Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Individualized Techniques of Implant Coating with an Antibiotic-Loaded, Hydroxyapatite/Calcium Sulphate Bone Graft Substitute
Authors Freischmidt H , Armbruster J, Reiter G, Grützner PA , Helbig L , Guehring T
Received 12 December 2019
Accepted for publication 28 June 2020
Published 29 July 2020 Volume 2020:16 Pages 689—694
DOI https://doi.org/10.2147/TCRM.S242088
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Holger Freischmidt,1 Jonas Armbruster,1 Gregor Reiter,1 Paul Alfred Grützner,1 Lars Helbig,2 Thorsten Guehring3
1Department of Trauma and Orthopedic Surgery, BG Trauma Center Ludwigshafen at Heidelberg University Hospital, Ludwigshafen am Rhein 67071, Germany; 2Clinic for Orthopedics and Trauma Surgery, Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Heidelberg University Hospital, Heidelberg 69118, Germany; 3Arcus Kliniken Pforzheim, Pforzheim 75179, Germany
Correspondence: Thorsten Guehring
Arcus Kliniken Pforzheim, Rastatter-Strasse 17-19, Pforzheim 75179, Germany
Email [email protected]
Background: The treatment of fracture- or non-union-related infections has persistently been a major challenge for both patients and treating surgeons. With rising aging of patients and increasing comorbidities, combined with the heterogeneity of germs and any number of multi-resistance against standard antibiotics, a successful treatment is increasingly difficult. One potential solution could be a custom-made individualized antibacterial coating of standard implants with a biphasic degradable biocarrier (Cerament G/V, supplied by Bonesupport AB, Lund, Sweden) that releases high doses of antibiotics around the bone-implant-interface. Here, we describe our technique of coating intramedullary nails, plates and press-fit shoulder endoprostheses which may prevent bacterial adhesion and biofilm formation. So far, there is very limited experience in individual coating of implants in hip or knee endoprostheses to prevent reoccurrence of surgical-site infection. Currently, no reports are available for coating of stems of shoulder prosthesis and nails or plates for fracture fixation.
Methods: Here, we show our first experiences with a new individualized surgical technique of coating these implants with a resorbable antibiotic-loaded hydroxyapatite/calcium sulphate biocomposite to prevent biofilm formation and thereby recurrence of bone or joint infection. We describe three cases for coating of plates and nails for fracture fixation and coating of stems of a shoulder prosthesis.
Results: No adverse events of the resorbable bone graft substitute were observed. In all of the cases, no recurrence of the infection was observed and osseointegration was achieved. After implant coating of the shoulder prosthesis, no radiological signs of loosening were detected.
Conclusion: We present a new surgical approach of a surface coating of plates, intramedullary nails or prostheses. The osteoconductive- and anti-inflammatory effect of the gentamicin- or vancomycin-loaded hydroxyapatite/calcium sulphate bone graft substitutes shows promising results.
Keywords: fracture-related infections, osteitis, osteosynthesis, local antibiotics, biodegradable biocomposites
Introduction
Fracture-related infection (FRI) or periprosthetic infection (PJI) still remain a major challenge in orthopedic and trauma surgery. Particularly patients with relevant comorbidities such as diabetes, renal failure, peripheral vasculopathy and known nicotine abuse have a high risk for implant- related infections.1
Numerous devices for local antibiotic application and several treatment options are already available. The aim of these devices is to release local antibiotics in high concentration over a prolonged time period,2 and a new generation of bio-degradable carriers for local antibiotic delivery have been recently emerging for utilization.3
One of the major difficulties arises from the ability of microorganisms to adhere to the implant and produce a biofilm layer which unfortunately hinder the treatment of implant-related infections.4–6
A promising solution could be a commercially available coating of implants such as tibia intramedullary nails. Currently, however, the “Expert tibial nail PROtect“ (Fa. DePuy Synthes) is the only factory-made antibiotic coated implant available on the market. However, the antibiotics delivery of this coated implant is limited to gentamicin, and it is rather expensive.
As one of the promising biodegradable biocomposites Cerament (supplied by Bonesupport AB, Lund, Sweden) is an injectable bone graft substitute, composed of 60% fast resorbing calcium sulphate and 40% calcium hydroxyapatite.7 The resorbable local antibiotic carrier provides two modes of actions. It remodels to bone and it provides a local antibiotic concentration which is above the minimal inhibitory concentration (MIC) for most sensitive microorganisms for at least 28 days with – at the same time – safe serum levels.8–10 On the other hand, after 30 days no antibiotic was detected in the urine, indicating that all antibiotic had been eluted at that time. The risk of low antibiotic concentrations over a long period of time, which occurs eg, with the use of PMMA, is minimized and thus the risk of development of resistance to antibiotics can thus be considered lower.11 The local administration of the antibiotic-containing ceramics promises a better anti-infectious effect than local injection or intravenous injection. Clinical trials demonstrated that Cerament is a viable replacement to bone grafting in filling voids and gaps following fractures.12 However, in recent literature there is clear evidence of lower infection rates after additional local antibiotics at least in grade III open fractures.13 Cerament is a biphasic material consisting of calcium sulphate and hydroxyapatite. The calcium sulphate component typically resorbs within four to six weeks, leaving behind the hydroxyapatite as an osteoconductive matrix in which blood vessels can migrate. The blood vessels allow for the transport of stem cells, which differentiate to osteoblasts. Osteoblasts are known to bind to the hydroxyapatite. Early histology of Cerament G in Ferguson et al3 shows nicely the CERAMENT G covered by an osteoid-like matrix produced by osteoblastic cells seen lining the bone graft substitute’s surface. Additionally, Cerament showed good tissue response in an animal model including growth of trabecular bone around the hydroxyapatite particles and complete embedment in bone tissue.14 Furthermore, Cerament is commercially available with two antibiotic-loaded options. It is marketed as Cerament G (17.5 mg gentamicin sulphate/mL paste) and Cerament V (66 mg vancomycin/mL paste) as 5 mL and 10 mL version.
The efficacy of Cerament G and Cerament V in treatment of infected bone defects has already been demonstrated in both osteomyelitis and fracture related infections,3,15,16 as well as in various animal models.17–19 In an in vitro study, the anti-biofilm activity of Cerament G was investigated, suggesting a benefit of this gentamicin-releasing biocomposite for both prophylaxis and treatment of bone infection or implant-related infection, respectively.20
However, clinical reports on the usage of Cerament G/V as off-label coating device are limited so far. The first clinical experience in using a calcium-based gentamicin or vancomycin loaded bone graft substitute as a surface coating on cementless prosthetic implants has been published by Logoluso et al. In this study 95% of patients treated for PJI with a two-stage revision showed healing without recurrence of infection and no signs of radiographic stem loosening appeared at 12 months follow up.1
In the here described method we aim to cover each individual implant with Cerament G or Cerament V. This method thus enables to individually coat the most suitable implant (ie, plates or nails in fracture treatment). In PJI the revision stems of the protheses can be augmented with the most suitable and effective antibiotics (ie, gentamicin or vancomycin) by augmenting the stem with Cerament G or V. After coating the stem is inserted in a press-fit technique.
In the following presentation of our operative technique, we provide three new surgical techniques to coat different implants with an antibiotic-loaded, hydroxyapatite/calcium sulphate bone graft substitutes in order to address the heterogeneity of both microorganism and surgical site.
Materials and Methods (Case Presentations)
First Case: Custom-Made Implant Coating of a Plate
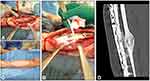
A 38-year-old male patient fell of a ride-on mower which then rolled over his left lower leg. This resulted in a second degree open fracture of the lower limb. Hence, wound debridement and external fixation were performed. The microbiologic results showed an infection with Enterobacter intermedius and Serratia fonticola, which have been treated with Cefuroxime and Metronidazole. Both microorganisms were sensitive to gentamicin. One month after the accident and several debridements later, the external fixator was removed and the tibia was stabilized with a low compression plate (LCP) (Figure 1A). In addition, the tibial defect was filled and the plate was augmented with 5 mL Cerament G to prevent infection (Figure 1B). Finally, the soft tissue defect was closed with an ALT flap by the plastic surgeons (Figure 1C).
Second Case: Implant Coating of an Intramedullary Nail in an Infected Non-Union
A 35-year-old male patient fractured his left femur shaft in a traffic accident in Egypt. Initially, the closed femoral shaft fracture was treated on site with an intramedullary nail. One year later bone healing could not be observed and the patient still suffered from pain due to a non-union. Therefore, we performed a resection of the non-union and the left femur was immobilized with an external transport fixator as there was a significant shortening of the leg. An infected non-union was diagnosed and a MRSA could be intraoperatively isolated from the non-union site.
A bone lengthening was approached for 2 months via the external fixator. However, the lengthening failed due to insufficient stability, and even though a rigid external fixator was subsequently applied for another 6 months, no bone healing could be achieved. Metal removal and complete en bloc resection of the non-union was done and a vancomycin loaded cement spacer was inserted intramedullary. No MRSA was detected during this procedure.
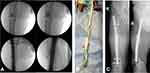
As a next step, the cement spacer was removed and a nail-osteosynthesis was inserted after the medullary canal had been reamed (LFN 360/16 mm, Fa Synthes). Before insertion, the nail was augmented with Cerament V (Figure 2B) and the medullary canal was filled up with the remaining amount of Cerament V using an extension tube. A total of 10 milliliters of Cerament V was utilized (Figure 2A). After the procedure an intravenous treatment with Vancomycin was done, in accordance with the resistogram.
Finally, two years after the accident, the nail was dynamically locked for dynamic compression of the non-union. Full weight bearing of the left lower extremity was possible immediately. After 22 months a complete bone healing was achieved (Figure 2C).
Third Case: Implant Coating of a Shoulder Prosthesis
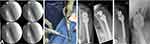
A 57-year-old female patient presented with a loosening of a proximal humeral nail (PHN, Fa. Synthes) after fractured left humeral head due to a fall on her shoulder. As reason for the loosening, an infection with extensive abscess formation around the implant was identified. The situation was complicated by the patient’s incompliance and comorbidities such as schizophrenia. In the first step, we removed the osteosynthesis and implanted a gentamicin and vancomycin loaded cement spacer (Figure 3). Swab examinations showed infection with a Staphylococcus lugdunensis, sensitive to vancomycin.
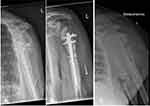 |
Figure 3 : (A) + (B) Removal of the loosened humeral nail; (C) Implantation of a cement spacer. |
During the second revision the shoulder joint was macroscopically free of infection, and thus an implantation of an inverse shoulder prosthesis was done (Fa Synthes DePuy) seven weeks later. During the operation the medullary canal was filled with Cerament V using an extension tube (Figure 4A). In addition, the stem of the prosthesis was augmented (Figure 4B) and thereafter the prosthesis stem was press-fit inserted. A total of 20 milliliters of Cerament V were used, in accordance with the resistogram. Followed by antibiotic treatment with Linezolid and Eremfat post-procedure.
Postoperatively, a dislocation of the prosthesis components occurred. Therefore, at 2 months a proximal enlargement of the stem with an augment was done (Figure 4C). The microbiological samples taken during this surgery were sterile after cultivation.
Results
So far, there have been no adverse events regarding the use of Cerament G or V as a surface coating during the follow up period.
First Case: Implant Coating of a Plate
Fifteen months after the augmentation of the LCP with Cerament G the tibia healed (Figure 1D), the patient is fully weight-bearing without pain. He has a free range of motion in the adjacent joints and no recurrence of the infection has been observed.
Second Case: Implant Coating of a Nail
No reinfection occurred 18 months after implantation of the augmented nail-osteosynthesis. Three months postoperatively the patient was allowed to fully weight-bear the right leg with a slightly limping gait remaining. At this time he did not have pain in the area of non-union and progressing, still incomplete bone healing could be detected in the CT scan. Twenty-two months after surgery a complete bone healing was achieved. The patient is actually full weight bearing without pain and a free range of motion in the adjacent joints.
Third Case: Implant Coating of a Shoulder Prosthesis
Fifteen months after implant coating of the shoulder prosthesis with Cerament V there were no signs for a recurrence of the infection. The shoulder prosthesis was fully integrated. No radiological signs of loosening were detected (Figure 4D). The patient is painless but with limited range of motion (flexion 80°, abduction 70°, external rotation 0°, free range of motion during internal rotation and adduction).
Conclusion
We present a new surgical approach of an individual off-label surface coating of plates, intramedullary nails or prostheses and could show promising initial results in these cases. To our knowledge, this is a relevant innovation in the therapy of fracture- and implant-associated infections. Most importantly no adverse effects of the resorbable bone graft substitute were observed and the osseointegration of the plates, nails and the prosthesis was observed. However, to gain further knowledge on the osteoconductive- and anti-inflammatory effect of the gentamicin- or vancomycin-loaded hydroxyapatite/calcium sulphate bone graft substitutes, further investigations and prospective clinical studies are required.
Abbreviations
ALT, anterolateral free flap; Cerament G, Cerament antibiotic loaded with gentamicin; Cerament V, Cerament antibiotic loaded with vancomycin; CT, computer tomography; FRI, fracture-related infection; LCP, low compression plate; LFN, lateral femoral nail; MRSA, multiresistant Staphylococcus aureus; PHN, proxima humerus nail; PJI, periprosthetic joint infection.
Ethics Approval and Consent to Participate
All researches were approved by the ethic commission of the medical association of Rhineland-Palatinate (2019-14425). We confirm that written informed consent was provided by the patients for the case details and any accompanying images published.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The study team wants to thank the Section of Septic Surgery of the trauma center for their cooperation and support.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. HF and TG participated in the study design and surgeries, analysis of the findings, and drafting of the final manuscript. GR participated in the study design and surgeries. PAG, JA and LH helped draft the final manuscript.
Disclosure
Prof. Dr. med. Thorsten Gühring is a consultant at Bone support and at Zimmer Biomet and reports personal fees from Bone Support and Zimmer Biomet, outside the submitted work. Holger Freischmidt reports that The BG Trauma Center Ludwigshafen is a “Center of Excellence” of the Bonesupport company. The remaining authors confirm that they have no conflicts of interest associated with this publication.
References
1. Logoluso N, Drago L, Gallazzi E, George DA, Morelli I, Romano CL. Calcium-based, antibiotic-loaded bone substitute as an implant coating: a pilot clinical study. J Bone Joint Infect. 2016;1:59–64. doi:10.7150/jbji.17586
2. Anagnostakos K, Schroder K. Antibiotic-impregnated bone grafts in orthopaedic and trauma surgery: a systematic review of the literature. Int J Biomater. 2012;2012:538061. doi:10.1155/2012/538061
3. Ferguson J, Athanasou N, Diefenbeck M, McNally M. Radiographic and histological analysis of a synthetic bone graft substitute eluting gentamicin in the treatment of chronic osteomyelitis. J Bone Jt Infect. 2019;4(2):76–84. doi:10.7150/jbji.31592
4. Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi:10.1126/science.3629258
5. Gristina AG, Naylor P, Myrvik Q. Infections from biomaterials and implants: a race for the surface. Med Prog Technol. 1988;14(3–4):205–224.
6. Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994;5(4):160–170.
7. Abramo A, Geijer M, Kopylov P, Tagil M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J Biomed Mater Res B Appl Biomater. 2010;92(1):281–286. doi:10.1002/jbm.b.31524
8. Colding-Rasmussen T, Horstmann P, Petersen MM, Hettwer W. Antibiotic elution characteristics and pharmacokinetics of gentamicin and vancomycin from a mineral antibiotic carrier: an in vivo evaluation of 32 clinical cases. J Bone Jt Infect. 2018;3(4):234–240. doi:10.7150/jbji.26301
9. Stravinskas M, Horstmann P, Ferguson J, et al. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: in vitro and clinical release studies. Bone Joint Res. 2016;5(9):427–435. doi:10.1302/2046-3758.59.BJR-2016-0108.R1
10. Stravinskas M, Nilsson M, Vitkauskiene A, Tarasevicius S, Lidgren L. Vancomycin elution from a biphasic ceramic bone substitute. Bone Joint Res. 2019;8(2):49–54. doi:10.1302/2046-3758.82.BJR-2018-0174.R2
11. Stravinskas M, Nilsson M, Horstmann P, Petersen MM, Tarasevicius S, Lidgren L. antibiotic containing bone substitute in major hip surgery: a long term gentamicin elution study. j bone jt infect. 2018;3(2):68–72. doi:10.7150/jbji.23901
12. Iundusi R, Gasbarra E, D’Arienzo M, Piccioli A, Tarantino U. Augmentation of tibial plateau fractures with an injectable bone substitute: CERAMENT. Three year follow-up from a prospective study. BMC Musculoskelet Disord. 2015;16(1):115. doi:10.1186/s12891-015-0574-6
13. Jahangir N, Niazi N, Aljawadi A, et al. The use of adjuvant local antibiotic hydroxyapatite bio-composite in the management of open Gustilo Anderson type IIIB fractures. A prospective review. J Orthopaedics. 2019;16(3):278–282. doi:10.1016/j.jor.2019.03.013
14. Nilsson M, Wang JS, Wielanek L, Tanner KE, Lidgren L. Biodegradation and biocompatibility of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg Br. 2004;86(1):120–125. doi:10.1302/0301-620X.86B1.14040
15. McNally MA, Ferguson JY, Lau ACK, et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite A PROSPECTIVE SERIES OF 100 CASES. Bone Joint J. 2016;98B(9):1289–1296. doi:10.1302/0301-620X.98B9.38057
16. Morgenstern M, Vallejo A, McNally MA, et al. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Joint Res. 2018;7(7):447–456. doi:10.1302/2046-3758.77.BJR-2018-0043.R1
17. Dvorzhinskiy APGCR, Van Der Meulen M, Ross F, Bostrom M, Yang N. Cerament bone void filler with gentamicin increases bone formation and decreases detectable infection in a rat model of debrided osteomyelitis. Bone Joint J. 2015;16:97.
18. Garcia P, Histing T, Holstein JH, et al. Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur Cell Mater. 2013;26:1–12. doi:10.22203/eCM.v026a01
19. Windolf CD, Meng W, Logters TT, MacKenzie CR, Windolf J, Flohe S. Implant-associated localized osteitis in murine femur fracture by biofilm forming Staphylococcus aureus: a novel experimental model. J Orthop Res. 2013;31(12):2013–2020. doi:10.1002/jor.22446
20. Butini ME, Cabric S, Trampuz A, Di Luca M. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf B Biointerfaces. 2018;161:252–260. doi:10.1016/j.colsurfb.2017.10.050
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.