Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Indocyanine Green Fluorescence Imaging in the Surgical Management of Skin Squamous Cell Carcinoma
Authors Zhou L, Gan Y, Wu Y, Xue D, Hu J, Zhang Y, Liu Y, Ma S , Zhou J, Luo G, Peng D, Qian W
Received 13 April 2023
Accepted for publication 28 October 2023
Published 15 November 2023 Volume 2023:16 Pages 3309—3320
DOI https://doi.org/10.2147/CCID.S413266
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ling Zhou,1,* Yu Gan,1,* Yanjun Wu,1 Dongdong Xue,1 Jianhong Hu,1 Yilan Zhang,2 Yang Liu,3 Siyuan Ma,1 Junyi Zhou,1 Gaoxing Luo,1 Daizhi Peng,1 Wei Qian1
1Institute of Burn Research, Southwest Hospital, State Key Laboratory of Trauma, Burns and Combined Injury, Army Medical University (Third Military Medical University), Chongqing, 400038, People’s Republic of China; 2Department of Oral and Maxillofacial Head and Neck Surgery, Army Medical Center of PLA/Daping Hospital, Army Medical University (Third Military Medical University), Chongqing, 400042, People’s Republic of China; 3Department of Urology, Urology Institute of PLA, Southwest Hospital, Army Medical University (Third Military Medical University), Chongqing, 400038, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daizhi Peng; Wei Qian, Email [email protected]; [email protected]
Introduction: Indocyanine green (ICG) fluorescence imaging has been used in the resection surgery and sentinel lymph node biopsy of many tumors. The aim of the present study is to verify the feasibility and effectiveness of ICG fluorescence imaging used for guiding the biopsy and resection of skin squamous cell carcinoma (SSCC).
Methods: Sixty patients were enrolled, including 18 patients of suspected SSCC and 42 patients of diagnosed SSCC on admission. The ICG fluorescence imaging-guided skin biopsy was performed preoperatively in the 18 cases of suspected SSCC. Fifty-three patients underwent ICG fluorescence imaging-guided radical excision.
Results: The results showed that 138 skin tissue samples in 60 patients with preoperative or intraoperative ICG fluorescence imaging-guide biopsy were collected. For a total number of 138 biopsies, 122 specimens were squamous cell carcinoma, and the accuracy rate was 88.4%, which was significantly higher than that of the group without preoperative ICG fluorescence imaging (41/62, 66.1%, P < 0.05). Fifty-three patients underwent surgery guided with ICG fluorescence imaging. Residual fluorescent signals in 24 patients were intraoperatively found and the excision was then expanded until the signals disappeared. Follow-up to November 2022, 12 patients died, of which 5 cases died from the tumor recurrence, and the others died due to advanced ages or other reasons. The recurrence rate was 9.4%, which was not significantly different from that of the group received routine radical resection (4/35, 11.4%, P > 0.05). Moreover, sentinel lymph nodes were successfully detected under ICG fluorescence imaging in the 4 patients with suspected lymph node metastases, and the location of lymph nodes can be precisely identified.
Conclusion: ICG fluorescence imaging technique can guide the pathology biopsy to improve the accuracy of pathological examination, and help to identify the boundaries of tumor tissues and sentinel lymph nodes to resect tumor radically during operation.
Keywords: indocyanine green, ICG, near-infrared fluorescence imaging, skin squamous cell carcinoma, biopsy, radical resection
Introduction
Skin is the largest organ of body, and the incidence of malignancy in skin is high. Many reports show that skin cancer is the most common cancer, accounting for about half of the total cancers, and its incidence is increasing year by year and globally.1,2 In 2016 and 2017, 67,867 patients with skin cancer were diagnosed in Japan, of which the proportions of basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanoma were 37.2%, 43.9% and 7.2%, respectively.3 In China, squamous cell carcinoma (SCC), as the most common skin cancer, originated in the epidermis of skin, occurs mainly in the exposed parts of the elderly. According to the statistics, SCC accounted for 80%–90% in skin cancers.1 However, the metastasis and malignancy of skin SCC (SSCC) are relatively low, and the general prognosis is good, but recent evidence suggests that the mortality of SSCC may be underestimated.4 SSCC secondary to burn scar, osteomyelitis sinus, urethral fistula, hairy sinus, syphilis, vaccinated scar and radiation ulcer, accounts for more than 70%.5 The malignant transformation rate of burn or other traumatic scar is 0.5% −2.0%.6 Furthermore, SSCC arising from a leg ulcer, burn scar, radiation dermatitis, and other chronic wounds has a reported metastatic risk of 26%.7
For the skin cancer, the treatment methods mainly include surgery, laser, radiotherapy, photodynamic therapy, etc. Among of them, surgical resection is still the most effective way.8 However, the precise positioning of the tumor has been plaguing clinicians and researchers for many years. During surgery, it is difficult to determine the edge of the tumor. Surgeons usually have to observe the tumor with their naked eyes and feel the tissues with their fingers. In many cases, this approach achieved tumor resection with negative margins accounted for only 50%.9,10 Among the patients with malignant tumors who underwent surgery, 20%–50% died of local recurrence,7 which was caused by tumor cells left during surgery, indicating that doctors failed to remove all the tumor tissues. During surgery, less resection may result in recurrence while more resection may cause harm to the patient.11 The surgeon has to balance the need for complete removal of the skin cancer against the conservation of surrounding healthy tissue.12 Determining the edge of the tumor is an enormous challenge, especially when the tumor infiltrates adjacent tissues, or the surrounding tissues changes due to vascular occlusion. Therefore, it is difficult to completely remove the tumor tissue via radical excision surgery, and the patients are more prone to local recurrence. Of course, the pathological consultation can also be used in the surgery. But there are many issues in the frozen section examination, including the technical challenges of the frozen tissue, the cost and the lack of real-time permanent validity. This surgical method is not only time consuming, but also wastes a lot of laboring and financial resources.
In recent years, near-infrared (NIR) fluorescence imaging technique of indocyanine green (ICG) has been used in experimental and clinical researches for breast cancer,13,14 lung cancer15,16 and hepatocellular carcinoma.17,18 This new technology is a low-energy method, so that it is relatively safe for the surgeons and the patients and considerably convenient for the application of bedside or the operating room. ICG, a traditional clinical near infrared (NIR) fluorescent dye, is currently approved by FDA.19 It can be intravenously administered for fluorescence imaging of the cancer tissue.16–19 It has no receptor specificity, but spreads due to the pressure difference between tumor blood vessels and lymphatic system.20 Several studies have reported that the tumor will emit fluorescence under the NIR light irradiation after the tumor tissues are labeled with NIR dyes, which effectively visualizes the tumor tissues and their edges.19,21 Preliminary studies of several cancer models and solid tumor cases have shown that ICG fluorescence imaging is indeed a reasonable method for determining solid tumors.19 It can achieve good visualization in surgery and has a good contrast between normal tissues and tumor tissues. Compared to other tumors, superficial skin tumors are easier to be observed by ICG fluorescence imaging. Recently, ICG fluorescence imaging has been used for detecting skin metastases of hypopharyngeal squamous carcinoma22 and sentinel lymph node biopsy in melanoma.23 This technique may provide a new method to improve the diagnosis and treatment of skin cancer. Therefore, in the present study, we aim to verify the feasibility and effectiveness of ICG fluorescence imaging used for guiding the biopsy and resection of skin squamous cell carcinoma (SSCC).
Materials and Methods
Patients
From November 2014 to April 2017, 60 patients with definitely diagnosed (42 cases) or highly suspected (18 cases) SSCC admitted to the Institute of Burn Research, Southwest Hospital, Army Medical University (Chongqing, China) were enrolled as the experimental subjects, including 44 males and 16 females, with an average age of 51.3 y ± 16.6 y. As a control, 38 patients without ICG fluorescence imaging were also analyzed. The demographic and clinical data were detailed in Table 1. The diagnosis of SSCC is primarily based on clinical features, and confirmed by skin biopsy.24
 |
Table 1 Demographic and Clinical Characteristics of the Patients with SSCC |
The exclusion criteria were as follows: (1) history of allergies to ICG, iodine and other serious allergies; (2) serious diseases of heart, lung, liver, kidney, hematopoietic system, nervous system and spirit; (3) contraindication for surgery with exclusion by ECG, echocardiography, pulmonary function and blood gas analysis.
Reagents and Equipment
Indocyanine green (ICG) for injection was purchased from Dandong Yichuang Pharmaceutical Co., Ltd (Dandong, China). The near-infrared fluorescence detector (FLUOPTICS 700, Grenoble, France) was provided by Shanghai Huijia Biological Instrument Co. Ltd (Shanghai, China).
Methods
ICG was administered intravenously to the patients at a dose of 0.5 mg/kg 24 hours prior to the biopsy or operation. According to the ICG fluorescence range and intensity under the excitation of near-infrared light, the skin lesions were marked and the tumor tissues were obtained for pathological examination preoperatively. Intraoperative ICG fluorescence imaging was then conducted to confirm whether fluorescence signals were still remained. If any fluorescence signal was found, the surgical resection would also be expanded accordingly under the navigation. Finally, the surgical specimens were biopsied guided by ICG fluorescence imaging for postoperative pathology.
Image Analysis
The real time fluorescence images can be directly observed on the computer screen during surgery. The margin between normal tissue and tumor tissue was easily assessed according to the intensity and scope of fluorescence signals, and the extent and depth of resection was subsequently adjusted. The fluorescence intensity was quantitatively analyzed by the software Image J 6.0 (National Institutes of Health, USA).
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 (IBM SPSS software, USA). The data were described as counts and relative frequency. The comparison for the two groups was conducted by chi-square test. P < 0.05 was considered statistically significant.
Results
Fluorescence Imaging
The total number of imaging times was 118 in 60 patients, including 60 times preoperative imaging for biopsy, 53 times intraoperative imaging for radical resection and biopsy, and 5 times imaging for follow-up. According to the type of fluorescent imaging, 60 patients were divided into three types (Figure 1), including 13 cases (21.7%) of complete type, 26 cases (43.3%) of partial type and 21 cases (35.0%) of peripheral type.
 |
Figure 1 Three types of ICG fluorescence imaging. |
Pathological Diagnosis of Skin Specimens
For imaging-guided skin biopsy (18 times preoperatively and 53 times intraoperatively), 138 specimens were obtained in 60 patients, of which 122 specimens (88.4%) were squamous cell carcinoma, and 16 specimens (11.6%) were chronic inflammation.
In preoperative biopsy, 32 specimens were obtained in 18 patients with strong fluorescence signals. The pathological diagnoses of 28 specimens were squamous cell carcinoma, and the rest were chronic inflammation, so the accuracy rate was 87.5% (28/32). In the same period, 62 specimens were obtained from 38 patients with naked-eye biopsy. The pathological results of 41 specimens were squamous cell carcinoma, and the rest were squamous cell hyperplasia (14 specimens) or chronic inflammation (7 specimens). Therefore, the biopsy accuracy was 66.1% (41/62) (χ2=4.938, P < 0.05). In surgery, 106 resected tissue specimens with strong fluorescence signals were obtained. The pathological results showed that 94 specimens were squamous cell carcinoma, and the accuracy rate was 88.7% (94/106).
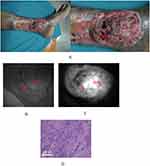
Typical case 1 A 68-year-old male was clinically diagnosed as scar cancer of the left medial malleolus. The patient was preoperatively biopsied under the navigation of ICG fluorescence imaging (Figure 2A–C). Two pieces of tissues with strong fluorescence signals were obtained for the pathological examination. The result revealed that the specimens were both squamous cell carcinoma (Figure 2D).
Intraoperative Real-Time Imaging and Recurrence
Fifty-three patients underwent intraoperative real-time ICG fluorescence imaging, with residual fluorescent signals in 24 cases and no residual signals in 29 cases. For the cases with residual fluorescent signals, the resection was expanded until the signals disappeared. Following up to November 2022, of the 24 cases, 3 cases were respectively of recurrence at 3, 5 and 5 months after operation. Of the 29 cases, 2 cases were respectively of recurrence at 4 and 6 months post-surgery. The recurrence rate was 9.4% (5/53) for the 53 cases. In the same period, 35 patients received routine radical resection without the navigation of ICG fluorescence imaging. Of the 35 cases, 4 cases were found recurrent, and the recurrence rate was 11.4% (4/35). Therefore, there was no significant difference in the recurrence rate between the two groups (χ2=0.157, P > 0.05) (Table 2).
 |
Table 2 Intraoperative Real-Time ICG Fluorescence Imaging (IFI) and Recurrence |
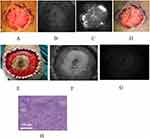
Typical case 2 A 43-year-old male, with scar carcinoma of left inguinal region and thigh, underwent intraoperative real-time ICG imaging for the wound after tumor resection (Figure 3A–D). Residual fluorescent signals were found in the left thigh (Figure 3D), and the resection was expanded until the signals disappeared under the navigation of ICG fluorescence imaging. The tissues in the margins of the wound after tumor resection were biopsied for the pathological examination. The result revealed that the specimens were all non-squamous cell carcinoma (Figure 3E–G). Follow-up to November 2022, no recurrence was found.
Typical case 3 A 60-year-old female, with squamous cell carcinoma of scalp, underwent preoperative ICG fluorescence imaging to provide reference for defining the surgical margin (Figure 4A–D). Intraoperative ICG fluorescence imaging was also performed for the wound after tumor resection (Figure 4E–G). No residual fluorescence signal was found (Figure 4G). The pathological examination of the resected tumor tissues revealed squamous cell carcinoma (Figure 4H). Follow-up to November 2022, no recurrence was found.
Lymph Nodes Examination
Fifteen cases with lymphadenopathy were found in the 60 patients after physical and radiologic examination (Table 3). Lymph node tissues of 9 cases with enlarged lymph nodes were biopsied for pathological examination. Five cases were diagnosed with lymph node metastasis, and the other 4 cases were chronic inflammation. The positive rate was 55.6% (5/9) and the metastasis rate was 8.3% (5/60). Four patients underwent sentinel lymph node localization under ICG fluorescence imaging. Eight lymph node specimens were obtained for pathological examination, of which 4 (50%) were squamous cell carcinoma metastasis and 4 (50%) were chronic inflammation. Five patients underwent lymph node exploration under ICG fluorescence imaging. Nine lymph node specimens were obtained for pathological examination, of which 5 (55.5%) were squamous cell carcinoma metastases and 4 (44.5%) were chronic inflammation. In the 5 cases with definite diagnosis of lymph node metastases, 4 patients had inguinal lymph node metastases, and 1 patient had popliteal lymph node metastases.
 |
Table 3 Lymph Nodes Examination |
Typical case 4 A 49-year-old male, was diagnosed as scar squamous cell carcinoma of the right heel. ICG fluorescence imaging was preoperatively performed on the sentinel lymph nodes of the right lower limb, and the location of the lymph nodes was delineated according to the imaging (Figure 5).
Typical case 5 A 58-year-old female, was diagnosed as well differentiated squamous cell carcinoma of the right leg with right inguinal lymph node metastases. Inguinal lymph node dissection was performed during radical operation, and ICG fluorescence imaging was also used to detect the specimens of the lymph nodes (Figure 6). A pathological examination of the lymph nodes revealed metastatic squamous cell carcinoma.
Discussion
Skin squamous cell carcinoma (SSCC) is a malignant tumor derived from epidermal or adnexal keratinocytes, and its incidence accounted for 20%–50% of skin cancers.25 SSCC has a complex etiology, high clinical misdiagnosis rate, malignant degree and strong destructiveness.26 In China, it was reported that the age of more than 60 years was the incidence peak.27 There were also domestic data showing that the cases aged 50–60 years accounted for 30.1%–35.3%, followed by 61–70 years (20.4%–28.0%), and the cases under 40 years old were rare, which is comparable with our study.28 In the present study, the ratio of male to female was 2.75: 1 (44/16), and the location distribution is 21 cases of head and neck (35.0%), 7 cases of upper limbs (11.7%), and 32 cases of lower limbs (53.3%), which are close to the published data.28
ICG fluorescence imaging, which can provide real-time visualization, has received more and more attention for its potential of clinic use.18,29,30 It can be utilized not only in surgery, but also for preoperative localization, pathological biopsy and intraoperative pathological biopsy.31 However at present, there are few reports on the application of this technology in the pathological biopsy and preoperative localization. Clinically, doctors employ the traditional method to biopsy tumor tissues, and sometimes multiple biopsies are required to make a definite diagnosis. As a result, in this study, the ICG fluorescence navigation system was innovatively applied in the preoperative localization and pathological biopsy. The results showed that the accuracy of preoperative pathological biopsy under ICG fluorescence imaging was 87.5%, which was significantly higher than that of the routine pathological biopsy in 38 cases in the same period (66.1%, P < 0.05). These results suggest that ICG fluorescence imaging is highly sensitive to SSCC and can significantly improve the accuracy of biopsy for SSCC diagnosis.
So far, ICG fluorescence imaging has been successfully applied in the detection and treatment of many tumors, such as lung, gastrointestinal, liver and breast tumors.32 The near infrared fluorescence imaging was believed to be an important supplement to the traditional imaging techniques, and it was possible to find some easily missed lesions. Theoretically, near-infrared light penetrates human tissues at a depth of about 10 mm,33 so the ICG fluorescence navigation technology in the present study can only be utilized to detect superficial skin tumor tissues. In fact, the restriction of the depth has little effect because some tumors that erode deeper tissues can be detected by imaging the wound after tumor resection in surgery.30,34 Compared with the traditional Mohs microsurgery, which requires repeated biopsy and frozen sections for pathological examination, the ICG fluorescence navigation technology possesses the advantages of simplicity, rapidity and real-time.35 In this study, the tumor boundaries of 53 patients were successfully oriented by ICG fluorescence imaging during operation, and the tumor tissues were resected radically according to the imaging results, which was consistent with the experimental results in the gastric cancers.36,37 A total of 5 cases of 53 patients detected by intraoperative ICG fluorescence imaging recurred, and the recurrence rate was 9.4%. In the same period, there were 35 cases of conventional surgery for SSCC without ICG fluorescence imaging, including 4 cases of recurrence, and the recurrence rate was 11.4% (χ2=0.157, P > 0.05). Previous reports showed that the 5-year recurrence rates of cutaneous SCC were 8% and 3%, respectively, after standard surgery and Mohs’ micrographic surgery.38 The relatively high recurrence rates in the present study should be interpreted carefully due to the following limitations. First, the number of cases was too small to distinguish the differences of the recurrence rates between the intraoperative ICG fluorescence imaging group and the conventional group without ICG fluorescence imaging. Second, due to the nature of the non-randomized concurrent controlled trial, there are inevitably some sample selection biases in the present study. Thus, large cases of multicenter randomized controlled long-term observation research are still needed to assess the efficacy of ICG fluorescence imaging in the resection of SSCC. However, with the more and more application of ICG fluorescence imaging,34,39,40 it is believed that this technology will become a promising method to identify tumor margins during SSCC resection and detect residual tumor tissues in the wound after resection.
At present, ICG fluorescence imaging has been widely used in the identification of sentinel lymph nodes of lung cancer, breast cancer, colorectal cancer, gastric cancer and melanoma.29,32,41–43 The detection rates of ranged from 70% to 99%, which might be attributed to ICG concentrations, injection timings, solidity ratios of nodules, and mechanical issues.32 However, the experiences of the exploration of sentinel lymph nodes in SSCC under ICG fluorescence imaging are quite few. In the present study, sentinel lymph nodes were successfully detected under ICG fluorescence imaging in the 4 patients with suspected lymph node metastases, and the location of lymph nodes can be precisely identified. Thus, ICG fluorescence imaging shows high effectiveness in positioning lymph nodes, determining regional lymph node status and guiding intraoperative lymph node dissection. In other words, during operation, under ICG fluorescence imaging, doctors can position swollen lymph nodes conveniently and quickly, and conduct lymph node dissection thoroughly with real-time image-based feedback.
In view of the clinical application of the ICG fluorescent navigation system, there are at least the following limitations that need to be addressed. First, the signal-to-background ratio of ICG fluorescence imaging is not high enough so that the images can only be observed under dark field. Second, due to the nonspecific distribution of ICG, the contrast between tumor tissues and inflammatory tissues is not very obvious. Third, the imaging system used in this study has also its own limitation. The strong background light source has a great interference to the imaging process so that a dark environment is required which is quite unfavorable for doctors to operate well. Recently, some researchers have conjugated ICG and polyethylene glycol (PEG) to form nanoparticles, which greatly improves the retention ability in tumor tissues.44 Other researches have shown that some specific targeting components, such as antibodies and ligands, were introduced into the structure of ICG to acquire a stronger tumor-targeting ability.35,45 However, the safety and effectiveness of modified ICG in the clinical trials still need to be further evaluated. It is also necessary to explore or construct novel near-infrared contrast agents with stronger tumor-targeting.
Conclusion
This study shows that ICG fluorescence imaging technique can guide the pathology biopsy to improve the accuracy of pathological examination, and help to identify the boundaries of tumor tissues and sentinel lymph nodes to resect tumor radically during operation. ICG fluorescence imaging can be popularized and applied widely in the diagnosis and treatment of SSCC.
Ethics Approval and Informed Consent
The present study was approved by the Ethics Review Committee of Southwest Hospital, Army Medical University (Third Military Medical University) (Chongqing, China) and complied with the Declaration of Helsinki. All patients provided written informed consents for participation in this study. The informed consents for publication of case details were also obtained from the patients, in line with the CARE guidelines.
Acknowledgments
The authors sincerely thank the medical staff at our center and the team of Prof. Chunmeng Shi (Institute of Combined Injury, State Key Laboratory of Trauma, Burns and Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University) for their help during the implementation of the present study. This work was supported by the Talent Program of Army Medical University (Grant No. XZ-2019-505-065). However, the funding source had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146:1–6. doi:10.1046/j.1365-2133.146.s61.2.x
2. Apalla Z, Nashan D, Weller RB, Castellsague X. Skin Cancer: epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol Ther. 2017;7(1):s5–s19. doi:10.1007/s13555-016-0165-y
3. Ogata D, Namikawa K, Nakano E, et al. Epidemiology of skin cancer based on Japan’s National Cancer Registry 2016–2017. Cancer Sci. 2023;114(7):2986–2992. doi:10.1111/cas.15823
4. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. doi:10.1016/j.jaad.2012.11.037
5. Samarasinghe V, Madan V. Nonmelanoma Skin Cancer. J Cutan Aesthet Surg. 2012;5(1):3–10. doi:10.4103/0974-2077.94323
6. Gul U, Kilic A. Squamous cell carcinoma developing on burn scar. Ann Plast Surg. 2006;56(4):406–408. doi:10.1097/01.sap.0000200734.74303.d5
7. Abigail W, Chrysalyne S. Cutaneous Squamous Cell Carcinoma. Hematol Oncol Clin N Am. 2019;33(1):1–12. doi:10.1016/j.hoc.2018.08.001
8. Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol. 2011;18(3):603–607. doi:10.1245/s10434-010-1442-0
9. Klimberg VS, Harms S, Korourian S. Assessing margin status. Surg Oncol. 1999;8(2):77–84. doi:10.1016/S0960-7404(99)00031-6
10. Vaidya A, Hawke C, Tiguert R, Civantos F, Soloway M. Intraoperative T staging in radical retropubic prostatectomy: is it reliable? Urology. 2001;57(5):949–954. doi:10.1016/S0090-4295(01)00904-9
11. Sim FW, Xiao HD, Bell RB. Margin analysis: squamous cell carcinoma of the oropharynx. Oral Maxillofac Surg Clin North Am. 2017;29(3):269–280. doi:10.1016/j.coms.2017.03.004
12. Rasmussen SM, Nielsen T, Hody S, Hager H, Schousboe LP. Photoplethysmography for demarcation of cutaneous squamous cell carcinoma. Sci Rep. 2021;11(1):21467. doi:10.1038/s41598-021-00645-4
13. Kang B, Lee JH, Lee J, et al. Comparative Study Between Radioisotope Uptake and Fluorescence Intensity of Indocyanine Green for Sentinel Lymph Node Biopsy in Breast Cancer. J Breast Cancer. 2022;25(3):244–252. doi:10.4048/jbc.2022.25.e27
14. Yang RQ, Wang PY, Lou KL, et al. Biodegradable Nanoprobe for NIR-II Fluorescence Image-Guided Surgery and Enhanced Breast Cancer Radiotherapy Efficacy. Adv Sci. 2022;9(12). doi:10.1002/advs.202104728
15. Nomori H, Yue C, Sugimura H. Utility and pitfalls of sentinel node identification using indocyanine green during segmentectomy for cT1N0M0 non-small cell lung cancer. Surg Today. 2016;46(8):908–913. doi:10.1007/s00595-015-1248-6
16. Tashiro Y, Aoki T, Hirai T, et al. Indocyanine Green Labeling of Tumors in the Liver Recurring After Radiofrequency Ablation Enables Complete Resection by Fluorescence-guided Surgery. Anticancer Res. 2022;42(3):1345–1350. doi:10.21873/anticanres.15603
17. Jianxi W, Xiongfeng Z, Zehao Z, et al. Indocyanine green fluorescence-guided laparoscopic hepatectomy versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: a single-center propensity score matching study. Front Oncol. 2022;12:930065. doi:10.3389/fonc.2022.930065
18. Inagaki FF, Takemura N, Ito K, Mihara F, Kurokawa T, Kokudo N. Intraoperative indocyanine green fluorescence navigation facilitates complete removal of lymph node metastases from hepatocellular carcinoma. Glob Health Med. 2021;3(6):406–408. doi:10.35772/ghm.2020.01097
19. Morales-Conde S, Licardie E, Alarcón I, Balla A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: recommendations based on the descriptive review of the literature and the analysis of experience. Cir Esp. 2022;100(9):534–554. doi:10.1016/j.ciresp.2021.11.018
20. Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. Annu Rev Med. 2010;61(1):359–373. doi:10.1146/annurev.med.60.052907.094936
21. Leiloglou M, Kedrzycki MS, Chalau V, et al. Indocyanine green fluorescence image processing techniques for breast cancer macroscopic demarcation. Sci Rep. 2022;12(1):8607. doi:10.1038/s41598-022-12504-x
22. Kusada T, Yogi A, Hirakawa H, et al. Different indocyanine green fluorescence patterns of two skin metastases of hypopharyngeal squamous carcinoma: a case report. Photodiagnosis Photodyn Ther. 2021;34:102211. doi:10.1016/j.pdpdt.2021.102211
23. Göppner D, Nekwasil S, Jellestad A, Sachse A, Schönborn KH, Gollnick H. Indocyanine green-assisted sentinel lymph node biopsy in melanoma using the “FOVIS” system. J Dtsch Dermatol Ges. 2017;15(20):169–178.
24. Madan V, Lear JT, Szeimies R-M. Non-melanoma skin cancer. Lancet. 2010;375(9715):673–685. doi:10.1016/S0140-6736(09)61196-X
25. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247. doi:10.1016/j.jaad.2017.08.059
26. Burton KA, Ashack KA, Khachemoune A. Cutaneous Squamous Cell Carcinoma: a Review of High-Risk and Metastatic Disease. Am J Clin Dermatol. 2016;17(5):491–508. doi:10.1007/s40257-016-0207-3
27. Wang XS, Liao KH. Yang Guoliang Dermatology. Vol. 12. Shanghai Scientific and Technological Literature Press; 2005:978–981.
28. Wu ZB. Chinese Surgical Pathology.
29. Bargon CA, Huibers A, Young-Afat DA, et al. Sentinel Lymph Node Mapping in Breast Cancer Patients through Fluorescent Imaging using Indocyanine Green - The INFLUENCE trial. Ann Surg. 2022;276(5):913–920. doi:10.1097/SLA.0000000000005633
30. Shen Y, Zheng M, Li J, et al. Clinical Application of Indocyanine Green Fluorescence Imaging in the Resection of Hepatoblastoma: a Single Institution’s Experiences. Front Surg. 2022;9:932721. doi:10.3389/fsurg.2022.932721
31. Huber D, Hurni Y. Sentinel Node Biopsy for Endometrial Cancer by Retroperitoneal Transvaginal Natural Orifice Transluminal Endoscopic Surgery: a Preliminary Study. Front Surg. 2022;9:907548. doi:10.3389/fsurg.2022.907548
32. Dai ZY, Shen C, Mi XQ, Pu Q. The primary application of indocyanine green fluorescence imaging in surgical oncology. Front Surg. 2023;10:1077492. doi:10.3389/fsurg.2023.1077492
33. Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191–2208. doi:10.2147/NDT.S78182
34. Achterberg FB, Sibinga Mulder BG, Meijer RPJ, et al. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann Transl Med. 2020;8(21):1448. doi:10.21037/atm-20-1999
35. Géczi T, Simonka Z, Lantos J, et al. Near-infrared fluorescence guided surgery: state of the evidence from a health technology assessment perspective. Front Surg. 2022;9:919739. doi:10.3389/fsurg.2022.919739
36. Ushimaru Y, Omori T, Fujiwara Y, et al. The feasibility and safety of preoperative fluorescence marking with indocyanine green (ICG) in laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2019;23(3):468–476. doi:10.1007/s11605-018-3900-0
37. Yoon BW, Lee WY. The oncologic safety and accuracy of indocyanine green fluorescent dye marking in securing the proximal resection margin during totally laparoscopic distal gastrectomy for gastric cancer: a retrospective comparative study. World J Surg Oncol. 2022;20(1):26. doi:10.1186/s12957-022-02494-5
38. Potenza C, Bernardini N, Balduzzi V, et al. A review of the literature of surgical and nonsurgical treatments of invasive squamous cells carcinoma. Biomed Res Int. 2018;2018:9489163. doi:10.1155/2018/9489163
39. Wu Z, Dong Y, Wang Y, Hu Q, Cai H, Sun G. Clinical application of indocyanine green fluorescence navigation technology to determine the safe margin of advanced oral squamous cell carcinoma. Gland Surg. 2022;11(2):352–357. doi:10.21037/gs-22-33
40. Lee EG, Kim SK, Han JH, Lee DE, Jung SY, Lee S. Surgical outcomes of localization using indocyanine green fluorescence in breast conserving surgery: a prospective study. Sci Rep. 2021;11(1):9997. doi:10.1038/s41598-021-89423-w
41. Wang Z, Yang X, Wang J, et al. Real-Time In Situ Navigation System With Indocyanine Green Fluorescence for Sentinel Lymph Node Biopsy in Patients With Breast Cancer. Front Oncol. 2021;11. doi:10.3389/fonc.2021.621914
42. Jiang K, Luo B, Hou Z, et al. Application of an indocyanine green surgical fluorescence imaging system in sentinel lymph node biopsy of acral malignant melanoma. Ann Transl Med. 2021;9(18):1456. doi:10.21037/atm-21-4366
43. Pameijer CR, Leung A, Neves RI, Zhu J. Indocyanine green and fluorescence lymphangiography for sentinel node identification in patients with melanoma. Am J Surg. 2018;216(3):558–561. doi:10.1016/j.amjsurg.2018.01.009
44. Xue K, Wei F, Lin J, et al. Tumor acidity-responsive carrier-free nanodrugs based on targeting activation via ICG-templated assembly for NIR-II imaging-guided photothermal-chemotherapy. Biomater Sci. 2021;9(3):1008–1019. doi:10.1039/D0BM01864C
45. Goto M, Ryoo I, Naffouje S, et al. Image-guided surgery with a new tumour-targeting probe improves the identification of positive margins. EBioMedicine. 2022;76:103850. doi:10.1016/j.ebiom.2022.103850
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.