Back to Journals » Medical Devices: Evidence and Research » Volume 16
Increasing Incremental Burden of Surgical Bleeding Associated with Multiple Comorbidities as Measured by the Elixhauser Comorbidity Index: A Retrospective Database Analysis
Authors Afolabi M, Johnston SS, Tewari P, Danker WA
Received 13 September 2023
Accepted for publication 17 November 2023
Published 4 December 2023 Volume 2023:16 Pages 237—249
DOI https://doi.org/10.2147/MDER.S434779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mosadoluwa Afolabi,1,* Stephen S Johnston,1,* Pranjal Tewari,2,* Walter A Danker3,*
1MedTech Epidemiology and Real-World Data Sciences, Johnson and Johnson, New Brunswick, NJ, USA; 2Decision Science, Mu Sigma, Bangalore, India; 3Franchise Health Economics and Market Access, Ethicon, Johnson and Johnson, Raritan, NJ, USA
*These authors contributed equally to this work
Correspondence: Stephen S Johnston, Real-World Data Analytics and Research, 410 George Street, New Brunswick, NJ, 08901, USA, Tel +1 443-254-2222, Email [email protected]
Purpose: Disruptive bleeding can complicate surgical procedures, increasing resource use, and impacting patients’ well-being. This study aims to elucidate the impact of comorbidity on the risk of disruptive surgical-related bleeding and selected transfusion-associated complications, as well as the incremental cost of such bleeding.
Patients and Methods: This retrospective analysis of the Premier Healthcare Database included patients who were age ≥ 18 years and who had a procedure of interest between 1-Jan-2019— 31-Dec-2019: cholecystectomy, coronary artery bypass grafting, cystectomy, hepatectomy, hysterectomy, pancreatectomy, peripheral vascular, thoracic, and valve procedures (first=index). The Elixhauser comorbidity index was assessed on index date and patients were grouped by cumulative comorbidity score (0, 1, 2, 3, 4, 5, ≥ 6). Outcomes, all measured as in-hospital during index, included bleeding (diagnosis and/or intervention for bleeding), transfusion-associated complications (diagnosis of infection, acute renal failure, or vascular events), and incremental total hospital costs associated with bleeding. Multivariable generalized linear models were used to examine the association of comorbidity/bleeding with outcomes.
Results: Of the 304,074 patients included, 7% experienced bleeding. The Elixhauser scores were distributed as follows: 0=29%, 1=23%, 2=18%, 3=12%, 4=8%, 5=5%, ≥ 6=5%. Odds of bleeding significantly increased with Elixhauser score: 1 comorbidity vs 0 (odds ratio [OR] =1.30, 95% confidence interval [95% CI] =1.19– 1.43), and this trend continued to surge (≥ 6 comorbidities [OR=3.22, 95% CI=2.94– 3.53]). Similarly, the odds of transfusion-associated complications significantly increased with comorbidities score: 1 comorbidity vs 0 (OR=2.14, 95% CI=1.88– 2.34), ≥ 6 comorbidities vs 0 (OR=12.37, 95% CI=10.80– 14.16). The incremental cost of bleeding also increased with comorbidities score; per-patient costs with and without bleeding were $18,132 vs $13,190, p < 0.001 among patients with 0 comorbidities and $28,952 vs $19,623, p < 0.001 among patients with ≥ 6 comorbidities.
Conclusion: Higher comorbidity burden was associated with significant increases in the risk of surgical bleeding, subsequent transfusion-related complications, and incremental cost burden of bleeding.
Keywords: bleeding complications, Elixhauser comorbidities, risk factors, observational, economic outcomes
Introduction
Disruptive bleeding is a common surgical complication that can occur during or after invasive or minimally invasive procedures.1 Surgery-related disruptive bleeding confers clinical burden through increased morbidity and mortality, and adds economic burden through increased use of expensive healthcare services (eg, critical/intensive care unit [CCU/ICU] admissions, extended operating room times), longer hospital stays, and greater need for repeat procedures, all of which contribute to higher all-cause direct healthcare costs.2–5 The nature of the surgical procedure and particular patient characteristics (eg, age, bleeding history, medication exposures) influence a given patient’s risk of intra- or post-operative bleeding, but the understanding of these risk factors is currently incomplete and still evolving. For instance, even as recently as 2018, the American College of Surgeons noted in its Guidelines for the Perioperative Management of Antithrombotic Medication that physicians should rely on their own experience to judge procedure-specific bleeding risk, with consideration for both the invasiveness of the procedure and the potential consequences if bleeding should occur.6
Despite the general lack of high-quality definitive evidence to quantify risk factors for surgical-related bleeding, researchers have developed a few formal, usually procedure-specific, bleeding risk assessments such as the Papworth Bleeding Risk Score for cardiac surgeries.7 To date, other bleeding risk scores have also been developed with user-friendly online assessments, such as the HAS-BLED and Atria tools, however, these have focused on guiding decision-making regarding the initiation of anticoagulant therapy rather than for assessing surgical bleeding risk per-se.8–10
Although some researchers have reported associations between comorbidity burden and surgical outcomes (eg, 30-day and 1-year mortality, 30-day complications, readmissions) and others have reported worse outcomes for patients admitted to hospital for acute gastrointestinal bleeding, to our knowledge the literature lacks data on the impact of comorbidities on the risk of disruptive peri-surgical bleeding and its clinical and economic consequences.11–13 The current study was conducted to narrow this knowledge gap and enhance the evidence available for physicians to rely on when considering each patient’s potential risk for disruptive surgical bleeding and how to prepare appropriately. Specifically, this study aims to elucidate the impact of comorbidity on risk of bleeding, the risk of selected transfusion-associated complications, and the incremental cost of bleeding. This study provides a broad perspective on these questions, having examined these outcomes for nine commonly performed surgical procedures.
Materials and Methods
Data Source and Study Population
In this observational study, we retrospectively analyzed real-world, discharge-level data from the all-payer Premier Healthcare Database (PHD).14 This United States (US) population-based research database contains inpatient and outpatient hospital billing records from nearly 1000 hospitals and health systems that participated in the Premier Healthcare Performance Improvement Alliance during the study period. Although this convenience sample excluded federally funded hospitals, at the time of this study it was considered to be nationally representative with respect to hospital bed size, geographic distribution, location (urban/rural) and teaching status. Hospital discharge-level information included patient demographics, primary and secondary International Classification of Diseases, 10th Revision, Clinical Modification diagnoses and procedures, information on medical devices/supplies, medications, length of hospital stay, and discharge disposition, as well as information on facility and physician provider characteristics. Hospital cost data corresponding to a date-stamped log of all billed items by cost-accounting department were also available for each encounter.
This analysis of the PHD was conducted under an exemption from Institutional Review Board oversight for US-based studies using de-identified healthcare records, as dictated by Title 45 Code of Federal Regulations (45 CFR 46.104(d)(4)(ii)).15 As the PHD does not contain direct identifiers of individuals, employers, households, or providers, Institutional Review Board approval is not required.
The study cohort included patients who were age 18 years or older at the time of their inpatient or outpatient encounter for a procedure of interest in calendar year 2019 (first = index encounter). The nine procedures of interest are commonly performed in the US and are generally considered by surgeons to carry a range of risk for disruptive bleeding: coronary artery bypass grafting (CABG), cholecystectomy, cystectomy, hepatectomy, hysterectomy, lung resection, pancreatectomy, peripheral vascular procedures (PVP), and valve repair/replacement; furthermore, with the exception of PVP, these procedures were also previously evaluated in a prior study of the incidence and impact of surgical bleeding conducted by Corral et al.2 Only patients whose index encounter occurred at a hospital that contributed data to the PHD for at least 90 days post-discharge were included. Patients whose index encounters occurred through transfer from a different hospital and those with missing discharge status or sex, or with zero or negative billed amounts for total, room and board, and/or supply costs were excluded.
Patient Classification, Covariates, and Outcome Variables
The Elixhauser comorbidity index, an unweighted, validated measure for defining comorbidities in real-world data, comprises 31 comorbidities and has been widely used in health outcomes research.16,17 Comorbidities were identified using International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnosis codes that were designated as being present on admission at the time of the index encounter. Patients were then classified for analysis as having 0, 1, 2, 3, 4, 5, or ≥6 comorbidities.
Covariates, which were measured to characterize the study cohort and used for adjustment in the multivariable models, included: index procedure type and procedural physician specialty; patients’ age, sex, race, ethnicity, marital status, payor; hospitals’ bed size, teaching status, region urban or rural location, and surgical volume for index procedure type.
The occurrence of disruptive bleeding events during the index encounter was indicated by the presence of at least one ICD-10-CM diagnosis for hemorrhage or hematoma complicating a procedure; ICD-10 Procedure Coding System (ICD-10-PCS) procedure code interventions to control bleeding; charges billed for use of Hemovac drainage devices; charges billed for use of erythropoietin, blood product transfusions, and charges billed for cryoprecipitates, fresh frozen plasma, red blood cells, plasma, platelets, and whole blood. Transfusion-associated complications during the index encounter were defined by the presence of ICD-10-CM diagnosis codes indicating infection, acute renal failure, or specific vascular events.3 The total hospital cost (ie, costs borne by the hospital) and length of stay for the index encounter were also assessed.
Statistical Analysis
Data for categorical variables were summarized using counts and percentages of patients in each category. Data for continuous variables were summarized using the mean and standard deviation of the variable’s distribution; medians and other distributional information were reported for variables with values that were not normally distributed.
Generalized linear models (GLM) were used to quantify the associations between comorbidity burden and outcomes, adjusting for all covariates listed above. GLMs were conducted to evaluate the association of comorbidity burden with risk of disruptive bleeding and, separately, the risk of transfusion-associated complications; these models used a log link and binomial error distribution. Furthermore, to examine whether comorbidity burden influences the incremental impact of disruptive bleeding on total hospital costs and length of stay for the index encounter, GLMs with an interaction term between comorbidity burden and presence of disruptive bleeding were also conducted; these models used a log link and gamma (cost) or negative binomial (length of stay) error distribution. Analyses of length of stay were restricted to procedure conducted in an inpatient setting. A p-value of ≤0.05 denoted statistical significance. Analyses were performed using StataSE 16 (StataCorp, College Station, Texas, US).
Results
From among 349,763 patients with at least one hospital discharge for one or more of the nine procedures of interest in the 2019 calendar year, 304,074 patients met full inclusion criteria (Figure 1). Table 1 and 2 show patient and hospital characteristics, respectively. Patients had a mean age of 53 years, 73% were female, and 77% were of white race. The majority of patients had either commercial or Medicare coverage (50% and 29%, respectively), while 13% of patients were enrolled in a Medicaid plan.
 |
Table 1 Selected Patient Characteristics |
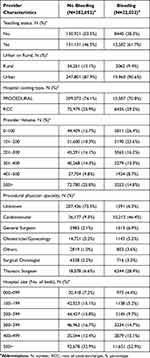 |
Table 2 Selected Provider Characteristics |
 |
Figure 1 Patient selection. |
Cholecystectomy was the most common procedure (40% of patients), followed by hysterectomy (36%), and CABG (11%), while each of the remaining procedures comprised 4% or less of the study cohort (Figure 2). In the study cohort overall, for most patients the index encounter was considered elective (75%) and performed in the outpatient setting (68%). The nature and setting did vary by procedure; however, the percentage of procedures deemed elective ranged from 61% in patients undergoing CABG to 91% in patients undergoing cystectomies. Cholecystectomy, hysterectomy, and a small number of peripheral vascular surgeries were the only procedures performed in the outpatient setting.
 |
Figure 2 Distribution of patients by Elixhauser score and index procedure. |
Over half of the study patients had an Elixhauser score of 0 or 1 while 5% had a score of 6 or greater (0=29%, 1=23%, 2=18%, 3=12%, 4=8%, 5=5%, ≥6=5%). The distribution of patients by Elixhauser score differed across procedure types (Figure 2). Patients with low comorbidity scores predominated among patients undergoing cholecystectomies and hysterectomies, while the majority of patients undergoing CABG, peripheral vascular surgeries, or valve procedures had an Elixhauser score of 3 or greater.
Overall, 22,022 patients experienced disruptive bleeding associated with their index procedure, the unadjusted proportion of patients with procedure-related disruptive bleeding ranged from 0.6% among patients who underwent cholecystectomy to 38.6% among patients who underwent valve procedures (Table 3). The multivariable-adjusted odds of disruptive bleeding significantly and monotonically increased with the number of comorbidities (Figure 3A and B). Specifically, patients with an Elixhauser score of 1 had 30% greater odds of disruptive bleeding compared with those who had a score of 0 (odds ratio [OR]=1.30, 95% confidence interval [95% CI]=1.19–1.43). This trend increased as scores increased, up to a more than 3-fold increase in odds among patients with scores of ≥6 [OR=3.22, 95% CI=2.94–3.53]). Similarly, the adjusted risk of transfusion-associated complications significantly increased as the number of comorbidities increased (Figure 4A and B). The adjusted odds of a transfusion-associated complication were two-fold higher in patients with an Elixhauser score of 1 versus those with a score of 0 (OR=2.14, 95% CI=1.88–2.34). The adjusted odds of a transfusion-associated complication among patients with scores of at least 6 was more than 12-fold that of patients with scores of 0 (OR=12.37, 95% CI=10.80–14.16).
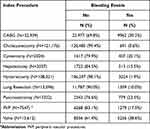 |
Table 3 Proportion of Patients with Disruptive Bleeding by and Index Procedure |
 |
Figure 3 Adjusted risk of bleeding, by Elixhauser score. (A) Absolute adjusted risks. (B) Adjusted odds ratios. |
 |
Figure 4 Adjusted risk of transfusion-associated complications, by Elixhauser score. (A) Absolute adjusted risks. (B) Adjusted odds ratios. |
Among patients undergoing a procedure in an inpatient setting, the presence of disruptive bleeding also had a substantial impact on the index encounter’s adjusted length of stay (Figure 5). At every level of comorbidity, patients who experienced disruptive bleeding had longer stays than those without bleeding. The greatest incremental difference in length of stay between patients with and without disruptive bleeding was in patients whose Elixhauser scores were at least 6 (12.2 days [95% CI 11.8–12.7] vs 8.5 days [8.3–8.7]).
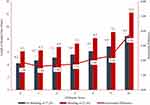 |
Figure 5 Adjusted incremental differences in index encounter length of stay among patients undergoing a procedure on the inpatient setting, by Elixhauser score and occurrence of disruptive bleeding. |
The adjusted incremental hospital cost of bleeding increased monotonically with the number of comorbidities (Figure 6). Among patients with an Elixhauser score of 0, those who had experienced disruptive bleeding during their index encounter incurred higher costs than those without disruptive bleeding ($18,132 [95% CI=$16,444–$19,820] vs $13,190 [95% CI=$12,785–$13,596]). The same pattern was observed for patients with an Elixhauser score of 3 (with bleeding vs without bleeding: $20,117 [95% CI=$19,205–$21,030] vs $14,827 [95% CI=$14,444–$15,209]). The greatest incremental hospital cost difference between patients with and without disruptive bleeding was highest among patients whose Elixhauser score was at least 6 ($28,952 [95% CI=$27,729–$30,175] vs $19,623 [95% CI=$19,009–$20,236]).
 |
Figure 6 Adjusted incremental differences in index encounter total hospital costs, by Elixhauser score and occurrence of disruptive bleeding. |
Discussion
Although some researchers have examined the role of patient comorbidity in influencing surgical outcomes and others have considered the relationship between bleeding (both condition- and surgery-related) and clinical and economic outcomes, to our knowledge, this is the first study to quantify the association between a general measure of patient comorbidity and the risk and impact of disruptive bleeding in a large, real-world surgical cohorts representing a diversity of procedures.1–5,11,12 We found that greater comorbidity burden had consistent and substantial associations with important negative clinical outcomes (disruptive bleeding, transfusion-associated complications) as well as increased length of stay and total hospital costs, both overall and in terms of the incremental impact of disruptive bleeding on these outcomes.
Even after adjusting for procedure type and numerous other potential confounders for which data were available, patients with an Elixhauser score greater than 0 had at least 30% higher odds of disruptive bleeding during the index encounter, with up to a 300% higher risk among patients in the highest comorbidity category. The impact was even more substantial for transfusion-associated complications, where patients with non-zero Elixhauser scores had at least twice the odds of having a transfusion-associated complication with 12 times higher risk among individuals who had Elixhauser scores of 6 or greater.
With respect to the distribution of Elixhauser score, across all procedures nearly one-third (29%) of patients had a score of zero. However, among those with at least one comorbidity, nearly half (43%) had an Elixhauser score ≥3. When reanalyzing the data using an Elixhauser score at least 3 as the top category, such patients had approximately two times the adjusted odds of disruptive bleeding (OR=1.96, 95% CI=1.74–2.21) and 6.19 times the adjusted odds of a transfusion-associated event (OR=6.19, 95% CI=5.44–7.04) as compared with those who had an Elixhauser score of 0. Consistent with the overall analysis, when comparing between patients with and without disruptive bleeding, those with an Elixhauser score of at least 3 also had the greatest incremental difference in length of stay (9.4 days [95% CI 9.1–9.7] vs 6.5 days [95% CI 6.4–6.7]) and incremental hospital cost difference ($23,642 [95% CI $22,647—$24,637] vs $16,069 [95% CI $15,651—$16,487]).
These findings clearly show that risk of disruptive bleeding and transfusion-associated complications is not uniform or independent of comorbidity burden. To date, our knowledge of risk factors for these outcomes has been incomplete and there is a paucity of pre-surgical risk assessment tools.7,8,10 In the absence of consensus about risk factors and the current lack of widely available generic risk assessment tools, the results of this study suggest that thoughtful consideration of each patient’s pre-surgical comorbidity burden might be a reasonable, easily implemented risk reduction approach with potentially important clinical benefits. Such consideration, paired with other standard pre-surgery protocols, may provide an additional perspective and insight to better tailor preparation of the operating room. For patients who may be at higher risk based on their comorbidity profile, such preparation may include ensuring the availability of optimal adjunctive hemostats and other equipment needed to efficiently address bleeding if it does occur during the procedure.
In addition to conferring additional risk for these key clinical outcomes, greater comorbidity burden and the presence of disruptive bleeding both contribute to substantially longer hospital stays and higher total hospital costs for surgical patients. The highest costs and greatest incremental costs between patients with and without disruptive bleeding were observed in patients with the greatest comorbidity burden. Although these clinically complex patients may require more intensive care or longer hospital stays regardless of what preparations are made to prevent or mitigate disruptive bleeding, pre-surgical consideration of the risk their comorbidity profile likely confers may still result in better quality and more efficient care.
An understanding of the limitations of the study data and methods provides important context for interpreting the results of this study. Among these considerations, we note that the accuracy of our patient selection, classification for analysis, and identification of index procedures, disruptive bleeding, and the other study outcomes depend to a large degree on the level of detail and accuracy of the information recorded in the source database (PHD). Data from the PHD undergo extensive curation and quality checks and is widely used for research, so although these data are primarily recorded to support reimbursement and costing analyses, the clinical and other details in this data source should be generally reliable. Finally, we note that although the Elixhauser score is not widely used or readily available in the routine care setting, it was primarily used in this study because it is an established and validated tool for quantifying comorbidity burden in real-world research.15,16 The level of rigor afforded by the Elixhauser score was needed in order to conduct a credible examination of the associations between comorbidity burden and the outcomes of interest in this study. Physician awareness of comorbidity burden as a risk factor for disruptive bleeding and related clinical and economic impact documented in this study may benefit both patients and the healthcare system. In the routine care setting, physicians assess comorbidities in a variety of ways, and the hope is that the results of this study will allow them to use that knowledge in considering the best risk reduction strategies to employ as they prepare for each surgery.
Conclusion
In conclusion, in this large cohort of patients who underwent a variety of inpatient and outpatient surgical procedures in the routine care setting, higher comorbidity burden was associated with a greater risk of disruptive bleeding, greater risks of subsequent transfusion-related complications, and a higher incremental burden of disruptive bleeding in terms of length of stay and total hospital costs.
Acknowledgments
Sally Wade (MPH), a partner in Wade Outcomes Research and Consulting (Salt Lake City, Utah), provided medical writing support for this manuscript. The abstract of this paper was presented at the 2022 Society of Advanced Blood Management (SABM) Annual Congress in Las Vegas, Nevada, with the title “Increasing Incremental Burden of Surgical Bleeding Associated with Multiple Comorbidities as Measured by the Elixhauser Comorbidity Index: A Retrospective Database Analysis” as an oral presentation with interim findings. The abstract was published in Anesthesia & Analgesia Journal. Mosadoluwa Afolabi, MPH; Stephen S Johnston, PhD; Pranjal Tewari, BE; Walter Danker, PhD. Increasing Incremental Burden of Surgical Bleeding Associated with Multiple Comorbidities as Measured by the Elixhauser Comorbidity Index: A Retrospective Database Analysis. Anesthesia and Analgesia. 2022; Vol. 135. No. 3 S_ SUPPL:77–77.
Funding
This study was funded by Johnson & Johnson.
Disclosure
SSJ and WAD are employees and stockholders of Johnson & Johnson. MA and PT provided data and analysis support under contract to Johnson & Johnson. PT was an employee of Mu Sigma during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Thomas AB, Shammas RL, Orr J, et al. An assessment of bleeding complications necessitating blood transfusion across inpatient plastic surgery procedures: a nationwide analysis using the national surgical quality improvement program database. Plast Reconstr Surg. 2019;143(5):1109e–1117e. doi:10.1097/PRS.0000000000005537
2. Corral M, Ferko N, Hollmann S, Broder MS, Chang E. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the Premier Perspectives Database. Clinicoecon Outcomes Res. 2015;7:409–421.
3. Johnston SS, Jamous N, Mistry S, et al. Association of In-hospital surgical bleeding events with prolonged hospital length of stay, days spent in critical care, complications, and mortality: a retrospective cohort study among patients undergoing neoplasm-directed surgeries in English Hospitals. Clinicoecon Outcomes Res. 2021;13:19–29. doi:10.2147/CEOR.S287970
4. Al-Attar N, Johnston S, Jamous N, et al. Impact of bleeding complications on length of stay and critical care utilization in cardiac surgery patients in England. J Cardiothor Surg. 2019;14(1):64. doi:10.1186/s13019-019-0881-3
5. Stone GW, Clayton TC, Mehran R, et al. Impact of major bleeding and blood transfusions after cardiac surgery: analysis from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Am Heart J. 2012;163(3):522–529.
6. Hornor MA, Duane TM, Ehlers AP, et al. American College of Surgeons’ guidelines for the perioperative management of antithrombotic medication. J Am Coll Surg. 2018;227(5):521–36.e1.
7. Vuylsteke A, Pagel C, Gerrard C, et al. The Papworth Bleeding Risk Score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. Eur J Cardiothorac Surg. 2011;39(6):924–930. doi:10.1016/j.ejcts.2010.10.003
8. Pisters R. HAS-BLED Score for major bleeding risk. MDCalc. Available from: https://www.mdcalc.com/calc/807/has-bled-score-major-bleeding-risk.
9. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi:10.1378/chest.10-0134
10. Singer DE. ATRIA Bleeding Risk Score. MDCalc. Available from: https://www.mdcalc.com/calc/1718/atria-bleeding-risk-score.
11. Potts J, Nagaraja V, Al Suwaidi J, et al. The influence of Elixhauser comorbidity index on percutaneous coronary intervention outcomes. Catheter Cardiovasc Interv. 2019;94(2):195–203. doi:10.1002/ccd.28072
12. Mehta HB, Dimou F, Adhikari D, et al. Comparison of comorbidity scores in predicting surgical outcomes. Med Care. 2016;54(2):180–187. doi:10.1097/MLR.0000000000000465
13. Siebenhüner K, Blaser J, Nowak A, et al. Comorbidities associated with worse outcomes among inpatients admitted for acute gastrointestinal bleeding. Dig Dis Sci. 2022;67(8):3938–3947. doi:10.1007/s10620-021-07197-7
14. Premier healthcare database white paper. Available from: https://offers.premierinc.com/rs/381-NBB-525/images/PremierHealthcareDatabaseWhitepaper.pdf.
15. Congress US. Protection of human subjects (45 CFR 46).104 -- Exempt research: Department of Health and Human Services; 2009. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/guidance/exempt-research-and-research-expedited-review/index.html.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi:10.1097/00005650-199801000-00004
17. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
