Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Increased Serum Trimethylamine N-Oxide Level in Type 2 Diabetic Patients with Mild Cognitive Impairment
Authors Xu N, Wan J, Wang C, Liu J, Qian C, Tan H
Received 10 April 2022
Accepted for publication 7 July 2022
Published 28 July 2022 Volume 2022:15 Pages 2197—2205
DOI https://doi.org/10.2147/DMSO.S370206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Nongzhang Xu,1,* Jianwei Wan,1,* Cuihong Wang,1 Jiatao Liu,2 Chenqai Qian,1 Hongyang Tan3
1Department of Pharmacy, Shanghai University of Medicine and Health Science Affiliated Zhoupu Hospital, Shanghai, People’s Republic of China; 2Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 3Clinical Research Center for Mental Disorders, Chinese-German Institute of Mental Health Shanghai Pudong New Area Mental Health Center, School of Medicine, Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongyang Tan, Clinical Research Center for Mental Disorders, Chinese-German Institute of Mental Health, Shanghai Pudong New Area Mental Health Center, School of Medicine, Tongji University, Shanghai, People’s Republic of China, Tel +86-18321133996, Email [email protected]
Purpose: Trimethylamine N-oxide (TMAO) is a metabolite of phosphatidylcholine in red meat and other diets, which is associated with cardiovascular and other diseases. The aim of this study is to evaluate the associations of serum TMAO with mild cognitive impairment (MCI) in the Chinese type 2 diabetes mellitus (T2DM) population.
Materials and Methods: A total of 253 hospitalized T2DM patients and 150 healthy controls were included in this cross-sectional study. Montreal Cognitive Assessment (MoCA) assessed the cognition function, and the 253 T2DM patients were divided into 74 subjects with MCI and 179 with non-MCI. Demographic data and biochemical test results were evaluated. Serum TMAO level was measured by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS).
Results: A higher serum TMAO level was observed in T2DM patients compared with the healthy controls (P < 0.001). Among all T2DM patients, the MCI group (n = 74) showed higher serum TMAO levels than the non-MCI group. Spearman correlation test showed that TMAO levels were significantly positively correlated with age (r = 0.147, P = 0.019), body mass index (BMI) (r = 0.153, P = 0.015), diabetes duration (r = 0.160, P = 0.011), HbA1c (r = 0.138, P = 0.029), triglyceride (TG) (r = 0.138, P = 0.029), creatinine (r = 0.184, p = 0.003), hs-CRP (r = 0.243, P < 0.001), and were negatively correlated with HDL-C (r = − 0.144, P = 0.022), BDNF (r = − 0.165, p = 0.009), and MoCA (r = − 0.386, P < 0.001) score (all P < 0.05). Multivariable Logistic regression identified high serum TMAO level as a significant independent factor of MCI in the T2DM patients (OR = 1.404, 95% CI = 1.255– 1.571; P < 0.001).
Conclusion: Our study showed that T2DM patients with MCI have elevated serum TMAO levels.
Keywords: trimethylamine N-oxide, type 2 diabetes mellitus, mild cognitive impairment, Montreal Cognitive Assessment, healthy control
Introduction
Type 2 diabetes mellitus (DM) is one of the most common severe public health problems in the 21st century globally.1 China has the highest number of Type 2 diabetes mellitus (T2DM) patients, accounting for over 90% of all diabetes mellitus patients locally.2 T2DM is amalgamated with a potential risk of dementia, cognitive impairment, and Alzheimer’s disease (AD).3,4 Mild cognitive impairment (MCI) is a transitional state between normal aging and dementia, and subjects have a gradual loss of memory and executive function. Consequently, different morphological changes in the central nervous system (CNS) include decreased hippocampal size and neurogenesis, brain tissue atrophy, and unusual neural electrical property changes.5–8 These disorders affect tremendous public health care and quality of life. Therefore, there is an urgent need to reveal early diagnostic biomarkers for MCI in T2DM patients.
Trimethylamine-N-oxide (TMAO) is a metabolite mainly generated from dietary choline and L-carnitine and its precursor trimethylamine (TMA). Both choline and L-carnitine undergo metabolization by the gut microbiota into TMA, which is further converted into TMAO by liver flavin-containing monooxygenase 3 (FMO3).9 A major concern in TMAO levels could be the result of dietary differences, suggesting that intestinal microbiota may play a significant role in the variation of TMAO levels.10 Several studies demonstrated that TMAO could be associated with cardio-renal disorders, including atrial fibrillation, heart failure, chronic kidney disease, and acute myocardial infarction.11–14 A recent study reported plasma TMAO level as a novel prognostic consequence of cardiovascular diseases (CVD).15 Previously, a meta-analysis showed a positive dose-dependent association of serum TMAO with the risk of diabetes. A high TMAO level could reduce glucose homeostasis consequences to the worst clinical result of diabetic complications.16,17 Thus, TMAO probably plays a significant role that was previously unrecognized in T2DM-associated cognitive impairment. However, it remains unexplored about the relationship between TMAO and cognitive function in diabetic patients. Therefore, we speculated that TMAO could influence the sensitivity to early cognitive impairment in T2DM patients.
This study aims to explore the significant link between serum TMAO levels and cognition function in T2DM patients.
Materials and Methods
Study Population
This cross-sectional study was conducted in the Department of Endocrinology of Shanghai Pudong Hospital from January 2018 to December 2020. We acquired written informed consent from participants or nearest relatives before the commencement of our study. The Research Ethics Committee approved this study protocol of the Hospital.
In the present study, a total of 253 hospitalized T2DM patients and 150 healthy people were recruited. The included T2DM patients had more than three years of diabetes history. All patients were diagnosed with T2DM based on the criteria of the WHO 1999.18 MoCA evaluated cognitive function. The recruited 253 T2DM patients were classified into 74 patients with MCI and 179 with normal cognition. All recruited patients were capable of understanding and cooperating with study procedures. The exclusion criteria were as follows: (1) severe diabetic complications; (2) dementia or depression; (3) other diseases with cognitive dysfunction; (4) major illnesses, such as severe heart failure, neoplasm, and severe infection; (4) use of cognition-impairing drugs in the previous 3 months. The control group came from 150 cases of healthy volunteers who received health examinations; they were aged 50–75 years (mean age 60.3 ± 7.3 year).
Data Collection
Clinical data were collected through inquiry with all subjects after admission, including name, age, gender, occupation, education, history of smoking or alcohol intake, and the history of diabetes mellitus and complications, cardiovascular disease (CVD), hypertension and hyperlipidemia. General biochemical indexes, including fasting blood glucose (FBG), HemoglobinA1c (HbA1c), triglyceride (TG), total cholesterol (TC), LDL-C, HDL-C, and Creatinine. Hs-CRP (DCRP00) and brain-derived neurotrophic factor (BDNF, DBD00) in serum were determined by the enzyme-linked immunosorbent assay (ELISA) method (R&D Systems, Minneapolis, MN, USA), and all protocols and steps were performed in strict accordance with the instructions.
The Montreal Cognitive Assessment (MoCA)
The MoCA test was applied to evaluate the cognitive function.19 The test content of MoCA scale mainly includes visual space and executive function (including alternate connection test, replication cube and clock drawing test), naming, abstraction, delayed recall, attention (including anterograde and retrograde digit span test, 100 continuous minus 7 calculation and striking Hit test), language (including sentence repetition and language fluency test), and orientation (including time orientation and place orientation). The MoCA score ranges from 0 to 30; a MoCA score is ≥26 for subjects with normal cognition. The MoCA is used to detect MCI in T2DM patients.20 The diagnostic criteria of MCI were based on the European MCI Working Group.21 We only included patients with a MoCA score of 20 and above to exclude dementia. After MoCA measurement, we divided subjects into MCI group and non-MCI group (normal cognition).
Trimethylamine N-Oxide (TMAO) Measurement
Overnight fasting blood was collected from 253 T2DM patients and 150 healthy controls. Blood was centrifuged immediately to separate serum and stored in a −80°C. Serum levels of TMAO were detected with stable isotope dilution high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS).22 Briefly, 80μL 10-μmol/L d9-TMAO was added to 20μL plasma, and the sample was vortexed for 1 minute. The supernatant was then centrifuged for 25 minutes at 15,000 g and transferred to a clean sampling bottle for testing. A supernatant of 10 mL was injected into the SiO2 column for analysis. The column temperature was 30°C; the flow rate was 0.8 mL/min, with mobile phase A with 0.1% formic acid aqueous solution and mobile phase B with 0.1% acetic acid in methanol. The concentration of TMAO and d9-TMAO was determined by positive multiple reaction monitoring mass spectrometry. Serum TMAO concentrations were determined through comparison with an established standard curve.
Statistical Analysis
Quantitative data were presented as medians and inter-quartile ranges (IQR), and category data were presented as numbers (percentage). All statistical analysis was performed using SPSS Software 20.0 (SPSS Inc., Chicago, Illinois, USA). The comparison between groups was performed by Mann–Whitney U-test (quantitative data) or χ2 test (category data). Correlation analysis was performed using the Spearman rank test. The independent risk factors for MCI were analyzed using multivariate Logistic regression. The receiver operator characteristic (ROC) curve was applied to obtain the cut-off value of TMAO. P < 0.05 is regarded as statistical significance.
Results
The Baseline Characteristics of the Study Group
The demographic and clinical characteristics of all 253 T2DM patients are presented in Table 1. The χ2 test results demonstrated no significant differences between the MCI and non-MCI groups for males, smoking, and drinking. Moreover, the Mann–Whitney U-test showed that MCI subjects were more likely to have hypertension, hyperlipidemia, retinopathy, and nephropathy than patients without MCI. Furthermore, MCI subjects tended to be older, shorter education time, higher BMI, more extended diabetes history, higher levels of TG, HDL-C, creatinine, hs-CRP, lower level of BDNF, and lower MoCA score (all P < 0.05).
 |
Table 1 Demographic and Clinical Characteristics of Type 2 Diabetic (T2DM) Patients |
Serum TMAO Level in T2DM Patients
We performed the Mann–Whitney U-test to analyze the serum TMAO between different groups. Serum TMAO level was significantly higher in T2DM patients [12.28 μmol/L (IQR 9.59–14.64)] compared to healthy controls [5.10 μmol/L (IQR 4.48–6.06)] (P < 0.001; Figure 1A). Among all T2DM patients, serum TMAO level was significantly higher in the MCI group [14.16 μmol/L (IQR 11.28–18.44)] than in the non-MCI group [11.75 μmol/L (IQR 9.27–13.8)] (P < 0.001; Figure 1B).
Correlation Analysis
Spearman rank correlation analyses were performed to uncover the associations of TMAO with other clinical variables (Table 2). Remarkable positive correlations were found between serum TMAO level and Age (r = 0.147, P = 0.019), BMI (r = 0.153, P = 0.015), diabetes duration (r = 0.160, P = 0.011), HbA1c (r = 0.138, P = 0.029), TG (r = 0.138, P = 0.029), creatinine (r = 0.184, p = 0.003), and hs-CRP (r = 0.243, P < 0.001). In addition, serum TMAO level showed negative correlations with HDL-C (r = −0.144, P = 0.022), BDNF (r = −0.165, p = 0.009), and MoCA (r = −0.386, P < 0.001). No statistically significant differences were found between serum TMAO and Education, FBG, TC and LDL-C.
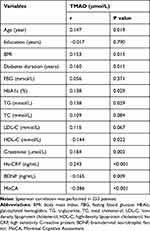 |
Table 2 The Correlations of Serum TMAO Level with Clinical Indicators in T2DM Patients |
Logistic Regression Models
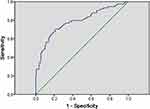
Multivariate logistic regression was performed to investigate whether serum TMAO is an independent factor of MCI in T2DM. MCI can be influenced by education year, diabetes duration, the presence of hyperlipidemia, hs-CRP, and TMAO (OR = 1.404, 95% CI = 1.255–1.571; P < 0.001) (Table 3). ROC curve showed the optimal cutoff value of serum TMAO is 14.14 μmol/L (sensitivity: 70.3%; specificity: 78.8%; AUC = 0.785, 95% CI 0.717–0.852; P < 0.001; Figure 2). T2DM patients with high serum TMAO levels (>14.14 μmol/L) had a significantly higher risk of MCI.
 |
Table 3 Multivariate Logistic Regression Evaluates the Risk of MCI in T2DM Patients |
Discussion
In our present study, we carried out an investigation to measure serum TMAO levels and their correlations with MCI in T2DM patients. We found a significant relationship between TMAO levels in T2DM patients with MCI. Our study showed (1) serum TMAO levels were higher in the MCI group compared to the non-MCI group in T2DM patients; (2) serum TMAO levels were positively associated with age, BMI, diabetes duration, HbA1c, TG, creatinine, and hs-CRP; (3) higher levels of serum TMAO were associated with the significant potential risk of MCI. Our findings speculate that higher serum TMAO levels may be associated with the deterioration of cognition in diabetes.
The previous study supports our results that diabetes could be a potential contributor to increased serum TMAO levels. In this study, a higher serum TMAO level was found in T2DM patients and diabetes with MCI. Thus, TMAO was considered a potential predictor for investigating reduced glucose tolerance and insulin resistance complications in the prediabetic state of the animal model Macaca mulatta.23 It was shown in a high-fat-diet mouse model that dietary TMAO elevates reduced glucose tolerance, inhibits the hepatic insulin signaling pathway, and consequently adipose tissue inflammation.24 Moreover, TMAO treatment reduced peripheral nerve abnormalities in the streptozotocin-induced diabetes model with no effect on diabetic hyperglycemia.25 It has also been shown that TMAO produces a macrophage phenotype, which affects the metabolism of cholesterol and sterol in macrophages, liver, and intestine.26 Previously, several studies demonstrated that higher serum TMAO levels were associated with cardiovascular disease and other diseases, including worse renal outcomes and other independent risk factors for diabetes.27 Considering these findings, our study found BMI, diabetes duration, HbA1c, TG, creatinine, and hs-CRP are associated with higher levels of TMAO in T2DM patients than healthy controls, which suggests further investigation of TMAO concentrations as a biomarker for cardiovascular diseases and in diabetes research.
We observed a positive relationship between serum TMAO levels with HbA1c. The HbA1c and diabetes duration enhanced the complications of MCI in T2DM patients, and a high concentration of HbA1c was related to cognitive impairment and less executive function in elderly adults.28,29 Our study confirmed these observations that patients with MCI had distinctly higher diabetes duration and higher concentrations of serum HbA1c compared to patients with no MCI. A recent study showed that diabetic and overweight people’s mean TMAO levels did not significantly change for 2 years, and due to TMAO levels, the authors noticed an intra-individual variability.30 The observed significant changes in TMAO concentrations in 2 years might be too short. For example, another research group reported age-dependent increase levels of TMAO for those below 40 years old and over 60 years old.31 Our study has also observed increased levels of TMAO with age, which is consistent with previously reported studies.
Diabetes is a group of disorders whose pathological processes are correlated with chronic inflammatory responses. However, hyperglycemia could activate through the inflammatory signal pathway NFκB and result in diabetic neuropathy and cognitive impairment.32 The NFκB is related to pathological neuroinflammation and is associated with proinflammatory cytokine expression.33 These proinflammatory cytokines are raised in patients with T2DM, including neutrophil/lymphocyte ratio, C-reactive protein, uric acid/HDL ratio, platelet/lymphocyte ratio, and mean platelet volume.34–38 In our study showed that the levels of hs-CRP were higher in MCI groups compared to non-MCI groups. A recent study reported that higher levels of TMAO induced the activation of NFκB pathway and improved the expression of the proinflammatory genes, such as inflammatory cytokines and chemokines.39 Moreover, through oxidative stress and NLRP3 inflammasome stimulation could be prompted by TMAO, whereas inflammatory cytokines (IL-18 and IL-1β) secretions were increased.40 In addition, increased TMAO could contribute to association with platelet hyperactivity, thrombosis, and cardiovascular disease.41–43 In our study, Spearman correlation test showed negative correlations between TMAO and HDL-C, BDNF. Thus, TMAO could be a potential therapeutic target to improve MCI through alleviation of inflammatory responses.
Limitations
There were different limitations to our study. (1) we used only the MoCA analysis tool to assess subjects’ cognitive cognition; more tools should be used to get more reliable and precise data. (2) The cognitive function was assessed within 7 days after admission, and the score can be affected by the mood and mental state of patients, which may affect the detection rate of cognitive impairment. (3) To define the correlation between TMAO levels and cognitive function were evaluated among the T2DM patients. More detailed studies are needed to be carried out on T2DM patients with MCI and no MCI groups. (4) A longitudinal investigation is required with this cross-sectional study to evaluate the change in TMAO levels in the early stage of T2DM.
Conclusion
The TMAO concentration increases in MCI patients and positively correlates with age, BMI, diabetes duration, HbA1c, TG, creatinine, hs-CRP, and is negatively associated with HDL-C and BDNF. Thus, the serum TMAO levels might be a biomarker for cognitive function and could be a potential predictor of MCI patients with T2DM.
Ethics Statement
All methods and procedures used in this study were in agreement with the ethical principles and standards of the institutional and national bioethical commission and with the Helsinki Declaration of 1964 and its later revisions. The Ethics Committee of Shanghai University of Medicine and Health Science Affiliated Zhoupu Hospital approved this study. All subjects provided informed consent to participate in the study.
Acknowledgment
We sincerely thank all of the participants in our study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; they took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work was supported by The work was supported by 1) Health plan scientific research project of Shanghai Pudong New Area Health Committee (pw2020A-63); 2)The Outstanding Clinical Discipline Project of Shanghai Pudong (Grant No. : PWYgy2021-02).
Disclosure
All authors in this article declared that they do not have any conflict of interest.
References
1. Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metabolic Syndrome Obesity. 2021;14:3567–3602.
2. Savonitto S, Morici N, Nozza A, et al. Predictors of mortality in hospital survivors with type 2 diabetes mellitus and acute coronary syndromes. Diab Vasc Dis Res. 2018;15(1):14–23.
3. Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942.
4. Wang G, Li W. Diabetes as a risk factor for abnormal cognition development. J Alzheimers Dis Rep. 2020;4(1):237–242.
5. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572.
6. Ramos-Rodriguez JJ, Molina-Gil S, Ortiz-Barajas O, et al. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PLoS One. 2014;9:e89229.
7. Wrighten SA, Piroli GG, Grillo CA, et al. A look inside the diabetic brain: contributors to diabetes-induced brain aging. Biochim Biophys Acta. 2009;1792:444–453.
8. Beauquis J, Saravia F, Coulaud J, et al. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol. 2008;210:359–367.
9. Subramaniam S, Fletcher C. Trimethylamine N-oxide: breathe new life. Br J Pharmacol. 2018;175(8):1344–1353.
10. Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60.
11. Tang WHW, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–1914.
12. Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726.
13. Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota- dependent trimethylamine N-oxide (TMAO) pathway contributes to both developments of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455.
14. Suzuki T, Heaney LM, Jones DJL, et al. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem. 2017;63(1):420–428.
15. Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose response meta-analysis. Eur Heart J. 2017;38(39):2948–2956.
16. Zhuang R, Ge X, Han L, et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes Rev. 2019;20(6):883–894.
17. Heianza Y, Sun D, Li X, et al. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS lost trial. Gut. 2019;68(2):263–270.
18. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553.
19. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
20. Alagiakrishnan K, Zhao N, Mereu L, et al. Montreal Cognitive Assessment is superior to Standardized Mini-Mental Status Exam in detecting mild cognitive impairment in the middle-aged and elderly patients with type 2 diabetes mellitus. Biomed Res Int. 2013;2013:186106.
21. Portet F, Ousset PJ, Visser PJ, et al. MCI Working Group of the European Consortium on Alzheimer’s Disease (EADC). Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714–718.
22. Wang Z, Levison BS, Hazen JE, et al. Measurement of Trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40.
23. Li X, Chen Y, Liu J, et al. Serum metabolic variables associated with impaired glucose tolerance induced by high-fat-high-cholesterol diet in Macaca mulatta. Exp Biol Med. 2012;237:1310–1321.
24. Gao X, Liu X, Xu J, et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high-fat diet. J Biosci Bioeng. 2014;118:476–481.
25. Lupachyk S, Watcho P, Stavniichuk R, et al. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–952.
26. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585.
27. Winther SA, Ollgaard JC, Tofte N, et al. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. 2019;42(8):1512–1520.
28. Gao Y, Xiao Y, Miao R, et al. The prevalence of mild cognitive impairment with type 2 diabetes mellitus among elderly people in China: a cross-sectional study. Arch Gerontol Geriatr. 2016;62:138–142.
29. Pappas C, Small BJ, Andel R, et al. Blood glucose levels may exacerbate executive function deficits in older adults with cognitive impairment. J Alzheimers Dis. 2019;67:81–89.
30. McEntyre CJ, Lever M, Chambers ST, et al. Variation of betaine, N, N dimethylglycine, choline, glycerophosphocholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem. 2015;52:352–360.
31. Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–910.
32. Muriach M, Flores-Bellver M, Romero FJ, et al. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014:102158.
33. Chen L, Hu L, Zhao J, et al. Chotosan improves Aβ1-42-induced cognitive impairment and neuroinflammatory and apoptotic responses through the inhibition of TLR-4/NF-κB signaling in mice. J Ethnopharmacol. 2016;191:398–407.
34. Duman TT, Aktas G, Atak BM, et al. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afri Health Sci. 2019;19:1602–1606.
35. Tekce H, Tekce BK, Aktas G, et al. Serum omentin-1 levels in diabetic and nondiabetic patients with chronic kidney disease. Exp Clin Endocrinol Diabetes. 2014;122:451–456.
36. Aktas G, Kocak MZ, Bilgin S, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2019;23:1–5.
37. Ilgun E, Akyurek O, Kalkan AO, et al. Neutrophil/Lymphocyte Ratio and Platelet/Lymphocyte Ratio in Fibromyalgia. Eur J Gen Med. 2016;13(2):100–104.
38. Ulasli SS, Ozyurek BA, Yilmaz EB, et al. Mean platelet volume as an inflammatory marker in acute exacerbation of chronic obstructive pulmonary disease. Pol Arch Med Wewnetrznej. 2012;122(6):284–290.
39. Ma G, Pan B, Chen Y, et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep. 2017;37(2):BSR20160244.
40. Boini KM, Hussain T, Li PL, et al. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol Biochem. 2017;44(1):152–162.
41. Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165(1):111–124.
42. Zhu W, Wang Z, Tang WHW, et al. Gut microbe-generated trimethylamine N-Oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135(17):1671–1673.
43. Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27(1):305–313.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.