Back to Journals » International Journal of Nanomedicine » Volume 11
Increased survival rate by local release of diclofenac in a murine model of recurrent oral carcinoma
Authors Will OM, Purcz N, Chalaris A, Heneweer C, Boretius S, Purcz L, Nikkola L, Ashammakhi N, Kalthoff H, Glüer CC, Wiltfang J, Açil Y, Tiwari S
Received 25 March 2016
Accepted for publication 30 July 2016
Published 12 October 2016 Volume 2016:11 Pages 5311—5321
DOI https://doi.org/10.2147/IJN.S109199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Olga Maria Will,1,* Nicolai Purcz,2,* Athena Chalaris,3 Carola Heneweer,4,5 Susann Boretius,1 Larissa Purcz,2 Lila Nikkola,6 Nureddin Ashammakhi,6 Holger Kalthoff,7 Claus-Christian Glüer,1 Jörg Wiltfang,2 Yahya Açil,2 Sanjay Tiwari1
1Section Biomedical Imaging, Clinic for Radiology and Neuroradiology, MOIN CC, 2Department of Oral and Maxillofacial Surgery, University Hospital Schleswig-Holstein, 3Institute of Biochemistry, Christian-Albrechts-Universität zu Kiel, 4Clinic for Radiology and Neuroradiology, University Hospital Schleswig-Holstein, Kiel, 5Institute for Diagnostic and Interventional Radiology, University Hospital Cologne, Cologne, Germany; 6Department of Biomedical Engineering, Tampere University of Technology, Tampere, Finland; 7Institute for Experimental Cancer Research, University Hospital Schleswig-Holstein, Kiel, Germany
*These authors contributed equally to this work
Abstract: Despite aggressive treatment with radiation and combination chemotherapy following tumor resection, the 5-year survival rate for patients with head and neck cancer is at best only 50%. In this study, we examined the therapeutic potential of localized release of diclofenac from electrospun nanofibers generated from poly(d,l-lactide-co-glycolide) polymer. Diclofenac was chosen since anti-inflammatory agents that inhibit cyclooxygenase have shown great potential in their ability to directly inhibit tumor growth as well as suppress inflammation-mediated tumor growth. A mouse resection model of oral carcinoma was developed by establishing tumor growth in the oral cavity by ultrasound-guided injection of 1 million SCC-9 cells in the floor of the mouth. Following resection, mice were allocated into four groups with the following treatment: 1) no treatment, 2) implanted scaffolds without diclofenac, 3) implanted scaffolds loaded with diclofenac, and 4) diclofenac given orally. Small animal ultrasound and magnetic resonance imaging were utilized for longitudinal determination of tumor recurrence. At the end of 7 weeks following tumor resection, 33% of mice with diclofenac-loaded scaffolds had a recurrent tumor, in comparison to 90%–100% of the mice in the other three groups. At this time point, mice with diclofenac-releasing scaffolds showed 89% survival rate, while the other groups showed survival rates of 10%–25%. Immunohistochemical staining of recurrent tumors revealed a near 10-fold decrease in the proliferation marker Ki-67 in the tumors derived from mice with diclofenac-releasing scaffolds. In summary, the local application of diclofenac in an orthotopic mouse tumor resection model of oral cancer reduced tumor recurrence with significant improvement in survival over a 7-week study period following tumor resection. Local drug release of anti-inflammatory agents should be investigated as a therapeutic option in the prevention of tumor recurrence in oral squamous carcinoma.
Keywords: tumor recurrence, oral squamous cell carcinoma, head and neck cancer, NSAIDs, drug releasing polymers, mouse model
Introduction
Head and neck cancers including those of the salivary glands, thyroid, mucosal lining of the oral cavity, pharynx, nasopharynx, and larynx are the sixth most common cancer in the world.1 Almost all the cases are head and neck squamous cell carcinoma (HNSCC), and the disease is strongly associated with tobacco and alcohol consumption and/or infection with oncogenic strains of human papilloma virus.2 Despite multimodal treatment that includes aggressive chemotherapy and/or radiation, prognosis of the patients is poor in general. Recurrence at the primary site contributes to the majority of cancer-related deaths in patients with HNSCC. In some cases, a second primary tumor arises in which the tumor does not share any genetic similarity to the original tumor.3 It is thought that molecular abnormal cells in the mucosal field adjacent to the tumor constitute a field of preneoplastic cells and subsequently acquire additional mutations that give rise to second field tumors. It has also been suggested that cancer stem cells, with their inherent properties of tumor initiation and resistance to cytotoxic agents, are responsible for field cancerization.4 Irrespective of the mechanism, preventing tumor recurrence in HNSCC holds the challenge of not only suppressing the progression of premalignant lesions to carcinogenesis but also eliminating the positive margins surrounding the surgical area.
Many epidemiological studies have demonstrated that nonsteroidal anti-inflammatory drugs (NSAIDs) reduce the risk of a wide range of tumors.5–8 The anti-inflammatory properties of NSAIDs mediated through inhibition of cyclooxygenase-2 (COX-2) are considered to be the mechanism responsible for the chemopreventive property. However, NSAIDs also have direct antitumor activity through inhibition of cancer cell proliferation and induction of apoptosis in a variety of cell types.9–11 Furthermore, inhibition of prostaglandin synthesis has been shown to inhibit the expansion of cancer stem cells in a mouse model of colon cancer.12 With its combined cytostatic and cytotoxic effects, NSAIDs can potentially eliminate malignant clones, inhibit progression of premalignant cells, and facilitate more effective combination therapy. Diclofenac, one of the well-known NSAID agents, inhibits both COX-1 and COX-2 activity.13 However, systemic administration of NSAIDs can cause serious cardiovascular and gastrointestinal side effects and are therefore not routinely recommended for these diseases.13–15
Improving the targeting strategies for NSAIDs will avoid the toxicity associated with systemic administration. A feasible strategy for the prevention of local tumor recurrence following resection in HNSCC is the implantation of a diclofenac-releasing polymer at the site of surgical resection. Biodegradable implantable drug devices have been reported to be successful in preventing or slowing tumor recurrence in the preclinical setting of other tumor types. Implantation of hydrogel, nano/microspheres, or their combination loaded with a chemotherapeutic agent has been used in murine resection models of breast cancer,16 glioma,17 and ovarian cancer.18 A polymer film as a chemotherapeutic delivery platform was utilized to prevent local recurrences in a subcutaneous murine model of Lewis lung carcinoma and sarcoma,19 and a silk film was utilized in an orthotopic neuroblastoma model with tumor cells implanted on the adrenal glands resulting in delayed tumor recurrence.20 Recently, a five-layer drug-loaded electrospun nanofibrous mat for one-sided local chemotherapy after hepatic cell carcinoma surgery was successfully utilized to prevent tumor recurrence.21
We have previously reported the generation of an electrospun nanofiber scaffold loaded with diclofenac.22 The scaffold was generated from poly(D,L-lactide-co-glycolide) (PDLGA80/20), which has a high inherent viscosity and a relatively slow degradation rate compared to other aliphatic polyesters. The scaffold consists of highly interconnected porous structures with the nanofiber diameter ranging from 0.9 μm to 1.25 μm. Diclofenac-loaded nanofibers contained bead-like structures along the nanofibers with an average diameter of 17.6±2.7 μm. No bead-like structures were observed with unloaded scaffolds. The release profile of diclofenac in vitro showed a release period for up to 60 days with an initial high release of 20 μg/mL/d, which decreased gradually to 1 μg/mL/d by day 23. As a reference, the lowest level in synovial fluid at 12 hours following oral administration of 75 mg diclofenac to arthritic patients was 0.12 μg/mL, considered to be the minimum therapeutic tissue concentration.23 We hypothesized that the diclofenac release profile from PDLGA80/20 scaffolds will be efficacious in preventing HNSCC tumor recurrence following tumor resection. We postulate that the early peak release of diclofenac will attenuate the inflammatory response in the postoperative surgical field and have antiproliferative and/or cytotoxic activity against residual tumor cells. Subsequent release will function as a chemopreventive agent in inhibiting inflammation-driven tumor recurrence. We have tested the efficacy of localized diclofenac release following implantation of PDLGA80/20 in the resected region in an orthotopic floor-of-mouth squamous cell carcinoma (SCC) mouse model. We show that this therapeutic strategy significantly prolonged survival in mice following tumor resection.
Methods
Cell lines
The human cell lines SCC-4, SCC-9, SCC-15, and SCC25, all derived from SCC of the tongue, were obtained from ATCC® (American Type Culture Collection, Manassas, VA, USA). Cells were cultivated in culture media consisting of Dulbecco’s Modified Eagle’s Medium with 10% fetal calf serum, 100 IE penicillin/mL, 100 μg streptomycin/mL, and 400 ng/mL hydrocortisone at 37°C with 5% CO2.
In vitro test
WST-1: 5×103 cells were seeded in 96-well plates and allowed to adhere overnight. The next day they were treated with diclofenac sodium at concentrations of 0.87–224 μg/mL (2.7–700 μM) for 48 hours. Cell viability was assessed using the cell proliferation kit WST-1 (catalog number 116446807001; Roche Diagnostics, Mannheim, Germany), and the procedure was carried out according to the manufacturer’s protocol.
Diclofenac-loaded electrospun nanofibers
Electrospun diclofenac sodium-releasing PDLGA80/20 scaffolds were generated as previously described.22 Briefly, PDLGA80/20 was dissolved in acetone/dimethylformamide to generate a 6% solution. Diclofenac was added to form a 20% w/v homogenous solution. Approximately 1 g of polymer/drug solution was spun onto the collector under electrostatic conditions. The distance between needle tip and sample collector was arranged to 10 cm, and an applied electric field of 2 kV/cm was applied. The resulting highly porous nanofiber scaffold was ~2 mm thick, and the diameter of nanofibers was ~130 nm.
In vivo experiment
All animal experiments were approved by the Ethics Committee for Animal Experiments at Christian-Albrechts-Universität-zu-Kiel (permission number V 312-72241.121-14 [89-8/11]) in accordance with the German Animal Welfare Act. A xenograft orthotopic tumor resection model of oral squamous cell cancer was established as follows. Tumors were induced in athymic nude mice (Hsd:Athymic Nude-Foxn1nu; Harlan Winkelmann, Borchen, Germany) by injection of SCC-9 cells in the floor of the mouth in between the two digastric muscles. A total of 1×106 cells in 25 μL Matrigel (BD Biosciences, San Jose, CA, USA) were applied. The injections were performed under general anesthesia with intraperitoneal application of midazolam (5 mg/kg body weight), fentanyl (0.05 mg/kg body weight), and medetomidin (0.5 mg/kg body weight). A high-resolution ultrasound system (Vevo770; VisualSonics) was used to localize the injections. Tumor growth was monitored by ultrasound weekly. Volume measurement was estimated by defining three–dimensional (3D) regions of interest using the VisualSonics image analysis software package. Two-dimensional (2D) images were initially acquired at regular spatial intervals that were parallel and uniformly spaced at 30 μm. The 3D image reconstruction was then performed automated by the Vevo 770 software.
Three weeks following tumor cell inoculation, tumor resection was carried out using an extraoral submandibular approach. Tumors were of a solid structure and could easily be identified during surgery. A complete resection with tumor-free margins was performed with the aid of surgical magnifying glasses. Biopsy samples to ensure margins were tumor free were not taken due to the anatomical restrictions in removing further tissue. Afterward, animals were allocated into four groups of ten mice, with equal distribution of mice per group according to the weight of the primary tumor.
The first group received no further treatment after tumor resection other than postoperative pain medication (buprenorphin 0.1 mg/kg body weight). This pain medication was given to all four treatment groups. The second group received control scaffolds with a mean weight of 15 mg. These scaffolds did not contain diclofenac. The scaffolds were positioned at the site of resection. The third group received diclofenac-loaded scaffolds with a mean weight of 14 mg. The scaffolds were positioned at the site of resection. Finally, the fourth group received diclofenac orally through the drinking water (po). The dosage was 30 mg/kg body weight based on the estimate that a mouse drinks ~3 mL water per day.24 All operations were carried out under general anesthesia with local application of Ultracain™ (20 μg/50 μL) to reduce the intraoperative and postoperative pain. Mice were euthanized if weight loss exceeded 20% or were in a moribund state or upon development of a large tumor size with the risk of perforation. Two mice in the control group and one mouse from the diclofenac-loaded scaffold group were sacrificed within the first 3 days following resection/implantation of the scaffold and were not included in data analyses. Due to adverse side effects with application of diclofenac in the drinking water (enteritis with consecutive peritonitis), six mice were sacrificed between day 1 and day 10, and diclofenac was removed from the drinking water from day 10 onward. The study was terminated 7 weeks after the resection after which tumors were removed and weighed and thereafter fixed in formalin and embedded in paraffin.
In vivo imaging of tumor recurrence
The first sonographic measurement after the operation was carried out 1 week after tumor resection to allow the mice to recover from the tumor operation. Thereafter, weekly measurements were performed. Magnetic resonance imaging (MRI) measurement was performed at week 4. A 7 T MRI for small animal imaging (Cliniscan, Bruker Biospin GmbH, Ettlingen, Germany) was used with a four-channel phased-array coil for signal reception and a birdcage resonator (inner diameter 70 mm) for excitation (both Bruker Biospin GmbH). T2-weighted images were obtained by a 2D turbo-spin-echo sequence (time repetition [TR]/time echo [TE] 3,430/8.9 ms, four echoes, spatial resolution 130×130 μm, slice thickness 300 μm, 30 slices) and oriented parallel to the mouse body. Proton density-weighted images were acquired by a spoiled 3D gradient-echo sequence (TR/TE 30/1.1 ms, flip angle 5°, matrix size 192×108×192) with a spatial resolution of 130×130×140 μm. MRI imaging was carried out with mice under general anesthesia with isoflurane.
Immunohistochemistry
Prior to staining, slides were deparaffinized using decreasing concentrations of xylol/ethanol. Antigen demasking was performed using citrate buffer, pH 6.0, and staining was performed using the EnVision™ G/2 Doublestain System for Rabbit and Mouse antibodies (DAB+/Permanent Red) (Dako Denmark A/S, Glostrup, Denmark). The procedure for staining was performed according to the manufacturer’s protocol. The primary antibodies used were monoclonal mouse anti-human cytokeratin antigen large spectrum, clone KL1, mouse IgG1, dilution 1:500 (Immunotech), and monoclonal mouse anti-human Ki-67 antigen, clone MIB-1, dilution 1:40 (Dako Denmark A/S). Counterstaining was performed with hematoxylin, and coverslips were mounted with Aquatex™ (Merck).
Determination of diclofenac release
Scaffolds extracted from mice following 7 weeks of implantation were briefly washed and incubated in 1.5 mL phosphate-buffered saline (PBS). The vials were placed in a shaker (100 rpm) at 37°C. The PBS from the vials was removed weekly and replaced with fresh PBS. Concentration of diclofenac was measured from collected samples using a UV spectrophotometer set at 280 nm with PBS used as the baseline reading. Measurements were made for a period of 4 weeks at which point no further release of diclofenac was observed. The concentration of diclofenac was measured using a standard curve with linear regression. The formula used for calculation with a range from 1 μg/mL to 100 μg/mL was Y=0.023x+0.051. For comparison with in vitro release, measurements of five 15 mg diclofenac-loaded scaffolds were made weekly for a period of 11 weeks, at which point no further release of diclofenac was observed. The total amount released after 7 weeks was used for comparison, and % cumulative release was determined from the total amount released after 11 weeks.
Results
Diclofenac inhibits cell growth and induces cell death in SCC cell lines
To determine the sensitivity of SCC cell lines to diclofenac, a WST-1 proliferation/viability assay was performed, which relies on the metabolism of formazan by metabolically active cells. The assay was performed on four different SCC cell lines derived from the tongue, SCC-4, SCC-9, SCC-15 and SCC-25. Cell viability was reduced in all cell lines at higher diclofenac concentrations with SCC-15 and SCC-25 showing similar sensitivity with an IC50 of 120 μM and 128 μM, respectively, followed by SCC-9 with an IC50 of 176 μM (Figure 1). SCC-4 was the least sensitive with a 50% reduction in viability not reached within 48 hours at the highest concentration of 704 μM. Although SCC-15 had the lowest IC50 of 120 μM, this value corresponded to 38 μg/mL, which is outside the range of diclofenac release by the scaffold, as previously determined. Furthermore, at the lowest concentration of 2.7 μM (0.87 μg/mL), diclofenac actually increased proliferation of the SCC-15 cell line. In contrast, SCC-9 cells had a significant reduction in viability at the lowest diclofenac concentration. Therefore, the daily release concentrations of diclofenac from PDLGA80/20 scaffolds is predicted to directly impact SCC-9 growth more than any of the other cell lines. For this reason, SCC-9 cell line was utilized for in vivo experiments.
Diclofenac-releasing implants decrease recurrence of oral tumors in an orthotopic mouse model
To investigate whether local release of diclofenac can inhibit tumor recurrence of oral SCC, a tumor resection mouse model was established. Guided by sonographic visualization, 106 SCC-9 cells were injected into the floor of the oral cavity between the two digastric muscles of Foxn1 nude mice. Three weeks post cell inoculation, the primary tumor volume was measured by ultrasound, resected and the primary tumor weighed to ensure that in the subsequent randomization all groups contain a similar distribution of mice with respect to the primary tumor size. In the respective experimental groups, the scaffolds with or without diclofenac were implanted at the site of the excised tumor. Control groups received diclofenac orally from the drinking water (po) or were left untreated after operation (placebo).
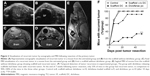
All animals were monitored for recurrent tumor growth by weekly sonographic scans. Tumors were identified by their hypoechogen (dark-grayish) appearance compared to surrounding tissue (Figure 2A). However, due to the presence of the scaffold, seen as anechoic (Figure 2B, black area), it was not possible to determine the cross-sectional area of the recurrent tumor. Therefore, ultrasound imaging was restricted only to the detection of recurrent tumor. Longitudinal ultrasound imaging revealed that most mice in the control group, the group that received scaffolds without diclofenac, and the group in which diclofenac was administered orally incurred tumor recurrence within 1 week of resection (Figure 2F). However, only 10% of the mice in the group with diclofenac-loaded scaffolds had detectable recurrent tumors. At the end of 7 weeks, only 33% of the mice of this group harbored recurrent tumor in contrast to 90%–100% in the other three groups.
Since oral cell carcinoma has a propensity for distant metastases, MRI imaging was performed at week 4 following resection to determine distant metastases in the head and neck region (Figure 2C–E). No metastases were detected, and we found no evidence that SSC-9 cells spread beyond the injection site.
Survival rate is increased in diclofenac-releasing scaffold group
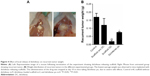
The group with diclofenac-releasing scaffold had a 7-week survival rate of 89% compared to 25% of the untreated group, 10% of the scaffold without diclofenac group, and 10% of the diclofenac po group (Figure 3A). Unexpectedly, six mice developed enteritis with consecutive peritonitis and were sacrificed in the diclofenac po group between day 1 and day 10. The administration of diclofenac po was stopped prematurely 10 days post operation for the remaining four mice in this group. To determine if there was any association of survival with the size of the primary tumor of individual mice, the survival time was plotted with the weight and volume of the primary resected tumor. No correlation was observed indicating that it is unlikely that survival time was correlated with unforeseen prior adverse effects (Figure 3B and C). For example, correlation of small primary tumors with decreased survival may indicate incomplete resection of the primary tumor. Alternatively correlation of large primary tumors with decreased survival may indicate poor welfare state of the individual mice.
Weight of recurrent tumors was lowest in the diclofenac-releasing scaffold group
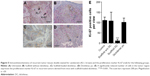
Following termination of the experiment at week 7 following resection, the recurrent tumors were removed from individual mice and weighed (Figure 4A). The recurrent tumor weights were lowest in the diclofenac-releasing scaffold group with an average of ~0.02 g (n=3, P<0.01). In the scaffold without diclofenac group and the untreated control group, an average weight of 0.12 g (n=9) and 0.16 g (n=8) were measured, respectively (Figure 4B). The average weight of recurrent tumors in the diclofenac po group was ~0.08 g (n=4, P<0.05). For this group, we cannot exclude that the weight of recurrent tumors was influenced by the premature stop of diclofenac po administration due to peritonitis.
Diclofenac-releasing scaffold reduces marker of proliferation in recurrent tumor
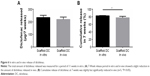
To investigate the effect of local diclofenac release at the cellular level, immunohistochemical (IHC) analyses were performed on tissues at the tumor sites. Pan cytokeratin (clone KL1) staining confirmed the presence of tumor cells. To assay the proliferation in the tumor sections, staining for the proliferation antigen Ki-67 was performed (Figure 5A–D). The percentage of Ki-67-positive cells in the tumor regions was significantly lower from the group with implanted diclofenac-releasing scaffolds (P<0.001; Figure 5E) compared to the other groups. Active caspase 3 IHC staining revealed no significant differences in the number of apoptotic cells in untreated mice and mice implanted with and without diclofenac-loaded scaffolds (data not shown). This suggests that the localized release of diclofenac after 30 days is not sufficient to induce tumor cell apoptosis.
Cumulative release of diclofenac in vitro exceeds in vivo measured release
To determine the release of diclofenac in vivo, diclofenac-loaded scaffolds were extracted following the termination of the experiments, washed briefly, and incubated in PBS, and diclofenac release was measured weekly until no further release was observed (a period of 4 weeks). The total released amount was determined and compared with diclofenac release in vitro. Scaffolds that produced a turbid solution were excluded from analyses. No significant difference in the amount of diclofenac released was found to occur in vivo compared to an equivalent time period in vitro (219.8±18.5 vs 234.6±17.7, respectively, P=0.23; Figure 6A). This amounted to a cumulative release of 77.5% in vivo compared to 82.7% in vitro, which was significantly different (n=5, P=0.017; Figure 6B).
Discussion
In this study, we have utilized a clinically relevant orthotopic HNSCC mouse model of tumor resection combined with imaging techniques to demonstrate that implantation of diclofenac-releasing polymer scaffolds leads to inhibition of tumor recurrence. Longitudinal imaging allowed for the noninvasive monitoring of tumor recurrence as well as verification of in situ positioning of the scaffold at the resection site. We show that mice with implanted diclofenac-loaded scaffolds have significantly increased survival times. Staining for the proliferation marker Ki-67 in the recurrent tumor biopsy samples indicated that anti-proliferative mechanisms contributed to anti-tumor activity. Overall, the study indicates that localized release of diclofenac from PDLGA80/20 scaffolds allows for the cancer-preventive and anti-tumoral effects of NSAIDs without the associated adverse side effects of a systemic administration.
Certain features of the diclofenac-loaded scaffolds are advantageous for prevention of locoregional recurrence. First, the bulky nature of the scaffolds allow for surgical mediated positional stability. The scaffolds were inserted in the tissue parenchyma of the resected area and embedded by adjacent mucosal tissue flaps by surgical suture. This procedure allowed for the selective placement of the scaffolds for the duration of the study. Second, the biodegradable polymer used in this study has a high molecular weight with a substantially slower degradation rate compared to other aliphatic polyesters. This has the effect of prolonging the release of the drug. Moreover, the biodegradable and bioresorpable properties of PDLGA circumvent a second surgery for scaffold removal. Finally, copolymers obtained from PDLGA are among the most biocompatible and nontoxic materials used for drug delivery, and a number of PDLGA drug-loaded microparticles have been approved by the US Food and Drug Administration for human use.25 Nevertheless, the polymer has a higher proportion of lactic acid to glycolic acid (80:20 ratio), which would be expected to lead to a slower rate of hydrolyses compared to a polymer containing a 50:50 ratio. Long-term studies are required to determine the effect of the scaffold on the exposed tissue and the biocompatibility of the degraded oligomers and particles.
We have previously shown in vitro that the release profile of diclofenac is biphasic with an initial burst followed by slow continued release.22 In vivo, the cumulative release of diclofenac was found to be slightly reduced compared to the measured in vitro release. It is unclear why this is so, but it may be that a biological film surrounding the scaffold may hinder degradation of the scaffold. Since implantation of any material will elicit an inflammatory response, which potentially can lead to increased tumor promotion, the initial burst release of diclofenac can crucially inhibit this process. In addition to dampening the inflammatory response, HNSCC cell line SCC9 is particularly sensitive to the anti-proliferative effects of diclofenac, as observed by the low concentrations in which proliferation is inhibited in vitro. The significantly reduced numbers of mice that develop recurrent disease within the first week in the group with diclofenac-loaded scaffolds support the anti-inflammatory and anti-proliferative role of diclofenac in vivo. Further support of this effect is provided by Ki-67 staining of recurrent tumor tissues. There was an approximately 10-fold decrease in the number of proliferating cells as determined by Ki-67-positive cells in mice with diclofenac-loaded scaffolds compared to the other groups.
There are limitations in this study. One limitation is that the plasma or tissue concentrations of diclofenac were not measured to directly validate diclofenac release from the scaffolds. A previous study using novel diclofenac nitrosothiol esters was able to detect as little as 4.5 nmol/mL diclofenac in plasma derived from 1 mL of blood from mice using high-performance liquid chromatography analyses.26 This is equivalent to 1.35 μg/mL diclofenac sodium in a 25 g mouse. It is uncertain whether this sensitivity would have been sufficient to detect diclofenac release at the termination of our experiment. However, reduced Ki-67 staining of tumor tissue combined with nonreduced levels of active caspase-3 indicate that low levels of diclofenac were released in the tumor microenvironment, which was not sufficient for apoptotic activity but sufficient for anti-proliferative effects. Nevertheless, future studies will need to perform comprehensive longitudinal pharmacokinetic analyses to determine bioavailability of diclofenac in vivo. A second limitation is that we have used only one cell line in the study in vivo. The SCC-9 cell line used was the most sensitive to diclofenac in vitro, and this sensitivity appears to be maintained in vivo. Proliferation assays suggest that the other HNSCC cell lines tested do not share this sensitivity to diclofenac at low concentrations. In particular, the SCC-15 cell line actually increased in proliferation in response to low concentrations of diclofenac although concentrations >10 μg/mL no longer induced this stimulatory effect. Tumor growth in vivo is dependent not only on the intrinsic properties of the tumor cells but also on the external signals from the microenvironment, which enhance proliferation, resistance to apoptosis, and angiogenesis. In this context, localized release of diclofenac may still restrict growth of tumors in vivo derived from cell lines not as sensitive to diclofenac as SCC-9, through inhibition of pro-inflammatory cytokine secretion from the supporting stromal cells. Moreover, it will be of interest to determine the intrinsic differences between SCC-15 and SCC-9 at the genomic and/or epigenetic level to understand the mechanism that accounts for the differences in response to diclofenac in vitro.
In addition to their application following tumor resection, diclofenac-loaded scaffolds also have potential applications in the clinical setting of unresectable tumors. Recent studies have identified a pivotal role of PGE2 synthesis in expanding the cancer stem cell phenotype.12 Cancer stem cells have intrinsic resistance to chemotherapy and radiation therapy through an increased capacity for drug efflux and deregulation of apoptotic signaling. Since the main effect of NSAIDs is the inhibition of COX-2, which catalyses the synthesis of prostaglandins, implantation of diclofenac-loaded scaffolds in the proximity of unresectable tumors may lead to a reduced number of tumor cells with the cancer stem cell phenotype. This may lead to a clinical benefit with greater susceptibility of the tumors to aggressive irradiation and chemotherapy. However, as seen in one of the cell lines in this study (SCC-15), caution needs to be applied since in some circumstances low concentration of NSAID may also promote tumor growth.
Next-generation sequencing has yielded insights into the genetic heterogeneity of HNSCC tumor cells in the malignant field,26,27 tumor cells evolve over time, location (tumor microenvironment), random genetic drifting, and genetic drifting in response to chemotherapy. This constant transformation of the genetic landscape enables those cells harboring unique mutational events to drive carcinogenesis. In the setting of recurrent disease, tumors with mutational similarity to the primary tumor are recurrences and those with no similarity are second primary tumors. Clearly treating a cancer by every mutation cannot be therapeutically addressed, and therefore, greater emphasis needs to be placed on cancer prevention. In this study, we have taken a novel approach to prevent tumor recurrence by implanting diclofenac-loaded electrospun scaffolds in the resected region in a preclinical tumor resection model of HNSCC. We show that long-term localized release of diclofenac decreases the recurrence of HNSCC. These results provide the basis for pursuing clinical studies to determine long-term survival and safety following tumor resection.
Acknowledgments
The authors are grateful to Gabriele Trompke, Gisela Refrath, and Gabi Neßenius for their excellent technical assistance. Financial support was provided by the research grant from the state of Schleswig-Holstein and the European Union ERDF-European Regional Development Fund (MOIN CC, Zukunftsprogramm Wirtschaft) and by the medical faculty of UK-SH, Campus Kiel (Faculty Grant, NP), Sonderforschungsbereich 877 (SFB 877), and the Kreitz Stiftung (NP and ST).
Disclosure
The authors report no conflicts of interest in this work.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. | ||
Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. | ||
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. | ||
Simple M, Suresh A, Das D, Kuriakose MA. Cancer stem cells and field cancerization of oral squamous cell carcinoma. Oral Oncol. 2015;51(7):643–651. | ||
Rostom A, Dube C, Lewin G, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146(5):376–389. | ||
Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysis of the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila). 2009;2(4):310–321. | ||
Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. | ||
Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23(6):1403–1415. | ||
Bock JM, Menon SG, Goswami PC, et al. Differential activity of sulindac metabolites against squamous cell carcinoma of the head and neck is mediated by p21waf1/cip1 induction and cell cycle inhibition. Cancer Biol Therapy. 2007;6(1):30–39. | ||
Li WZ, Huo QJ, Wang XY, Xu F. Inhibitive effect of celecoxib on the adhesion and invasion of human tongue squamous carcinoma cells to extracellular matrix via down regulation of MMP-2 expression. Prostaglandins Other Lipid Mediat. 2010;93(3–4):113–119. | ||
Ondrey FG, Juhn SK, Adams GL. Inhibition of head and neck tumor cell growth with arachidonic acid metabolism inhibition. Laryngoscope. 1996;106(2 pt 1):129–134. | ||
Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149(7):1884–1895.e1884. | ||
Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–1102. | ||
Masso Gonzalez EL, Patrignani P, Tacconelli S, Garcia Rodriguez LA. Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum. 2010;62(6):1592–1601. | ||
Garcia Rodriguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am College Cardiol. 2008;52(20):1628–1636. | ||
Jaiswal M, Naz F, Dinda AK, Koul V. In vitro and in vivo efficacy of doxorubicin loaded biodegradable semi-interpenetrating hydrogel implants of poly (acrylic acid)/gelatin for post surgical tumor treatment. Biomed Mater. 2013;8(4):045004. | ||
Ozeki T, Kaneko D, Hashizawa K, Imai Y, Tagami T, Okada H. Combination therapy of surgical tumor resection with implantation of a hydrogel containing camptothecin-loaded poly(lactic-co-glycolic acid) microspheres in a C6 rat glioma model. Biol Pharm Bull. 2012;35(4):545–550. | ||
Gilmore D, Schulz M, Liu R, et al. Cytoreductive surgery and intraoperative administration of paclitaxel-loaded expansile nanoparticles delay tumor recurrence in ovarian carcinoma. Ann Surg Oncol. 2013;20(5):1684–1693. | ||
Liu R, Wolinsky JB, Catalano PJ, et al. Paclitaxel-eluting polymer film reduces locoregional recurrence and improves survival in a recurrent sarcoma model: a novel investigational therapy. Ann Surg Oncol. 2012;19(1):199–206. | ||
Chiu B, Coburn J, Pilichowska M, et al. Surgery combined with controlled-release doxorubicin silk films as a treatment strategy in an orthotopic neuroblastoma mouse model. Br J Cancer. 2014;111(4):708–715. | ||
Liu S, Wang X, Zhang Z, et al. Use of asymmetric multilayer polylactide nanofiber mats in controlled release of drugs and prevention of liver cancer recurrence after surgery in mice. Nanomed Nanotechnol Biol Med. 2015;11(5):1047–1056. | ||
Nikkola L, Morton T, Balmayor ER, et al. Fabrication of electrospun poly(D,L lactide-co-glycolide) 80/20 scaffolds loaded with diclofenac sodium for tissue engineering. Eur J Med Res. 2015;20:54. | ||
Todd PA, Sorkin EM. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1988;35(3):244–285. | ||
Mayorek N, Naftali-Shani N, Grunewald M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS One. 2010;5(9):e12715. | ||
Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125(3):193–209. | ||
Bandarage UK, Chen L, Fang X, et al. Nitrosothiol esters of diclofenac: synthesis and pharmacological characterization as gastrointestinal-sparing prodrugs. J Med Chem. 2000;43(21):4005–4016. | ||
Hedberg ML, Goh G, Chiosea SI, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Investigat. 2016;126(1):169–180. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.