Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Individualized Treatment Algorithm Using Hyaluronic Acid Fillers for Lifting, Contouring and Volumizing the Midface
Authors Di Gregorio C, Gauglitz G, Partridge J
Received 21 December 2021
Accepted for publication 18 March 2022
Published 14 April 2022 Volume 2022:15 Pages 681—690
DOI https://doi.org/10.2147/CCID.S353878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Carlo Di Gregorio,1 Gerd Gauglitz,2 Jackie Partridge3
1Plastic Surgery Unit Clinica Candela, Palermo, Italy; 2Haut- und Laserzentrum im Glockenbachviertel, Munich, Germany; 3The Aesthetic Consultancy, Edinburgh, Scotland
Correspondence: Carlo Di Gregorio, Plastic Surgery Unit Clinica Candela, via Villareale 54, Palermo, 90141, Italy, Tel +39 91 586592, Email [email protected]
Purpose: Patients seeking treatment for the midface are individuals with different objectives and characteristics of skin and soft tissue. In this study, patients were treated in the midface with one of three products with different properties; HARV, HARD, or HARL, using a treatment algorithm based on primary need for treatment (volumizing, lifting or contouring) and tissue coverage (thin or thick tissue). The aim was to optimize the treatment outcome and to provide an individualized treatment approach that distinguishes different types of patients seeking midfacial treatment.
Materials and Methods: Subjects were treated in the midface at baseline to achieve optimal aesthetic results (≤ 2 mL per side of the face), and touch-up was allowed after 4 weeks (≤ 1 mL per side of the face). Study visits were scheduled at Weeks 8, 16 and 24. Assessments included aesthetic improvement of the midface, evaluation of midface fullness, and subject satisfaction. Safety evaluations included local tolerability symptoms collected at 4 weeks after treatment and adverse events.
Results: A total of 90 subjects were included in the study, mean age was 45 years (range 29– 55) and 82% of subjects were female. Mean total injected volume for the products was 4.4 mL (HARV and HARL) and 4.2 mL (HARD). At least 92% of subjects were assessed as aesthetically improved throughout the study. Assessment of midface fullness showed ≥ 90% of subjects being responders until Week 24. In addition, subject satisfaction was high throughout the study. Tenderness, bruising and swelling were the most reported local tolerability symptom for all study products, and no adverse events related to study product/treatment were reported.
Conclusion: Midface treatment with either HARV, HARD, or HARL using a treatment algorithm to guide the choice of product to individual subject needs was effective and safe for up to 24 weeks after treatment.
Keywords: aesthetic improvement, MMVS, subject satisfaction, natural-looking, nasolabial fold, safety
Introduction
A broad range of injectable products including hyaluronic acid (HA) fillers are currently available to provide aesthetic treatments of the midface.1,2 The concept of Restylane® (Galderma, Uppsala, Sweden) gel technology is individualized treatment with a complete portfolio of HA fillers from two complimentary manufacturing technologies, resulting in a broad range of products with distinct properties.3 From the two technologies, the injectors can optimize their choice based on the gel’s rheological properties from firm to flexible and the patient’s individual needs.4
HA fillers manufactured using the NASHA™ technology are firm gels with a high G’, adapted for deep injections, providing distinct lifting capacity and support. The OBT™ technology (XpresHAn in the US) is characterized by soft, flexible gels with varying degrees of cross-linking, that provide contouring and natural expression in dynamic areas.
Restylane Lyft (HARL) with lidocaine, manufactured using NASHA technology is the firmest of the Restylane full range of gels, thereby providing lift and structure for a pronounced effect. Restylane Defyne (hereafter HARD) with lidocaine has the highest degree of firmness among the OBT gels, and is designed to provide contouring and maintain dynamic expression in areas of high movement in the face.5–7 Restylane Volyme (hereafter HARV) also containing lidocaine, is a softer OBT gel than HARD, an optimal gel for restoring a soft contour and natural looking volume with diffuse tissue integration.8
Patients seeking treatment for the midface are individuals with different demographics, treatment objectives and characteristics of skin and soft tissue. A thorough and accurate facial analysis is therefore key to ensuring optimal aesthetic outcomes. Also, while some patients may need to restore volume loss due to aging, others may wish to enhance shape and contours of the face. In this study, patients were treated in the midface with one of the products HARV, HARD or HARL based on specific treatment objectives and skin characteristics to provide an individualized treatment approach that distinguishes different types of patients presenting to aesthetic health care practitioners. The intention of the treatment was to improve the appearance of the subjects compared to before treatment and to achieve a result that was perceived as natural-looking. To our knowledge, this is the first study that combines three products with different properties to optimize the treatment outcome based the individual skin characteristics.
Materials and Methods
This was a multi-center, evaluator-blinded, 24-week study conducted in Italy, Germany and Scotland. The study protocol conformed to the Declaration of Helsinki and was approved by independent ethics committees (Comitato Etico Palermo 1, Ref. no. 10/2018; Ethik-Kommission der Bayerischen Landesärztekammer, Ref. no. 18054; Scotland A Research Ethics Committee, Ref. no. 18/SS/0102). Subjects provided a written informed consent for participation in the study and for the use of photographs in scientific literature. Study period was 09 Nov 2018 to 30 Aug 2019. Data from this clinical trial is presented in the Clinicaltrial.gov database (NCT03869450). No other data sharing will be made.
Subjects and Treatment
Eligible subjects were 25–55 years of age, in need of lifting, contouring or volumization of the midface and with Medicis Midface Volume Scale9 (MMVS) score 2–4 on each side of the face (same on both sides), defined as mild to substantial loss of midface fullness. Main exclusion criteria included treatment in the facial area with hyaluronic acid or collagen during the last 12 months, or subjects with permanent facial tissue augmentation. Product selection was based on a predefined treatment algorithm covering main treatment goal, ie volumizing or lifting/contouring, followed by investigator assessment of subject tissue coverage (thin or thick), presented in Figure 1. Thin tissue cover was defined as: skin in the cheek area which was, based on the investigator’s opinion, comprising soft tissue that may have a thinner vs thicker dermis, and relatively less vs more subcutaneous fat. Thick tissue cover was defined as: skin in the cheek area which was, comprising soft tissue that may have a thicker vs thinner dermis, and relatively more vs less subcutaneous fat. The pinch test and slide test in the cheek area could help influence the decision regarding tissue coverage.
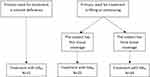 |
Figure 1 Treatment algorithm. Abbreviations: HARV, Restylane Volyme; HARD, Restylane Defyne; HARL, Restylane Lyft. |
Subjects were treated in the midface at baseline (≤2 mL per side of the face) to achieve optimal aesthetic results, and touch-up was allowed after 4 weeks (≤1 mL per side of the face) to further improve the aesthetic appearance. Optimal aesthetic improvement was defined as the best possible aesthetic result that could be obtained for an individual subject, as agreed by the treating investigator and subject (HARD and HARL), or at least 1-grade improvement on MMVS, assessed by the treating investigator (HARV). The injection technique was chosen at the discretion of the treating investigator and was in accordance with the Instruction for use for the study product. Separate sterile needles or cannulas were used for each injection. The investigational product contained lidocaine hydrochloride, but additional local anesthesia could be used at the discretion of the investigator to further reduce pain on injection. Aseptic technique and standard practice to prevent cross-infections were observed at all times.
Midface was defined as the area inferior to the maxillary prominence, superior to the plane of nasal alae, including the area from the attachment of the ear to the face to the medial canthus and lateral to the nose on the subject’s right and left sides. Treatment area was lateral/superior to the nasolabial folds that thus were not treated. Subjects were followed for 24 weeks after treatment with 3 visits scheduled at Weeks 8, 16 and 24.
Study Assessments
Endpoints for effectiveness included: 1) Assessment of aesthetic improvement of the midface using Global Aesthetic Improvement Scale (GAIS) by subjects and investigators at 8, 16 and 24 weeks after treatment and by blinded evaluation at Week 8 by comparing the appearance at follow-up against a photograph taken before treatment. The photographs were presented on a computer monitor (preferably the same monitor throughout the study). Live assessment could help the investigator and the blinded evaluator and a mirror could help the subject; 2) Live evaluation of midface fullness using the validated MMVS by investigators at 8, 16 and 24 weeks after treatment and by blinded evaluation at Week 8; 3) Subject satisfaction using a questionnaire as well as the FACE-Q Psychological function, indicating how subjects felt about their facial appearance from 0 (worst) to 100 (best) at baseline and at Weeks 8, 16 and 24; 4) Naturalness of treatment results was assessed by subjects and investigators at 8, 16 and 24 weeks after treatment and by blinded evaluation at Week 8, and was based on review of baseline photographs and live assessment; 5) Effect on lifting and contouring using a questionnaire was assessed by investigators (only for HARD and HARL) at Weeks 8, 16 and 24; 6) Nasolabial fold wrinkle severity was assessed live at Week 8 by treating investigators and blinded evaluators at baseline and Week 8 using the validated Wrinkle Severity Rating Scale10 (WSRS) (only applicable for subjects with a WSRS score of ≥2 for both nasolabial folds at baseline).
Endpoints for safety included local tolerability symptoms as expected after injection with HA fillers for cheek augmentation,2 collected by direct questioning to subjects at 4 weeks after first treatment and touch-up. Adverse events were collected throughout the study.
Statistical Analysis
Two analysis populations were defined for the study; the Safety population included all subjects who were injected at least once in any side of the midface, and the modified Intention-to-treat (mITT) population consisted of all subjects who were injected in both right and left side of the midface. All analysis for effectiveness were based on the mITT population and the safety analysis was performed based on the Safety population. GAIS was summarized in frequency tables by treatment group and visit. In addition, the proportion of improved subjects (improved, much improved and very much improved) was calculated together with a 95% confidence interval. MMVS was summarized in frequency tables by treatment group and visit. MMVS score and change from baseline was also summarized at each visit using mean and standard deviation. Change from baseline was analyzed with Wilcoxon signed-rank test. In addition, the proportion of improved subjects (decrease in MMVS of at least one grade compared to baseline) was calculated together with a 95% confidence interval. Subject satisfaction questionnaire, FACE-Q, evaluation of naturalness, and the investigator questionnaire regarding the effect of lifting and facial contouring were presented descriptively by treatment group and visit. WSRS was summarized in frequency tables by treatment group and visit. In addition, the proportion of improved subjects (decrease in WSRS of at least one grade compared to baseline) was calculated together with a 95% confidence interval. WSRS score and change from baseline was also summarized at each visit using mean and standard deviation. Local tolerability symptoms and adverse events were analyzed descriptively by treatment group.
Results
Demographics and Treatment
A total of 90 subjects were enrolled in the study, all were included in the Safety and mITT populations. All subjects except 3 that were lost to follow-up completed the study. Subject disposition according to the treatment algorithm is shown in Figure 1. Mean age for the whole study population was 45 years (range 29–55) and a majority of subjects were female (82%). All subjects except one were White; one was Black. Baseline MMVS scores for the whole study population were 3 (moderate loss) for the majority of subjects (64.4%), followed by 2 (mild loss) for 21.1% and 4 (substantial loss) for 14.4% of subjects. The MMVS scores showed moderate to substantial loss of volume in all subjects in the HARV group, whereas subjects in the other two groups mainly had mild to moderate loss. This was in line with the treatment algorithm of HARV being intended for subjects needing treatment for volume deficiency. Subject demographics for the different study products are presented in Table 1. Similar mean injection volume was used for all products at initial and touch-up treatment; mean total injected volume was 4.4 mL (HARV and HARL), 4.2 mL (HARD). Details are presented in Table 2. Injections were generally at the supraperiosteal depth for HARV and at the supraperiosteal or subcutis depth for HARD and HARL. Bolus injection was the most common injection technique for all 3 products at initial treatment, while bolus or linear threading were mainly used at touch-up.
 |
Table 1 Subject Demographics |
 |
Table 2 Injection Characteristics, Both Sides |
Effectiveness
Aesthetic improvement was high overall; at least 92% of subjects were assessed as aesthetically improved by subjects, investigators and by blinded evaluation throughout the study until Week 24 (Figure 2A). Also, a majority of subjects were improved at all visits when evaluated by study product, as shown in Figure 2B for investigator assessment and blinded evaluation. Representative subject photographs are presented in Figures 3–5.
Investigator assessment of midface fullness using MMVS showed at least 90% of subjects overall being responders (≥1-grade improvement from baseline) on both sides of the face until Week 24. MMVS responder rate was high throughout the study also when analyzed by study product, (Figure 6). In addition, the change from baseline in MMVS scores, based on the treating investigator’s assessments, demonstrated statistically significant improvement at all time points after treatment with all 3 study products on both sides of the face (p<0.001).
FACE-Q total scores indicating how the subjects felt about themselves after treatment were statistically significantly higher compared to baseline (p<0.001) at all visits throughout the study in the subject population as a whole, with the largest change from baseline occurring at Week 24. Subject satisfaction with treatment was high for all 3 study products, and at Week 24 at least 70% of subjects agreed or strongly agreed that the treatment makes you look younger, makes you feel better about yourself, improves your attractiveness, improves your self-esteem/self-confidence, makes you look the way you feel, and improves your facial symmetry/balance (Figure 7). At Week 24, 97% of all subjects replied they would do the treatment again and recommend the treatment to a friend.
 |
Figure 7 Subject satisfaction, Week 24. Abbreviations: HARV, Restylane Volyme; HARD, Restylane Defyne; HARL, Restylane Lyft. |
Both treating investigators and blinded evaluators assessed the treatment results as looking natural for ≥95% of the subjects overall. For each product, the proportion of subjects that was assessed by the treating investigators and blinded evaluator as looking natural at Week 8 was 100% for subjects injected with HARD and ≥94% for subjects injected with HARV and HARL.
The investigators assessed that the treatment had achieved a lifting effect for a majority of subjects (HARD: ≥92%, HARL: ≥67%) as well as facial contouring improvement (HARD ≥96%), HARL: ≥94%) throughout the study.
Midface treatment also resulted in improvement in nasolabial fold wrinkle severity for subjects having an MMVS score of ≥2 at baseline (all subjects except 1), as assessed by treating investigator and blinded evaluator at Week 8 (Figure 8).
Safety
All treatments were well tolerated, no adverse events related to study product/treatment were reported. Tenderness, bruising and swelling were the most reported local tolerability symptom for all study products (Table 3). All symptoms were mild or moderate, except one case of severe bruising after treatment with HARV, and all symptoms were resolved within 2 weeks.
 |
Table 3 Local Tolerability Symptoms After Initial Treatment |
Discussion
Key aspects to ensure optimal aesthetic results are an accurate facial analysis and choice of product. This study was conducted to demonstrate the application of a simple treatment algorithm that distinguishes different skin types ie thin or thick tissue coverage, and treatment needs (volume, lifting, contouring) to guide individual treatment choice. The study products included in the treatment algorithm have unique gel properties; HARL being the firmest, suitable for lifting and projection, HARD is also firm but more flexible, and HARV is a soft gel providing natural looking volume. All three products were effective in terms of improving midface fullness, aesthetic improvement, and with high levels of subject satisfaction. Treatment with HARD and HARL also achieved lifting effect and facial contouring for a majority of subjects (HARV was not evaluated for this parameter).
A slightly lower MMVS improvement rate that was observed for HARL than HARV could be due to the mentioned moderate to substantial loss of volume in all subjects in the HARV group at baseline compared to mainly mild to moderate loss for HARL subjects. Interestingly, midface treatment also resulted in improvement in nasolabial fold wrinkle severity in a majority of subjects as assessed by investigators and blinded evaluators, except for HARV where investigators assessed 42% of subjects as improved (61% for blinded evaluators). It could be speculated that this kind of effect from a softer product was not expected by the treating (unblinded) investigators.
A similar study has previously been published where 30 female subjects with midface contour deficiency were treated with HARL or HARV based on the treating investigator’s clinical evaluation of skin thickness.11 In line with the current study it was concluded that the use of a treatment algorithm may improve outcomes for patients seeking injectable treatments for midfacial volume loss and contour deficiencies and that the concepts may be applied to other families of fillers.
A thorough facial assessment including skin quality, facial shape and contours as well as proportion, symmetry and appearance of the face will provide an individualized treatment with the appropriate hyaluronic acid filler for each patient.12
Limitations with the current study included the lack of objective evaluations eg ultrasound to confirm the subjective clinical examination of thin/thick tissue coverage that was used to match subjects to treatment.11 In addition, it could have been of interest to standardize injection technique used and injection depth to further differentiate the treatment effect of the different products.
Conclusion
Midface treatment with either HARV, HARD, or HARL, using a treatment algorithm to guide the choice of product to individual subject needs was effective and safe for up to 24 weeks after treatment and all 3 study products helped to achieve optimal results in accordance to patient needs. It is suggested that the treatment algorithm could be used by health care practitioners for the purpose of deciding the best possible treatment allocation for each individual subject.
Acknowledgments
The authors would like to thank Maria Norberg, PhD for medical writing assistance. Galderma funded the study and provided medical writing support.
Disclosure
Carlo Di Gregorio is chief of and reports grants from Galderma Italian Scientific Faculty. Gerd Gauglitz and Jackie Partridge have no conflicts of interest to declare in this work.
References
1. Bertucci V, Lin X, Axford-Gatley RA, et al. Safety and effectiveness of large gel particle hyaluronic acid with lidocaine for correction of midface volume loss. Dermatol surg. 2013;39(11):1621–1629. doi:10.1111/dsu.12340
2. Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol surg. 2016;42(6):699–709. doi:10.1097/DSS.0000000000000771
3. Segura S, Anthonioz L, Fuchez F, et al. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol. 2012;11(1 Suppl):s5–8.
4. Öhrlund Å. Evaluation of rheometry amplitude sweep cross-over point as an index of flexibility for HA fillers. J Cosmet Dermatol Sci Appl. 2018;08:47–54. doi:10.4236/jcdsa.2018.82008
5. Philipp-Dormston WG, Schuster B, Podda M. Perceived naturalness of facial expression after hyaluronic acid filler injection in nasolabial folds and lower face. J Cosmet Dermatol. 2020;19(7):1600–1606. doi:10.1111/jocd.13205
6. Philipp-Dormston WG, Wong C, Schuster B, et al. Evaluating perceived naturalness of facial expression after fillers to the nasolabial folds and lower face with standardized video and photography. Dermatol surg. 2018;44(6):826–832. doi:10.1097/DSS.0000000000001419
7. Dayan S, Fabi S, Nogueira A. Lay rater evaluation of naturalness and first impression following treatment of lower face wrinkles with hyaluronic acid fillers. J Cosmet Dermatol. 2021;20(4):1091–1097. doi:10.1111/jocd.13927
8. Talarico S, Meski AP, Buratini L, et al. High patient satisfaction of a hyaluronic acid filler producing enduring full-facial volume restoration: an 18-month open multicenter study. Dermatol surg. 2015;41(12):1361–1369. doi:10.1097/DSS.0000000000000549
9. Lorenc ZP, Bank D, Kane M, et al. Validation of a four-point photographic scale for the assessment of midface volume loss and/or contour deficiency. Plast Reconstr Surg. 2012;130(6):1330–1336. doi:10.1097/PRS.0b013e31826d9fa6
10. Day DJ, Littler CM, Swift RW, et al. The wrinkle severity rating scale: a validation study. Am J Clin Dermatol. 2004;5(1):49–52. doi:10.2165/00128071-200405010-00007
11. Andreas Nikolis KME, Lazarova D, Sampalis J. The role of clinical examination in midface volume correction using hyaluronic acid fillers: should patients be stratified by skin thickness? Aesthet Surg J Open Forum. 2020;2020:12.
12. Di Gregorio C, Rogers J, D’Arpa S. Hyaluronic acid-based two-stage medical therapy to unfold the aged face: the centrifugal approach. J Cosmet Dermatol. 2021;20(3):798–803. doi:10.1111/jocd.13623
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






