Back to Journals » OncoTargets and Therapy » Volume 12
Individualized Treatment Analysis Of Breast Cancer With Chronic Renal Failure
Received 18 July 2019
Accepted for publication 10 September 2019
Published 20 September 2019 Volume 2019:12 Pages 7767—7772
DOI https://doi.org/10.2147/OTT.S223729
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Wei Liu,1,2,* Jin-fu Peng,1,2,* Meng-jie Tang1
1Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410013, People’s Republic of China; 2Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha 410013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meng-jie Tang
Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410013, People’s Republic of China
Email [email protected]
Abstract: We report the case of a breast cancer patient with chronic renal failure (CRF). The clinical pharmacist adjusted the chemotherapy regimen and dosage according to the patient’s renal function after reviewing the literature and analyzing the pharmacological and pharmacokinetic characteristics of the patient’s antineoplastic drugs. To the best of our knowledge, this is the first report of successful multimodal treatment of breast cancer in a patient with CRF in China. The purpose of this case report is to optimize breast cancer therapy in patients with CRF and provide a reference for clinicians and clinical pharmacists to use antineoplastic drugs rationally.
Keywords: breast cancer, chronic renal failure, treatment regimen, dose adjustment
Introduction
Breast cancer is one of the most common malignant tumors in women.1 Renal failure is a difficult comorbidity in patients with breast cancer because the kidney metabolizes most chemotherapy drugs. Oncologists have limited knowledge about whether patients with renal failure can metabolize the chemotherapy drugs, which makes it difficult to develop appropriate treatment plans. At present, there are very few studies on the treatment of breast cancer in patients with end-stage renal disease (ESRD), except the case report by Modi et al,2 and these patients can rarely successfully complete a series of the standard treatments of surgery, adjuvant chemotherapy, and endocrine therapy. This report describes the case of a patient with breast cancer and comorbid CRF to explore the successful treatment strategy.
Case Report
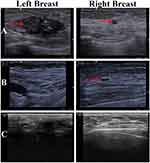
A 53-year-old Chinese woman presented to our hospital in March 2017 with a left breast mass. Breast ultrasonography revealed a 27 × 12-mm primary lesion in the left breast at the 3 o’clock position and assessed as category 5 by the Breast Imaging-Reporting and Data System (BI-RADS) and a 5 × 4-mm primary lesion in the right breast at the 12 o’clock position assessed as category 4B by the BI-RADS (Figure 1A). The biopsy pathology report showed that the left breast mass was invasive ductal carcinoma (IDC). The right breast mass was an intraductal papillary lesion with atypical hyperplasia of the ductal epithelium, and carcinoma needed to be excluded. Chest computed tomography (CT) and abdominal ultrasonography did not reveal any signs of metastasis.
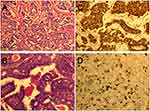
On March 29, 2017, the patient underwent breast-conserving surgery and sentinel lymph node biopsy for the right breast cancer and a modified radical mastectomy for left breast cancer. The postoperative pathological findings were left breast IDC grade III with ~30% high-grade ductal carcinoma in situ (DCIS), and 3/25 left axillary lymph nodes had evidence of metastasis. Immunohistochemistry (IHC) showed an estrogen receptor positive (ER +) frequency of 50%, progesterone receptor positive (PR +) frequency of 50%, human epidermal growth factor receptor 2 positive (HER2 +) status of 3 +, and Ki-67 index frequency of 30% (Figure 2). The right breast showed intraductal papillary carcinoma with low-grade DCIS. The TNM stage of the tumor in the left breast was pT2N1M0, stage IIA. Postoperative recovery was good. The patient had started peritoneal dialysis for treatment of renal failure and uremia in January 2016. Based on the tumor pathological characteristics, prognostic factors, age, and patient’s performance status, four cycles of adjuvant chemotherapy with a TH regimen was started in May 2017: docetaxel 80 mg/m2 with trastuzumab 8 mg/kg for the first dose and 6 mg/kg for the maintenance dose. Afterwards, four cycles of sequential adjuvant chemotherapy with the EC regimen were performed: epirubicin 75 mg/m2 and cyclophosphamide 500 mg/m2. Since both trastuzumab and epirubicin are cardiotoxic drugs, trastuzumab was temporarily discontinued during chemotherapy with the EC regimen; however, the total treatment cycle of trastuzumab was still 1 year. After completion of adjuvant chemotherapy, the patient underwent 3 cycles of radiotherapy. The patient received adjuvant endocrine therapy with tamoxifen 10 mg twice a day beginning in December 2017. Each chemotherapy session was started after the dialysate was changed, and the chemotherapy drug remained in the patient’s body for more than 8 hrs before the next peritoneal dialysis treatment. The patient tolerated the treatment well, and no serious toxicity issues were noted. The patient remains in remission.
Discussion
Breast Cancer Treatment Regimens
The National Comprehensive Cancer Network(NCCN), European Society for Medical Oncology (ESMO), and American Society of Clinical Oncology (ASCO) guidelines all indicate that for patients with ER +/PR +/HER2 + invasive breast cancer and positive lymph nodes, an anthracycline + cyclophosphamide → taxanes + trastuzumab (AC → TH) regimen is recommended for adjuvant chemotherapy; after chemotherapy, patients with ER + or PR + should receive adjuvant endocrine therapy.3 Based on the patient’s renal function status and the pharmacodynamics and safety of various drugs, we chose epirubicin and docetaxel for this patient’s chemotherapy regimen. The anthracyclines commonly used in breast cancer chemotherapy are doxorubicin and epirubicin. Renal excretion of both is minimal, but those with renal insufficiency have a higher risk of hyperuricemia with doxorubicin than with epirubicin,4 and the latter is significantly less cardiotoxic.5 Taxanes include paclitaxel and docetaxel, both of which have shown very good results in the adjuvant treatment of breast cancer. The ECOG-1199 clinical trial suggested that paclitaxel was suitable for a weekly schedule and docetaxel was suitable for a 3-weekly schedule.6 There are reports of mild renal toxicity with paclitaxel, but none with docetaxel;7 and the incidence and severity of adverse reactions such as anaphylaxis were lower with docetaxel compared to paclitaxel.8
For this patient, clinical pharmacists suggested that clinicians reverse the regimen sequence of EC → TH to TH → EC for two reasons. First, the Early Breast Cancer Trialists’ Collaborative Group meta-analysis suggested that taxane-containing regimens were significantly more effective than anthracycline-containing regimens.9 The results of the CALGB-9344 and BCIRG-001 clinical trials laid the foundation for the role of taxanes in the adjuvant treatment of node-positive breast cancer.10,11 Second, epirubicin and cyclophosphamide are more nephrotoxic than docetaxel and trastuzumab; it was unclear whether this patient could tolerate subsequent complete cycles of chemotherapy, and it is safer to give TH regimens with less nephrotoxicity first.
Dose And Administration Time Adjustments
Minimizing non-renal systemic toxicity is a particular problem for patients on chronic hemodialysis or peritoneal dialysis, especially if the details of drug metabolism and elimination are not fully understood. Dialyzed drug removal must be considered when choosing the appropriate timing of chemotherapy for dialysis-treated patients. Chemotherapy should be given after dialysis to avoid the loss of efficacy of drugs that are removed by dialysis, but not at the time of dialysis for drugs that are not removed by dialysis.12
Epirubicin is excreted in the urine in small amounts. Although Aronoff et al recommended that epirubicin or other anthracyclines need not be reduced in patients with renal failure,13 the US instructions for use (IFU) recommend that epirubicin dosage be reduced in patients with severe renal impairment (serum creatinine >5 mg/dL). One study suggested that epirubicin does not require dose adjustment in patients undergoing hemodialysis or peritoneal dialysis and should be administered after dialysis.14 The need for cyclophosphamide dose reduction in patients with renal insufficiency is controversial. Some investigators do not recommend dose modification on the grounds that renal function is not related to cyclophosphamide clearance or hematologic toxicity.15 However, one group suggested that dosage adjustment of cyclophosphamide is required in patients with creatinine clearance (CrCl) <30 mL/min,16 some investigators advocate a 25% dose reduction in patients with CrCl <10 mL/min, while others recommend 10% and 20% dose reductions in patients with CrCl values <55 and <20 mL/min, respectively.13 The Ontario Cancer Care Canada guidelines recommend a 25% dose reduction in patients receiving cyclophosphamide with CrCl 30 mL/min. The IFUs for the two cyclophosphamide marketed in China provide recommendations for different dose adjustments. One suggests a 50% dose reduction when the glomerular filtration rate (GFR) is <10 mL/min. Another suggests that the cyclophosphamide dose should be reduced to 1/2 or 1/3 of the therapeutic dose when renal insufficiency develops. In patients with ESRD, cyclophosphamide is moderately cleared by hemodialysis and should be administered after hemodialysis.14,17 Docetaxel is minimally excreted by the kidney, and limited data show that docetaxel can be safely administered to patients with renal insufficiency without dose adjustment.13 Docetaxel can be safely administered at standard doses to patients on chronic peritoneal or hemodialysis.18 Trastuzumab is not removed by hemodialysis, but there is no reliable information on whether it is cleared by peritoneal dialysis. Renal excretion of trastuzumab is very low, and renal dysfunction did not affect the disposition of trastuzumab in a population pharmacokinetic analysis. There are no dialysis dose adjustments in the US prescribing information for trastuzumab. One study suggested that tamoxifen does not require dose adjustment in patients with CRF and breast cancer receiving regular dialysis.19 Tamoxifen is excreted by the intestine, is not filtered by the kidneys, and is not easily removed by dialysis treatment.
The patient had a GFR of 5.087 mL/min and a clinical diagnosis of CRF. Based on this, we decreased the dosage of epirubicin from 90–100 to 75 mg/m2 and lowered the dosage of cyclophosphamide from 600 to 500 mg/m2; dose adjustments were not required for docetaxel, trastuzumab, and tamoxifen.
Safety And Effectiveness
The patient tolerated the treatment well. Neither neutropenia or thrombocytopenia were noted, chemotherapy induced grade I-II anemia, and clinical assessment and left ventricular ejection fraction (LVEF) measurement did not reveal any cytotoxicity. After the first chemotherapy session, the patient continued to undergo peritoneal dialysis according to the original plan. During subsequent chemotherapy and follow-up, the patient’s serum creatinine and urea values were not significantly changed compared with those before chemotherapy, indicating that the TH → EC dose-adjusted chemotherapy regimen had no significant effect on the patient’s renal function (Table 1).
 |
Table 1 Biochemical And Peripheral Blood Results |
The patient underwent whole breast ultrasound 9 and 16 months after surgery and right breast X-ray molybdenum target 16 months postoperatively.3 No progression was observed in the either breast, and no abnormal enlarged lymph nodes were observed in the bilateral armpit or supraclavicular area (Figure 1B and C). Serum levels of carbohydrate antigens 125 and 153 (CA125 and CA153) were significantly higher than normal when the patient was diagnosed with breast cancer in March 2017, but tumor marker levels all decreased to normal after surgery and were stable during adjuvant chemotherapy and endocrine therapy (Table 2). Other routine examinations showed no abnormalities. The patient was in good general condition during the follow-up period. No relevant evidence of disease progression or recurrence has been found to date. The goals of postoperative adjuvant chemotherapy and endocrine therapy were achieved.
 |
Table 2 Serum Tumor Marker Indicators In Patients |
Conclusion
In summary, CRF is not a contraindication to breast cancer surgery and systemic therapy. The improved TH → EC regimen may be a safe and effective chemotherapy regimen in the patient population. In general hospitals, breast cancer patients with CRF can still complete standard treatment and have the same potential for cure with multidisciplinary cooperation, individualized treatment plan selection, and close monitoring of renal function.
Ethics approval and consent to participate
Consent was obtained from the patient for the use of their images and medical information in this study.
Acknowledgment
This study was supported by the youth fund of the National Natural Science Foundation of China (Grant number: 81603192).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
2. Modi G, Madabhavi I, Patel A, Anand A. Treatment of breast cancer in a patient of alport syndrome-induced chronic renal failure: a triumph story. J Cancer Res Ther. 2018;14(2):462–464.
3. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(3):310–320.
4. Mohamed N, Goldstein J, Schiff J, John R. Collapsing glomerulopathy following anthracycline therapy. Am J Kidney Dis. 2013;61(5):778–781.
5. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75.
6. Schneider BP, Li L, Radovich M, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21(22):5082–5091.
7. Li YF, Fu S, Hu W, et al. Systemic anticancer therapy in gynecological cancer patients with renal dysfunction. Int J Gynecol Cancer. 2007;17(4):739–763.
8. Kadari A, Pooja D, Gora RH, et al. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur J Pharm Biopharm. 2018;132:168–179.
9. Palmieri C, Jones A. The 2011 EBCTCG polychemotherapy overview. Lancet. 2012;379(9814):390–392. doi:10.1016/S0140-6736(11)61823-0
10. Sartor CI, Peterson BL, Woolf S, et al. Effect of addition of adjuvant paclitaxel on radiotherapy delivery and locoregional control of node-positive breast cancer: cancer and leukemia group B 9344. J Clin Oncol. 2005;23(1):30–40. doi:10.1200/JCO.2005.12.044
11. Pajares B, Pollan M, Martin M, et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast Cancer Res. 2013;15(6):R105. doi:10.1186/bcr3572
12. Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–1384. doi:10.1002/cncr.22904
13. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children.
14. Janus N, Thariat J, Boulanger H, Deray G, Launay-Vacher V. Proposal for dosage adjustment and timing of chemotherapy in hemodialyzed patients. Ann Oncol. 2010;21(7):1395–1403. doi:10.1093/annonc/mdp598
15. Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function. Cancer Treat Rev. 1995;21(1):33–64.
16. Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int. 2002;61(4):1495–1501. doi:10.1046/j.1523-1755.2002.00279.x
17. Perry JJ, Fleming RA, Rocco MV, et al. Administration and pharmacokinetics of high-dose cyclophosphamide with hemodialysis support for allogeneic bone marrow transplantation in acute leukemia and end-stage renal disease. Bone Marrow Transplant. 1999;23(8):839–842. doi:10.1038/sj.bmt.1701646
18. Heijns JB, van der Burg ME, van Gelder T, et al. Continuous ambulatory peritoneal dialysis: pharmacokinetics and clinical outcome of paclitaxel and carboplatin treatment. Cancer Chemother Pharmacol. 2008;62(5):841–847. doi:10.1007/s00280-007-0671-9
19. Langenegger T, Wahl P, Schiesser D, Thurlimann B. Plasma levels of tamoxifen, N-desmethyl tamoxifen and anastrozole in a patient with metastatic breast cancer and chronic hemodialysis. Breast Cancer Res Treat. 2006;100(2):177–181. doi:10.1007/s10549-006-9243-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.