMS calcd for C21H19F3N5O2S + 486.1212, found 486.1201.
Part 2
 |
Table S1 Compounds possess antibacterial activity against B. subtilis generated from cell-based screening assay |
Part 3
Part 4
 |  |  |
Table S2 Antibacterial activity of CZ74 against clinical isolated MRSA |
Reference
1. Hong W, Li J, Chang Z, et al Synthesis and biological evaluation of indole core-based derivatives with potent antibacterial activity against resistant bacterial pathogens. The Journal of Antibiotics. 2017;70(7):832.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


