Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
Increased serum TRAIL and DR5 levels correlated with lung function and inflammation in stable COPD patients
Authors Wu Y, Shen Y, Zhang J, Wan C, Wang T, Xu D, Yang T, Wen F
Received 12 July 2015
Accepted for publication 6 September 2015
Published 6 November 2015 Volume 2015:10(1) Pages 2405—2412
DOI https://doi.org/10.2147/COPD.S92260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Yanqiu Wu,1,2,* Yongchun Shen,1,2,* Junlong Zhang,3 Chun Wan,1,2 Tao Wang,1,2 Dan Xu,1,2 Ting Yang,1,2 Fuqiang Wen1,2
1Department of Respiratory and Critical Care Medicine, West China Hospital of Sichuan University, 2Division of Pulmonary Diseases, State Key Laboratory of Biotherapy of China, 3Department of Laboratory Medicine, West China Hospital of Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Background: Chronic obstructive pulmonary disease (COPD) is associated with abnormal systemic inflammation, and apoptosis is one of the pathogenic mechanisms of COPD. Several studies have suggested that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and its receptors were not only involved in diseases associated with apoptosis but also in inflammatory diseases. However, limited data about the possible relationship between COPD and TRAIL/TRAIL-receptors are available.
Objective: To evaluate the potential relationship between TRAIL/TRAIL-receptors and COPD.
Methods: Serum levels of TRAIL, decoy receptor 5 (DR5), C-reactive protein, and tumor necrosis factor-α were analyzed using multiplex enzyme-linked immunosorbent assay kits. Then, serum levels of TRAIL and DR5 in 57 COPD patients with 35 healthy controls were compared and correlated with lung function and systemic inflammation.
Results: Mean levels of serum TRAIL and DR5 were significantly higher in COPD patients than those in controls (50.17±17.70 versus 42.09±15.49 pg/mL, P=0.029; 48.15±22.88 versus 38.94±10.95 pg/mL, P=0.032, respectively). Serum levels of TRAIL and DR5 correlated inversely with forced expiratory volume in 1 second % predicted, an index of lung function in COPD (r=-0.354, P=0.007 for TRAIL; r=-0.394, P=0.002 for DR5) in all participants (r=-0.291, P=0.005 for TRAIL; r=-0.315, P=0.002 for DR5), while DR5 correlated positively with C-reactive protein (r=0.240, P=0.021 for total subjects) and TRAIL correlated positively with tumor necrosis factor-α (r=0.371, P=0.005 for COPD; r=0.349, P=0.001 for total subjects).
Conclusion: Our results suggested that circulating TRAIL and DR5 increased in COPD patients and were associated with lung function and systemic inflammation in COPD. Future studies are needed to verify whether and how TRAIL and its receptors play roles in COPD.
Keywords: chronic obstructive pulmonary disease, tumor necrosis factor-related apoptosis-inducing ligand, decoy receptor 5, apoptosis, inflammation
Introduction
Chronic obstructive pulmonary disease (COPD), a kind of chronic airway inflammatory disease, has been considered as the third most life-threatening disease, with high morbidity and mortality.1 The pathogenesis and pathology of COPD are complicated, but abnormal airway inflammation is the most important hypothesis, combined with imbalance between protease/antiprotease system, oxidative stress, and immunological mechanisms.2 In addition, it has been treated not only as a respiratory disease, but also as a systemic process, which could cause target organ injury, leading to various comorbidities of COPD patients.3,4 Over the past few years, a growing number of studies have reported that the apoptotic alveolar epithelial and endothelial cells were significantly increased in the lungs of COPD patients, which suggested that apoptosis might be involved in the pathogenesis of COPD.5–7 However, the functional relationship between apoptosis and systemic inflammation in COPD pathogenesis remains unclear.8
Tumor necrosis factor (TNF) plays an important role in the pathogenesis of COPD. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a member of the TNF family and mainly expressed by CD11c+ dendritic cells, airway epithelial cells, human peripheral blood T-lymphocytes, natural killer cells, eosinophils, neutrophils, and monocytes can be activated by cytokine or antigen exposure.9–13 Decoy receptor 5 (DR5) is one of the most important receptor of TRAIL, the cytoplasmic death domains of which can activate nuclear factor kappa B cells (NF-κB), inducing apoptotic cell death.14,15 Moreover, DR5 has been confirmed to be the highest affinity receptor for TRAIL, and it plays a vital role in TRAIL-induced apoptosis.16
Previous studies mainly focused on the function of TRAIL in tumors, since it could activate the extrinsic apoptotic pathway in immortalized cell lines and primary tumor cells.17–19 In recent years, some studies have found that TRAIL and its receptors played important roles in certain inflammatory responses.20–22 Furthermore, the underlying roles of the TRAIL/TRAIL-receptor system in the conditions of chronic pulmonary diseases, such as asthma,11,23 lung fibrosis,24 and pulmonary hypertension,25 have also been investigated. It has been shown that the TRAIL/TRAIL-receptors system was one of various mechanisms implicated in alveolar apoptosis of emphysematous lung.26 Morissette et al27 previously reported that DR5 was upregulated in the lung tissues of patients with emphysema when compared with healthy controls (smokers and nonsmokers). They also discovered that cigarette smoking and oxidative stress could sensitize alveolar epithelial cell to TRAIL-mediated apoptosis, and TRAIL-mediated apoptosis was abnormally increased in the lung of patients with emphysema.28 Despite these findings, studies concerning the association between TRAIL/TRAIL-receptors system and COPD are still limited. Therefore, the main objectives of this study were to measure the circulating TRAIL and DR5 in stable COPD patients and analyze their correlations with pulmonary function and systemic inflammatory markers of COPD.
Methods
Subjects
Between January 2013 and October 2014, 57 patients with COPD were recruited from the outpatient department of West China Hospital. They all underwent a chest X-ray and a standard pulmonary function test using a Spirotel® spirometer purchased from Mir Medical International Research Srl (Rome, Italy). COPD was diagnosed and classified for this research in accordance with Global Initiative for Chronic Obstructive Lung Disease criteria.1 Patients with COPD had to have a forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) less than 70%, and a less than 12% increase in FEV1 after inspiring bronchodilator, and they should not have had an exacerbation within the last 3 months prior to the study. None of the patients had ever received inhaled corticosteroids or antibiotics. They were divided into nonsmokers with COPD and smokers with COPD. Thirty-five smoking history, sex, and age-matched subjects with normal pulmonary function were recruited as controls, and they also underwent a chest X-ray and a standard pulmonary function test as the COPD patients did. We collected all the subjects’ basic health information through questionnaires and physical examinations. Patients with kidney disease, heart disease, liver disease, diabetes mellitus, cancer, and autoimmune disease were excluded. To avoid the bias caused by a long time of research, we recruited a limited number of subjects and could not divide subjects into multigroups in more diverse ways. The Institutional Review Board for Human Studies of West China Hospital of Sichuan University, People’s Republic of China, approved the study, and all the subjects provided written consent.
Measurement of TRAIL, DR5, TNF-α, and CRP
Peripheral blood samples were obtained from all subjects, and serum was separated immediately and stored at −80°C until processing. Levels of TRAIL, DR5, TNF-α, and C-reactive protein (CRP) were analyzed using Human Magnetic Luminex Screening Assay (LXSAHM; R&D Systems, Inc., Minneapolis, MN, USA) on Bio-Plex 200 platform (Bio-Rad, Irvine, CA, USA). Manufacturer-specified limits of detection were 9.65 pg/mL for TRAIL, 2.2 pg/mL for DR5, 1 pg/mL for TNF-α, and 116 pg/mL for CRP. Technicians performing tests were blinded to the clinical details of the subjects.
Statistical analysis
Data were expressed as mean ± standard deviation. After normal distribution of these data was been confirmed, differences between groups were statistically analyzed using an unpaired Student’s t-test and, for multiple comparisons, one-way analysis of variance was used. The nonnormal data were transformed to be normally distributed through log transformation. Correlations between variables were analyzed using Pearson’s correlation analysis. The indication of statistically significant difference was P<0.05. Data were analyzed using SPSS 19.0 for Windows (IBM, Chicago, IL, USA). Pictures were created using Graphpad Prism5.0 (GraphPad Software, San Diego, CA, USA).
Results
Clinical characteristics of subjects
Demographic and clinical characteristics (including smoking pack-years, body mass index [BMI], and serum levels of TRAIL and DR5) of patients with COPD and healthy controls are summarized in Tables 1 and 2. The age, sex, BMI, blood oxygen saturation, proportion of smoking, and smoking pack-years of both groups were similar. There were significant reductions in FEV1, FEV1% predicted, and FEV1/FVC in the COPD group compared with those in the control group, suggesting that the COPD group had airflow limitation. In multigroup analysis, age, BMI, blood oxygen saturation of all the groups were similar. Even though the sex composition and smoking pack-years were different between COPD smokers with COPD nonsmokers and healthy nonsmokers, they were similar between smoking COPD group and healthy smokers.
Serum levels of TRAIL and DR5 increase in COPD patients
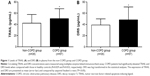
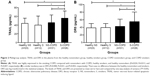
The circulating TRAIL levels in COPD patients were significantly upregulated in comparison with healthy volunteers (50.17±17.70 versus 42.09±15.49 pg/mL, P=0.029, Figure 1A). Similar result was found in DR5 (48.15±22.88 versus 38.94±10.95 pg/mL, P=0.032, Figure 1B). In addition, in multigroup analysis, we found that TRAIL and DR5 expressions were higher in smokers with COPD than those in nonsmokers with COPD (55.68±16.44 versus 45.55±17.65 pg/mL, P=0.024; 55.06±25.26 versus 42.35±19.22 pg/mL, P=0.008, respectively), healthy smokers (41.03±21.00 pg/mL, P=0.013; 39.38±10.64 pg/mL, P=0.021, respectively), and healthy nonsmokers (42.65±12.22 pg/mL, P=0.007; 38.71±11.34 pg/mL, P=0.002, respectively). However, there was no difference observed among the healthy nonsmokers, healthy smokers, and nonsmokers with COPD (Figure 2A and B).
TRAIL and DR5 are correlated with lung function, TNF-α, and CRP
We analyzed the correlations of TRAIL and DR5 with FEV1% predicted, FEV1/FVC, CRP, and TNF-α in COPD patients and in all subjects, and found that TRAIL was inversely correlated with FEV1% predicted (r=−0.354, P=0.007, Figure 3A) and FEV1/FVC (r=−0.32, P=0.013, Figure 3B) in COPD. Similar results were also found in all participants (r=−0.291, P=0.005; r=−0.321, P=0.002; respectively, Figure 3A and B). We also observed that DR5 had a negative correlation with FEV1% predicted (r=−0.394, P=0.002 for COPD; r=−0.315, P=0.002 for total subjects, Figure 3C) and FEV1/FVC (r=−0.316, P=0.017 for COPD; r=−0.358, P=0.000 for subjects, Figure 3D). More importantly, we still found a positive relationship between TRAIL and TNF-α in COPD and in all subjects (r=0.371, P=0.005; r=0.349, P=0.001, respectively; Figure 4A) and a positive correlation between DR5 and CRP in subjects (r=0.240, P=0.021, Figure 4B). All these correlations are shown in the Tables 3 and 4.
Discussion
In this study, we explored the serum levels of TRAIL and DR5 in 57 COPD cases and 35 controls using multiplex enzyme-linked immunosorbent assay (Luminex platform). We found that serum TRAIL and DR5 levels were significantly higher in patients with COPD than those in healthy controls. Moreover, in COPD patients, circulating TRAIL and DR5 had relationships with smoking history. Further analyses indicated that both TRAIL and DR5 concentrations correlated negatively with lung function, while TRAIL and DR5 correlated positively with TNF-α and CRP, respectively. To the best of our knowledge, this is the first study to assess the association between TRAIL/DR5 and COPD.
It has been proven that circulating TRAIL and DR5 play important roles in many diseases, such as cancer,29–31 asthma,32 allergic rhinoconjunctivitis,33 scleroderma,34 and chronic hepatitis B.35 Recent studies have also connected TRAIL and its receptors to COPD. It has been reported that alveolar epithelial cell was sensitive to TRAIL-mediated apoptosis due to the presence of cigarette smoke extract and H2O2, while recombinant TRAIL could activate caspase-3 and caspase-8 of emphysematous lung but decrease those apoptosis-related proteins in the nonemphysematous lung,28 which might be a new mechanism for persistent alveolar destruction after smoking cessation. Moreover, TRAIL could induce the proliferation of smooth muscle cells through activating NF-κB and inducing insulin-like growth factor-1 receptor, which may play a role in vascular remodeling in COPD.36,37 Besides, it could also activate proinflammatory signaling pathways, like the MAPK and phosphoinositoide 3-kinase pathways, which are classic signal pathways in COPD.38–40 Similarly, a previous investigation has shown that DR5 levels were higher in the lung of subjects with emphysema than normal smokers and nonsmokers, regardless of smoking cessation, and that oxidative stress increased DR5 expression in A549 cells.27 DR5 expression in alveolar epithelial cells was upregulated in idiopathic pulmonary fibrosis with overexpression of proapoptotic marker p53.24 All of the accumulated evidences suggested that TRAIL and DR5 are also expressed in the cells of lung tissue and had strong relationships with chronic pulmonary inflammatory disease, including COPD.
Therefore, our study took a further step toward exploring the involvements of TRAIL and DR5 in COPD. In this study, we found higher serum TRAIL and DR5 concentrations in COPD patients compared with those in healthy subjects, which was in accordance with previously published results. Meanwhile, in the analysis of stratification, both TRAIL and DR5 levels were higher in smoking COPD than those in nonsmoking COPD patients, healthy smokers, and healthy nonsmokers, indicating that circulating TRAIL/DR5 levels may have relations with smoking history and that different mechanisms may exist between smokers COPD and nonsmokers COPD, although the exact reason remains unclear. The differences between COPD smokers and COPD nonsmokers or healthy nonsmokers might attribute to the differences in either the sex or pack-years. However, there were no differences between smoking COPD group and healthy smokers regarding the sex composition and smoking pack-years. It can be deduced that TRAIL and DR5 may only contribute to the COPD induced by smoking cigarette, as cigarette smoking is responsible for the apoptosis of alveolar structural cells. More interestingly, the apoptosis progress would not stop after smoking cessation, indicating that other mechanisms perpetuate apoptosis in COPD. Whether the TRAIL/TRAIL-receptors play any role in these mechanisms remains an open question and deserves further research.
More importantly, we observed that either TRAIL or DR5 had a negative relationship with pulmonary function (FEV1% predicted) in COPD patients, suggesting that TRAIL and DR5 may be related to the severity of airway obstruction. Last but not least, we found that serum CRP and TNF-α levels in COPD patients were elevated relative to controls, and TRAIL and DR5 correlated positively with TNF-α and CRP, respectively. It is well established that circulating TNF-α and CRP levels were elevated in COPD, thus considered as appropriate biomarkers of systemic inflammation in COPD,41–43 and were associated with the presence of airflow obstruction.44,45 Therefore, our results suggested that TRAIL and DR5 might function as biomarkers of the severity of systemic inflammation in COPD. However, the underlying mechanisms could not be clarified in the present study. It is well known that TRAIL and its receptors are apoptosis-related proteins. As mentioned earlier, several disease mechanisms contribute to the development of COPD such as inflammation, proteinase/antiproteinase imbalance, oxidative stress, etc. There are a series of complicated interactions between apoptosis and all other pathways.7 Our findings might be further evidence of the relationship between apoptosis and inflammation in COPD. Nevertheless, further experiments should be performed in order to clarify how TRAIL and DR5 are induced in COPD and which signaling pathways they involve.
Our research also had some limitations. First, we recruited relatively small number of samples, which might lead to an inexact assessment of serum TRAIL and DR5 concentration. Second, most of our patients had relatively mild COPD, so this result might not be applicable for patients with more severe airway obstruction. Furthermore, we measured the serum levels of TRAIL and DR5 only once and chose the stable COPD patients, so it was unlikely to assess their dynamic levels during the progression of COPD and their expressions in the acute exacerbation of COPD. Finally, only two systemic inflammatory indicators (CRP and TNF-α) were analyzed, thus we could not gain more detailed insights into whether and how TRAIL/DR5 contribute to the disease.
In conclusion, this is the first study to research the correlations of serum TRAIL and DR5 with lung function and systemic inflammation in COPD. The results indicate that circulating TRAIL and DR5 may serve as blood-based markers for COPD severity and systemic inflammation and further confirm the roles of TRAIL and its receptors in COPD. However, more fundamental researches to demonstrate how TRAIL/TRAIL-receptors contribute to the pathogenesis of COPD should be made.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81230001, 81300032, 81470236, and 31171103), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China during the “12th Five-Year Plan” (2012BAI05B02), and projects in the Science and Technology Pillar Program from the Department of Science and Technology of Sichuan province (2015SZ0151). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
All authors contributed toward data collection, statistical analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Vijayan VK. Chronic obstructive pulmonary disease. Indian J Med Res. 2013;137(2):251–269. | ||
Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl). 2010;123(23):3467–3478. | ||
MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45(3):291–300. | ||
Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28(5):555–562. | ||
Mouded M, Egea EE, Brown MJ, et al. Epithelial cell apoptosis causes acute lung injury masquerading as emphysema. Am J Respir Cell Mol Biol. 2009;41(4):407–414. | ||
Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7(1):53. | ||
Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD. 2007;4(4):347–353. | ||
Robertson NM, Rosemiller M, Lindemeyer RG, Steplewski A, Zangrilli JG, Litwack G. TRAIL in the airways. Vitam Horm. 2004;67(20):149–167. | ||
Kayagaki N, Yamaguchi N, Nakayama M, et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163(4):1906–1913. | ||
Weckmann M, Collison A, Simpson JL, et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat Med. 2007;13(11):1308–1315. | ||
Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14(3–4):337–348. | ||
Daigle I, Simon HU. Alternative functions for TRAIL receptors in eosinophils and neutrophils. Swiss Med Wkly. 2001;131(17–18):231–237. | ||
Schneider P, Thome M, Burns K, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7(6):831–836. | ||
Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12(6):611–620. | ||
Truneh A, Sharma S, Silverman C, et al. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;275(30):23319–23325. | ||
Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–798. | ||
Bilski A, Pasz-Walczak G, Kubiak R, et al. TRAIL protein expression in breast cancer cells correlates with nuclear grade. Arch Med Sci. 2010;6(4):545–551. | ||
Yildiz Y, Yaylim-Eraltan I, Arikan S, Ergen HA, Kucucuk S, Isbir T. Is there any correlation between TNF-related apoptosis-inducing ligand (TRAIL) genetic variants and breast cancer? Arch Med Sci. 2010;6(6):932–936. | ||
McGrath EE, Marriott HM, Lawrie A, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011;90(5):855–865. | ||
Bem RA, Bos AP, Wosten-van Asperen RM, et al. Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection. Am J Respir Cell Mol Biol. 2010;42(6):697–705. | ||
Collison A, Foster PS, Mattes J. Emerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responses. Clin Exp Pharmacol Physiol. 2009;36(11):1049–1053. | ||
Tisato V, Garrovo C, Biffi S, et al. Intranasal administration of recombinant TRAIL down-regulates CXCL-1/KC in an ovalbumin-induced airway inflammation murine model. PLoS One. 2014;9(12):e115387. | ||
Akram KM, Lomas NJ, Forsyth NR, Spiteri MA. Alveolar epithelial cells in idiopathic pulmonary fibrosis display upregulation of TRAIL, DR4 and DR5 expression with simultaneous preferential over-expression of pro-apoptotic marker p53. Int J Clin Exp Pathol. 2014;7(2):552–564. | ||
Hameed AG, Arnold ND, Chamberlain J, et al. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med. 2012;209(11):1919–1935. | ||
Morissette MC, Parent J, Milot J. Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know. Int J COPD. 2009;4:19–31. | ||
Morissette MC, Vachon-Beaudoin G, Parent J, Chakir J, Milot J. Increased p53 level, Bax/Bcl-x(L) ratio, and TRAIL receptor expression in human emphysema. Am J Respir Crit Care Med. 2008;178(3):240–247. | ||
Morissette MC, Parent J, Milot J. The emphysematous lung is abnormally sensitive to TRAIL-mediated apoptosis. Respir Res. 2011;12:105. | ||
Ganten TM, Sykora J, Koschny R, et al. Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J Mol Med (Berl). 2009;87(10):995–1007. | ||
Toiyama D, Takaha N, Shinnoh M, et al. Significance of serum tumor necrosis factor-related apoptosis-inducing ligand as a prognostic biomarker for renal cell carcinoma. Mol Clin Oncol. 2013;1(1):69–74. | ||
Bisgin A, Kargi A, Yalcin AD, et al. Increased serum sTRAIL levels were correlated with survival in bevacizumab-treated metastatic colon cancer. BMC Cancer. 2012;12:58. | ||
Yalcin AD, Bisgin A, Kargi A, Gorczynski RM, Akdis CA. Serum-soluble TRAIL levels in patients with severe persistent allergic asthma: its relation to omalizumab treatment. Med Sci Monit. 2012;18(3):11–15. | ||
Yalcin AD, Bisgin A. Soluble trail as a marker of efficacy of allergen-specific immunotherapy in patients with allergic rhinoconjunctivitis. Med Sci Monit. 2012;18(10):617–621. | ||
Azab NA, Rady HM, Marzouk SA. Elevated serum TRAIL levels in scleroderma patients and its possible association with pulmonary involvement. Clin Rheumatol. 2012;31(9):1359–1364. | ||
Du J, Wang L, Han J, Gao L, Ma C, Liang X. Serum soluble death receptor 5 concentration in patients with chronic hepatitis B is associated with liver damage and viral antigen level. Clin Biochem. 2012;45(10–11):845–847. | ||
Kavurma MM, Schoppet M, Bobryshev YV, Khachigian LM, Bennett MR. TRAIL stimulates proliferation of vascular smooth muscle cells via activation of NF-kappaB and induction of insulin-like growth factor-1 receptor. J Biol Chem. 2008;283(12):7754–7762. | ||
Secchiero P, Zerbinati C, Rimondi E, et al. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci. 2004;61(15):1965–1974. | ||
Stagg HW, Bowen KA, Sawant DA, Rodriguez M, Tharakan B, Childs EW. Tumor necrosis factor-related apoptosis-inducing ligand promotes microvascular endothelial cell hyperpermeability through phosphatidylinositol 3-kinase pathway. Am J Surg. 2013;205(4):419–425. | ||
Degli-Esposti M. To die or not to die – the quest of the TRAIL receptors. J Leukoc Biol. 1999;65(5):535–542. | ||
Liu J, Qu X, Xu L, et al. Phosphoinositide 3-kinase/Akt and nuclear factor kappaB pathways are involved in tumor necrosis factor-related apoptosis-inducing ligand resistance in human gastric cancer cells. Mol Med Rep. 2010;3(3):491–496. | ||
Higashimoto Y, Iwata T, Okada M, Satoh H, Fukuda K, Tohda Y. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med. 2009;103(8):1231–1238. | ||
Tanni SE, Pelegrino NR, Angeleli AY, Correa C, Godoy I. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patients. J Inflamm (Lond). 2010;7:29. | ||
Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19(2):104–108. | ||
Chiang CH, Chuang CH, Liu SL. Transforming growth factor-beta1 and tumor necrosis factor-alpha are associated with clinical severity and airflow limitation of COPD in an additive manner. Lung. 2014;192(1):95–102. | ||
Pinto-Plata VM, Mullerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61(1):23–28. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.