Back to Journals » Risk Management and Healthcare Policy » Volume 13
Increased Risk of Clopidogrel-Induced Gastric Mucosal Erosion in Elderly Chinese Men Harboring the ABCB1 3435T Allele
Authors Duan L , Li M, Wang F, Cai Y, Li H, Zhou W, Li Y, Chen Q, Bai J, Liu H
Received 19 May 2020
Accepted for publication 30 July 2020
Published 20 August 2020 Volume 2020:13 Pages 1237—1244
DOI https://doi.org/10.2147/RMHP.S263625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Marco Carotenuto
Lei Duan, Man Li, Fan Wang, Yulun Cai, Huiying Li, Wenli Zhou, Yuerui Li, Qiang Chen, Jing Bai, Hongbin Liu
Department of Geriatric Cardiology, National Clinical Research Center for Geriatric Diseases, The Second Medical Center of PLA General Hospital, Beijing 100853, People’s Republic of China
Correspondence: Hongbin Liu Department of Geriatric Cardiology
National Clinical Research Center for Geriatric Diseases, The Second Medical Center of PLA General Hospital, 28 Fuxing Road, Beijing 100853, People’s Republic of China
Tel +86 010 66876349
Email [email protected]
Background: It is uncertain whether long-term use of clopidogrel alone can cause gastric mucosal injury. This study aimed to evaluate the relationship between ABCB1 C3435T polymorphisms, which could affect the intestinal absorption of clopidogrel, and gastric mucosal erosion in elderly Chinese men who used clopidogrel alone.
Methods: We selected 298 male patients (aged between 68.2 and 89.5 years, average age 78); 201 of them constituted the control group, and 97 constituted the case group. Patients taking clopidogrel alone who had undergone endoscopic screening for gastric erosion were analyzed for ABCB1 C3435T polymorphisms by a TaqMan assay.
Results: The proportion of people carrying the ABCB1 3435T allele (n = 63, 64.9% vs n = 97, 48.3%, p = 0.007) was significantly higher in the case group than in the control group. After adjustments for significant factors were made, ABCB1 3435T allele carrier (OR 2.14, 95% CI 1.43– 3.84, p < 0.01) was found to be associated with gastric mucosal erosion in people who used clopidogrel alone.
Conclusion: Carrying the ABCB1 3435T allele may be a useful genetic predictor for clopidogrel-induced gastric mucosal erosion in elderly Chinese men.
Keywords: clopidogrel, polymorphism, single nucleotide, ABCB1 C3435T, gastric mucosa, risk factor
Introduction
Cardio-cerebral vascular diseases are more common in the elderly population who have higher disability and mortality rates than affected populations of younger ages. In China, aspirin and clopidogrel are widely used in the prevention and treatment of ischemic cardiovascular and cerebrovascular diseases. In recent years, a number of studies have shown that aspirin’s bleeding risk offsets its benefit to some extent.1–3 Gastrointestinal injury not only limits the scope of application of aspirin, however, but also leads to serious clinical events.4
Clopidogrel works by blocking the P2Y12 receptors on platelets. Due to its better tolerability profile, it can be applied to aspirin-intolerant people, and it remands the first-line treatment option for secondary prevention in stroke patients.5 P-glycoprotein and hepatic cytochrome P450 2C19 affect the intestinal absorption and the efficiency of the active conversion of clopidogrel, which encoded by ABCB1 and CYP2C19, respectively. Therefore, mutations in these two protein-coding genetic loci may alter the bioavailability of clopidogrel.6
Whether clopidogrel causes gastric mucosal injury is currently controversial. Some studies have reported that patients treated with clopidogrel but not aspirin had similar or increased risks of gastrointestinal bleeding, while others reported decreased risks of gastrointestinal disease.7–10 Gastric mucosal injury is a continuous process. The initial stage of gastric mucosal injury involves scattered spots of bleeding and erosion and then progresses to gastric ulcers, stomach bleeding and even gastric perforation. Considering the increasing application of clopidogrel in real-world settings, as well as the elderly population, who are characterized by a gradual weakening of the body and a poor prognosis of the disease, early identification of clopidogrel-induced gastric mucosal injury is particularly important. The purpose of this study was to explore whether CYP2C19 and ABCB1 gene polymorphisms were associated with gastric mucosal erosion in elderly Chinese males who used clopidogrel alone.
Methods
Study Population
This retrospective case–control study analyzed 298 male patients (aged between 68.2 and 89.5 years, average age 78) who took 75 mg of clopidogrel daily between 2013 and 2018 for cardio-cerebral vascular disease due to aspirin intolerance or other reasons and underwent annual gastric mucosal endoscopy at the Second Medical Center of People’s Liberation Army of China (PLA) General Hospital. A total of 201 patients constituted the control group (modified Lanza score 0–1), and 97 constituted the case group (modified Lanza score 2–3) according to the gastroscopy results. The inclusion criteria were as follows: no abnormal gastric mucosa detected by gastroscopy before 2013; and consistent gastroscopy results; and no gastric ulcer or stomach bleeding between 2013 and 2018. The exclusion criteria were as follows: a history of cancer; severe liver and kidney dysfunction; the use of other antiplatelet or anticoagulant drugs; and long-term use of nonsteroidal anti-inflammatory drugs or hormone drugs. Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Helsinki declaration and its later amendments. This study was approved by the Medical Ethics Committee of PLA General Hospital (S2016-070-02).
Clinical Data and Laboratory Data Collection
The 2018 physical examination data of the population were collected through the electronic case system of the hospital and included age, body mass index, systolic blood pressure, diastolic blood pressure, smoking and drinking status, history of gastric ulcer, diabetes mellitus, hypertension, hyperlipidemia, ischemic heart disease, cerebrovascular disease, and proton pump inhibitor (PPI) and statin use. Diabetes mellitus, hypertension and hyperlipidemia were all defined based on hospital records or drug prescriptions. Ischemic heart disease was defined base on a medical history of percutaneous coronary intervention and/or coronary artery bypass grafting. Cerebrovascular disease was defined as a previous ischemic stroke based on hospital records. Blood samples were collected intravenously 12 hours after fasting.
The platelet count was measured by an automatic hematology analyzer (Nihon Kohden MEK-7222K, Japan). Thromboelastograms were used to determine the platelet inhibition rate induced by ADP (Haemonetics Corporation). The serum creatinine (SCr) level was determined by enzyme catalysis with an automatic biochemical analyzer (Hitachi 7400, Japan). H. pylori status was detected using the C13 urea breath test. The estimated glomerular filtration rate (eGFR) was used to evaluate renal function and was calculated as follows: eGFR (mL/min/1.73m2)= standard SCr (mg/dl)×175–age×1.234–0.179. [standard SCr (mg/dl)= SCr (mg/dl) (detected by enzyme catalysis)×0.795+0.29].11,12 All tests were performed by trained personnel in the same laboratory according to the standards of the World Health Organization reference laboratory. The clinical data were collected and collated by trained internal medicine clinicians.
Endoscopic Evaluation of Gastric Mucosal Injury
The modified Lanza score was used to evaluate the grade of gastric mucosal injury.13 There are five grades in the scoring system: grade 0 is normal gastric mucosa, grade 1 is only erythema or petechiae, grade 2 is 1–2 erosive lesions, grade 3 is 3–10 erosive lesions, and grade 4 is more than 10 erosive lesions or ulcers.
DNA Isolation and Genotyping
Two hundred microliters of venous blood was drawn from each patient and collected into an EDTA anticoagulant tube (Biotend, Shanghai, China). The sample DNA was extracted using the blood genomic DNA isolation kit (DP318, TIANGEN Biotech, Beijing, China), and a Q3000 ultraviolet spectrophotometer (Quawell, San Jose, CA, USA) was used to measure the concentration of the DNA samples. According to standard protocols, the TaqMan method and sequence analysis were used to determine genotype (Applied Biosystems, Foster City, Calif). Genotyping for CYP2C19*2 G681A (rs4244285) (forward primer 5′- CAATGTGATCTGCTCCATTATTTTC-3′, reverse primer 5′- GTCCCGAGGGTTGTTGATGT-3′); CYP2C19*3 G636A (rs4986893) (forward primer 5′-CTGCAATGTGATCTGCTCCA-3′, reverse primer 5′-TTAAGTAATTTGTTATGGGTTCCCG-3′); CYP2C19*17 C806T (rs12248560) (forward primer 5′-TTATGAACAGGATGAATGTGGTAT-3′, reverse primer 5′- GGGATTTGAGCTGAGGTCTT-3′) and ABCB1 C3435T (rs1045642) (forward primer 5′-CGACTGAATGTTCAGTGGCTC-3′, reverse primer 5′- TCCCAGGCTGTTTATTTGAAG −3′) were performed for each sample.
Statistical Analysis
The one-sample Kolmogorov–Smirnov test was used to test the normality of the distribution of continuous variables. Categorical variables are expressed as numbers and percentages and were compared by the chi-square test; continuous variables are expressed as the means ± standard deviations (SDs) and were compared by the two-sample t-test. The odds ratio (OR) and 95% confidence interval (CI) were obtained with Mantel–Haenszel statistics. After testing for multicollinearity, variables with statistical significance after single factor analysis were included in multivariable logistic regression analysis model to screen for risk and protection factors. Differences in the genotype frequencies between the two groups and the Hardy–Weinberg equilibrium of the allele frequencies at individual loci were evaluated by the chi-squared test or Fisher’s exact probability test by comparing the expected and observed genotype frequencies. Two-tailed p <0.05 was considered to be statistically significant. Analyses were performed using SPSS software version 20.0 (SPSS IBM Corporation, Armonk, NY, USA).
Results
Subject Characteristics
We enrolled 298 male patients (68.2–89.5 years old; average age: 78.0 years). According to the modified Lanza score classification criteria, 201 were placed into the control group, and 97 were placed into the case group. The baseline clinical characteristics according to the endoscopy results are presented in Table 1. The platelet inhibition rate (54.0 ± 23.7 vs 42.8 ± 21.4%, p = 0.02), a history of gastric ulcers (n = 33, 34.0% vs n = 38, 18.9%, p = 0.004), diabetes mellitus (n = 37, 38.1% vs n = 52, 25.9%, p = 0.03) and ischemic heart disease (n = 41, 42.3% vs n = 59, 29.4%, p = 0.027) were found to be significantly higher in the case group than in the control group. However, hyperlipidemia (n = 8, 8.2% vs n = 39, 19.4%, p = 0.013) and statin use (n = 43, 44.3% vs n = 126, 62.7%, P = 0.003) were lower in the case group than in the control group. These baseline clinical factors contributed to clopidogrel-induced gastric mucosal erosion to a significantly great extent in the univariable analysis.
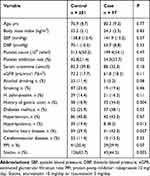 |
Table 1 Demographic and Clinical Characteristics of the Study Patients |
CYP2C19 and ABCB1 Genotype Distribution
All patients were successfully genetically tested. Hardy–Weinberg disequilibrium was not found to be significant for any of the tested genotypes (P > 0.05). There were no significant differences in the frequency of the CYP2C19*2, CYP2C19*3 and CYP2C19*17 alleles between the two groups. The proportion of people carrying the ABCB1 3435T allele was significantly increased in the case group (n = 63, 64.9% vs n = 97, 48.3%, p = 0.007) (Table 2).
 |
Table 2 Allele and Genotype Frequencies of CYP2C19 and ABCB1 in Patients Taking Clopidogrel |
Factors Associated with Gastric Mucosal Erosion
After adjusting for significant factors in the univariable analysis, a history of gastric ulcers (OR 3.11, 95% CI 1.84–5.05, p <0.01), diabetes mellitus (OR 2.37, 95% CI 1.49–4.60, p <0.05) and ABCB1 3435T allele carrier (OR 2.14, 95% CI 1.43–3.84, p <0.01) were significantly positively correlated with gastric mucosal erosion in multiple logistic regression analysis. However, statin use (OR 0.58, 95% CI 0.36–0.93, p <0.05) was significantly negatively correlated with gastric mucosal erosion (Table 3).
 |
Table 3 Association Between Various Related Factors and Gastric Mucosal Erosion in Patients Taking Clopidogrel |
Discussion
Aspirin and clopidogrel are commonly used antiplatelet drugs in the prevention and treatment of ischemic cardiovascular and cerebrovascular diseases. The gastric mucosal injury complications of aspirin are caused by a combination of topical mucosal injury and systemic effects via the inhibition of protective gastric prostaglandin synthesis. It is currently recommended that aspirin be used in combination with PPI to prevent gastric mucosal injury. Clopidogrel can be used alone by patients who require secondary stroke prevention or have and intolerance of aspirin and by children.5,14,15 It mainly works by blocking the platelet P2Y12 receptor to exert antiplatelet effects. Anderson et al found that the antiplatelet effect of clopidogrel activated before standard accepted biotransformation pathways.16 Another study speculated that clopidogrel also interfered with arachidonic acid-mediated platelet stimulation.17
Whether clopidogrel causes gastric mucosal injury is still controversial. Grove et al considered clopidogrel use to be associated with an increased risk of adverse gastric mucosal injury events such as gastritis, ulcers and bleeding, yet the risk was only modest.18 However, Whittemore et al showed that clopidogrel alone did not cause gastrointestinal bleeding or ulceration.19 Some articles could not explain the relationship between clopidogrel alone and gastrointestinal injury due to the combination of other antiplatelet drugs in the study designs.20,21
To our knowledge, this study is the first to assess the association of ABCB1 C3435T locus gene polymorphisms in elderly Chinese men with gastric mucosal erosion induced by clopidogrel alone. The results showed that ABCB1 3435T allele carrier status, a history of gastric ulcers and diabetes mellitus were risk factors for gastric mucosal erosion caused by clopidogrel and that the use of statins was a protective factor. In addition, PPI had no preventive effect on gastric mucosal erosion. Considering the serious threat of gastric ulcers and even stomach bleeding to elderly individuals, this study was of great significance for the early identification of gastric mucosal injury in elderly men taking clopidogrel alone.
Multiple experiments showed that diabetes mellitus increased the incidence of gastrointestinal mucosal injury and delayed the healing of ulcers.22,23 Harsch et al showed that the prolongation of gastric ulcer healing in diabetic rats was associated with an increase in gastric mucosal expression and release of TNFα, interleukin-1 and heat shock protein 70 and the suppression of vascular endothelial growth factor and platelet endothelial cell adhesion molecule-1.24 Studies have also suggested that reduced gastric mucosal blood flow, decreased insulin-like growth factor-1, and loss of antioxidant stress protein function caused by advanced glycation end products are also involved in the delayed healing of gastric mucosa injury.25,26
Our study found that the incidence of gastric mucosal erosion was lower in patients with hyperlipidemia among whom the proportion of statin use was higher than among people with normal blood lipids. We speculated that this phenomenon may be attributed to the gastric mucosal protective effect of statins. Animal experiments have found that the protective effect of hydroxymethylglutaryl coenzyme A reductase inhibitor on gastric mucosa is related to its inhibition of neutrophil activity, reduction of oxidative stress, increased the amount of nitric oxide and prostaglandin E2, and maintenance of the integrity of the vascular endothelium.27 However, our previous research did not find that statins had a protective effect on gastric mucosal injury caused by aspirin.28 In addition, our study did not find that PPI had a preventive effect on gastric mucosal injury induced by clopidogrel. Therefore, it could be speculated that gastric acid and the ‘aspirin-like’ effect of clopidogrel might not play a dominant role in the mechanisms of gastric mucosal erosion caused by clopidogrel and that the mechanisms of gastric mucosal injury between clopidogrel and aspirin might be different.
Clopidogrel is absorbed through the digestive tract and is converted to active metabolites in the liver. A key protein involved in clopidogrel absorption is the intestinal efflux pump P-glycoprotein, which is encoded by the ATP-binding cassette, subfamily B, member 1 gene (ABCB1). Therefore, the ABCB1 gene polymorphism can change the P-glycoprotein transport activity and affect the blood concentration of clopidogrel. Clopidogrel requires oxidation by hepatic cytochrome enzymes to metabolize into active substances to play its antiplatelet role. The difference in clopidogrel efficacy among individuals is related to the degree of biotransformation. Increasing evidence shows that this situation is mainly related to the genetic polymorphisms of CYP2C19. Among them, CYP2C19 *2 and CYP2C19 *3 are known as functional deletion gene mutations, which weaken the ability of clopidogrel to convert into its active metabolites. In contrast, CYP2C19 *17, known as a functional acquired gene mutation, is associated with an increased risk of bleeding.29
Our study did not observe that the CYP2C19 2 *3 * and 17 * alleles were associated with gastric mucosal erosion but found that ABCB1 3435T allele carriers had a higher incidence of gastric mucosal erosion after long-term clopidogrel use. ABCB1 is also known as the multidrug resistance gene MDR1, and its encoded P-glycoprotein can pump harmful substances or substrates, such as drugs, out of the cell. Therefore, its overexpression prevents the drug from being absorbed into the blood, which is one of the mechanisms of multidrug resistance.30 There are more than 50 genetic locus polymorphisms in ABCB1, of which the ABCB1 C3435T (rs1045642) polymorphism is considered to be the most clinically significant, but the current study conclusions are controversial. Simon et al and Mega et al suggested that T allele carriers had a higher risk of ischemia among patients with acute coronary syndrome than C allele carriers.31,32 However, Wallentin et al and Zhang et al took the opposite view and suggested that the mutated T allele increased clopidogrel absorption in the small intestine and reduced the incidence of clinical ischemic events.33,34 Another experiment also suggested that the ABCB1 C3435T genotype did not influence the antiplatelet response of clopidogrel.35 Wang et al stated that the 3435C> T substitution might be related to decreased mRNA stability and reduced mRNA and protein levels.36 Kimchi-Sarfaty et al suggested that the 3435C> T substitution might affect the timing of the cotranslational folding and insertion of P-glycoprotein into the membrane, resulting in a decrease in the specific binding ability of P-glycoprotein to the substrate and the function of the outflow pump.37
Our study found that the proportion of T allele carriers and the platelet inhibition rate were both higher in the case group than in the control group. We speculated that compared with the wild-type C allele, the 3435C> T substitution could weaken P-glycoprotein outflow pump function, increase intestinal absorption of clopidogrel, and finally enhance antiplatelet activity. Two mechanisms may be at play; first, clopidogrel might inhibit the normal accumulation of platelets in the area of the gastric mucosal injury and delay the repair of small ulcers. Second, clopidogrel might lead to the inhibition of gastric epithelial cell proliferation via the epidermal growth factor receptor-ERK signal transduction pathway and, of angiogenesis of gastric mucosa via the vascular endothelial growth factor-vascular endothelial growth factor receptor 2-ERK signal transduction pathway and to attenuation of gastric mucosal epithelial barrier function via the p38 MAPK pathway. All these factors weaken gastric mucosal microvascular system regeneration and reduce platelet-associated growth factor production, which is involved in the repair of gastric mucosal injury.38–40
Gastric mucosal injury is a dynamic imbalance between pathogenic factors and protective factors. When the aggressive factors overwhelm the defense and healing factors, mucosal injury is intensified, and gastric mucosal erosion relapses. Some findings also supported the conclusion of our study. Clopidogrel did not inhibit the effect of cyclooxygenase and did not induce gastric mucosal injury in healthy people.10 However, it is not safe for people who have suffered from gastric ulcer bleeding caused by aspirin to use clopidogrel instead in the prevention and treatment of cardiovascular disease, even if their ulcers had been confirmed to have healed by gastroscopy.41,42
The first limitation of the present study was that it only enrolled elderly Chinese men. Because gene polymorphisms might be influenced by different genetic backgrounds or environments, it might be inappropriate to generalize the results to other ethnic populations and women. Second, combined with the increase in the platelet inhibition rate, we speculated that mutations in the T allele might increase the absorption of clopidogrel. However, we did not measure the blood concentration of clopidogrel, so we could not prove a mutation in the T allele caused a decline in the function of the P-glycoprotein outflow pump directly.
Conclusion
Carrying the ABCB1 3435T allele may be a useful genetic predictor for clopidogrel-induced gastric mucosal erosion in elderly Chinese men. A history of gastric ulcers and diabetes mellitus are clinical risk factors for gastric mucosal erosion. Statin use may be a protective factor, but PPI has no protective effect.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Hongbin Liu reports grants from The General Logistics Department of Chinese People’s Liberation Army (PLA), during the conduct of the study. The authors report no other conflict on interest in this work.
References
1. Bowman L, Mafham M, et al.; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529–1539. doi:10.1056/NEJMoa1804988.
2. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–1518. doi:10.1056/NEJMoa1805819
3. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–1528. doi:10.1056/NEJMoa1803955
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036–1046. doi:10.1016/S0140-6736(18)31924-X
5. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi:10.1161/STR.0000000000000024
6. Frelinger AL, Bhatt DL, Lee RD, et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol. 2013;61(8):872–879. doi:10.1016/j.jacc.2012.11.040
7. Tsai TJ, Lai KH, Hsu PI, et al. Upper gastrointestinal lesions in patients receiving clopidogrel anti-platelet therapy. J Formos Med Assoc. 2012;111(12):705–710. doi:10.1016/j.jfma.2011.11.028
8. Mangalpally KK, Kleiman NS. The safety of clopidogrel. Expert Opin Drug Saf. 2011;10(1):85–95. doi:10.1517/14740338.2011.532485
9. Harker LA, Boissel JP, Pilgrim AJ, Gent M. Comparative safety and tolerability of clopidogrel and aspirin: results from CAPRIE. CAPRIE steering committee and investigators. Clopidogrel versus aspirin in patients at risk of ischaemic events. Drug Saf. 1999;21(4):325–335. doi:10.2165/00002018-199921040-00007
10. Fork FT, Lafolie P, Tóth E, Lindgärde F. Gastroduodenal tolerance of 75 mg clopidogrel versus 325 mg aspirin in healthy volunteers. A gastroscopic study. Scand J Gastroenterol. 2000;35(5):464–469. doi:10.1080/003655200750023705
11. Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi:10.1681/ASN.2006040368
12. Zhang LX, Zuo L, Xu GB, et al. Community-based screening for chronic kidney disease among populations older than 40 years in Beijing. Nephrol Dial Transplant. 2007;22(4):1093–1099. doi:10.1093/ndt/gfl763
13. Sogabe M, Okahisa T, Nakasono M, et al. Investigation of gastroduodenal mucosal injury in Japanese asymptomatic antiplatelet drug users. Medicine. 2015;94(26):e1047. doi:10.1097/MD.0000000000001047
14. Soghomonyan S, Abdel-Rasoul M, Zuleta-Alarcon A, et al. Clopidogrel IBS patients have higher incidence of gastrointestinal symptoms influenced by age and gender. Dig Dis Sci. 2017;62(10):2728–2743. doi:10.1007/s10620-017-4707-7
15. Gentilomo C, Huang YS, Raffini L. Significant increase in clopidogrel use across U.S. children’s hospitals. Pediatr Cardiol. 2011;32(2):167–175. doi:10.1007/s00246-010-9836-0
16. Anderson RA, Bundhoo S, James PE. A new mechanism of action of thienopyridine antiplatelet drugs a role for gastric nitrosthiol metabolism? Atherosclerosis. 2014;237(1):369–373. doi:10.1016/j.atherosclerosis.2014.08.045
17. Sambu N, Warner T, Curzen N. Clopidogrel withdrawal: is there a “rebound” phenomenon? Thromb Haemost. 2011;105(2):211–220. doi:10.1160/TH10-08-0554
18. Grove EL, Würtz M, Schwarz P, Jørgensen NR, Vestergaard P. Gastrointestinal events with clopidogrel: a nationwide population-based cohort study. J Gen Intern Med. 2013;28(2):216–222. doi:10.1007/s11606-012-2208-0
19. Whittemore JC, Mooney AP, Price JM, Thomason J. Clinical, clinicopathologic, and gastrointestinal changes from administration of clopidogrel, prednisone, or combination in healthy dogs: a double-blind randomized trial. J Vet Intern Med. 2019;33(6):2618–2627. doi:10.1111/jvim.15630
20. Hallas J, Dall M, Andries A, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case–control study. BMJ. 2006;333(7571):726. doi:10.1136/bmj.38947.697558.AE
21. García Rodríguez LA, Lin KJ, Hernández-Díaz S, Johansson S. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation. 2011;123(10):1108–1115. doi:10.1161/CIRCULATIONAHA.110.973008
22. Lim JH, Kim SG, Choi J, Im JP, Kim JS, Jung HC. Risk factors of delayed ulcer healing after gastric endoscopic submucosal dissection. Surg Endosc. 2015;29(12):3666–3673. doi:10.1007/s00464-015-4123-z
23. Zhong HJ, Yuan Y, Xie WR, Chen MH, He XX. Type 2 diabetes mellitus is associated with more serious small intestinal mucosal injuries. PLoS One. 2016;11(9):e0162354. doi:10.1371/journal.pone.0162354
24. Harsch IA, Brzozowski T, Bazela K, et al. Impaired gastric ulcer healing in diabetic rats: role of heat shock protein, growth factors, prostaglandins and proinflammatory cytokines. Eur J Pharmacol. 2003;481(23):249–260. doi:10.1016/j.ejphar.2003.09.019
25. Naito Y, Takagi T, Oya-Ito T, et al. Impaired gastric ulcer healing in diabetic mice: role of methylglyoxal. J Physiol Pharmacol. 2009;60(Suppl 7):123–130.
26. Takagi T, Naito Y, Oya-Ito T, Yoshikawa T. The role of methylglyoxal-modified proteins in gastric ulcer healing. Curr Med Chem. 2012;19(1):137–144. doi:10.2174/092986712803413971
27. Tariq M, Khan HA, Elfaki I, et al. Gastric antisecretory and antiulcer effects of simvastatin in rats. J Gastroenterol Hepatol. 2007;22(12):2316–2323. doi:10.1111/j.1440-1746.2007.05021.x
28. Duan L, Bai YY, Li M, Li H, Li Y, Liu H. Increased risk of aspirin-induced gastric mucosal erosion in elderly Chinese men harboring SLCO1B1*1b/*1b while using aspirin and an ACEI or ARB concomitantly. BMC Med Genet. 2019;20(1):183. doi:10.1186/s12881-019-0918-4
29. Galeazzi R, Olivieri F, Spazzafumo L, et al. Clustering of ABCB1 and CYP2C19 genetic variants predicts risk of major bleeding and thrombotic events in elderly patients with acute coronary syndrome receiving dual antiplatelet therapy with aspirin and clopidogrel. Drugs Aging. 2018;35(7):649–656. doi:10.1007/s40266-018-0555-1
30. Yun UJ, Lee JH, Koo KH, et al. Lipid raft modulation by Rp1 reverses multidrug resistance via inactivating MDR-1 and Src inhibition. Biochem Pharmacol. 2013;85(10):1441–1453. doi:10.1016/j.bcp.2013.02.025
31. Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360(4):363–375. doi:10.1056/NEJMoa0808227
32. Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON–TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1319. doi:10.1016/S0140-6736(10)61273-1
33. Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376(9749):1320–1328. doi:10.1016/S0140-6736(10)61274-3
34. Zhang JH, Tang XF, Zhang Y, et al. Relationship between ABCB1 polymorphisms, thromboelastography and risk of bleeding events in clopidogrel-treated patients with ST-elevation myocardial infarction. Thromb Res. 2014;134(5):970–975. doi:10.1016/j.thromres.2014.08.017
35. Jaitner J, Morath T, Byrne RA, et al. No association of ABCB1 C3435T genotype with clopidogrel response or risk of stent thrombosis in patients undergoing coronary stenting. Circ Cardiovasc Interv. 2012;5(1):82–8, S12. doi:10.1161/CIRCINTERVENTIONS.111.965400
36. Wang D, Johnson AD, Papp AC, Kroetz DL, Sadée W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15(10):693–704. doi:10.1097/01.fpc.0000175600.26893.fa
37. Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–528. doi:10.1126/science.1135308
38. Luo JC, Huo TI, Hou MC, et al. Clopidogrel delays gastric ulcer healing in rats. Eur J Pharmacol. 2012;695(13):112–119. doi:10.1016/j.ejphar.2012.07.054
39. Luo JC, Peng YL, Chen TS, et al. Clopidogrel inhibits angiogenesis of gastric ulcer healing via downregulation of vascular endothelial growth factor receptor 2. J Formos Med Assoc. 2016;115(9):764–772. doi:10.1016/j.jfma.2015.07.022
40. Wu HL, Gao X, Jiang ZD, et al. Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 MAPK activation. Toxicology. 2013;304:41–48. doi:10.1016/j.tox.2012.11.020
41. Ng FH, Wong SY, Chang CM, et al. High incidence of clopidogrel-associated gastrointestinal bleeding in patients with previous peptic ulcer disease. Aliment Pharmacol Ther. 2003;18(4):443–449. doi:10.1046/j.1365-2036.2003.01693.x
42. Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352(3):238–244. doi:10.1056/NEJMoa042087
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
