Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Increased Mortality in HIV Infected Individuals with Tuberculosis: A Retrospective Cohort Study, Addis Ababa, Ethiopia
Authors Seyoum E , Demissie M , Worku A, Mulu A, Berhane Y , Abdissa A
Received 7 January 2022
Accepted for publication 13 March 2022
Published 25 March 2022 Volume 2022:14 Pages 143—154
DOI https://doi.org/10.2147/HIV.S354436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Eleni Seyoum,1 Meaza Demissie,2 Alemayehu Worku,3 Andargachew Mulu,4 Yemane Berhane,5,* Alemseged Abdissa4,*
1Institute of Public Health, University of Gondar, Gondar, Ethiopia & Epidemiology and Evaluation Department, Addis Continental Institute of Public Health, Addis Ababa, Ethiopia; 2Public Health Department, Addis Continental Institute of Public Health, Addis Ababa, Ethiopia; 3Department of Preventive Medicine, School of Public Health, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 4Bacterial and Viral Diseases Research Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia; 5Epidemiology and Evaluation Department, Addis Continental Institute of Public Health, Addis Ababa, Ethiopia
*These authors contributed equally to this work
Correspondence: Eleni Seyoum, Institute of Public Health, University of Gondar, Gondar, Ethiopia & Epidemiology and Evaluation Department, Addis Continental Institute of Public Health, P.O.Box 10433, Addis Ababa, Ethiopia, Tel +251-091 160 9275, Email [email protected]
Background: Tuberculosis is one of the commonest coinfections and leading causes of death among people living with HIV in resource-limited countries. There is limited evidence on the short- and long-term mortality rate in people receiving antiretroviral therapy and coinfected by tuberculosis in sub-Saharan Africa, where the burden of coinfection is highest.
Purpose: This study aimed to compare mortality among HIV positives with and without tuberculosis coinfection receiving antiretroviral therapy in Addis Ababa, Ethiopia.
Methods: HIV positives’ medical records were reviewed between 2011 to 2018 and identified 7038 HIV-positive adults enrolled for antiretroviral therapy in Addis Ababa. The outcome of interest for this study was death. A parametric Gompertz regression model was applied to compare mortality between HIV with tuberculosis coinfection versus HIV without tuberculosis.
Results: Overall, 1123 (15.96%, 95% CI: 15.11– 16.83%) individuals with HIV had tuberculosis coinfection at antiretroviral therapy enrollment. After adjusting for age, sex, education, marital status, cotrimoxazole therapy, body mass index, baseline CD4 cell count, and year in ART enrollment, HIV positives with tuberculosis coinfection had more than twice a higher overall mortality risk than HIV positives without tuberculosis coinfection (AHR: 2.53; 95% CI 1.63– 3.91, p < 0.001).
Conclusion: This large retrospective cohort study reveals significantly higher mortality in HIV and tuberculosis coinfected group. This suggests the need for enhanced utility of integrated HIV and tuberculosis health services in sub-Saharan Africa where tuberculosis prevalence is highest.
Keywords: antiretroviral therapy, HIV, coinfections, death
Background
Tuberculosis (TB) is a preventable and treatable disease. Yet, it continues to claim millions of lives each year. In 2020, an estimated 10 million people (127/100,000) were ill of TB, and 1.3 million TB deaths occurred among HIV-negative people.1 About 8% of all incident cases of TB worldwide occur among people living with HIV (PLHIV), where Africa stands the highest.1 TB is the leading cause of death in PLHIV, with an estimated 210,000 TB-related deaths in 2019.2 Ethiopia ranks among the high burden TB and TB+HIV coinfection with an estimated TB incidence of 132 (92–178) per 100,100, and HIV+TB incidence of 8.6 (6–12) per 100,000, and 2.2 (1.3–3.2) per 100,000 HIV associated TB deaths in 2020.3 According to the national SPECTRUM estimation the overall AIDS related death in Addis Ababa was 39.89 (29.61–53.69) per 100,000 in 2020.4
TB and HIV have synergetic effects, where TB increases HIV replication and accelerates HIV progression.5 On the other hand, HIV lowers the immunity against TB leading, to increased occurrence of active TB infection, reinfection, or reactivation. HIV also increases the risk of recurrent TB due to the increased risk of reinfection.6,7 TB co-infected HIV positives maintain high viral load compared with HIV positives without TB coinfection even after curing of TB.8,9 Some of the associated risk factors that lead to the development of TB in PLHIV are compromised immunity (CD4<200 cell count at enrollment), poor nutritional status, and lower body mass index (BMI), and bedridden conditions.10–12 In response to the burden of these two intertwined infectious diseases, the World Health Organization (WHO) has been promoting integrated TB and HIV interventions including; HIV testing, TB screening, isoniazid preventive therapy (IPT) initiation, viral load testing, and antiretroviral therapy (ART).13 The three 95ʹs target set by UNAIDS also stipulates; 95% of PLHIV, including those coinfected with HIV+TB, to know their HIV status, to put 95% of those who know their status on ART and to achieve 95% viral suppression (viral load below 1000 copies) among those on ART.14 WHO identified 30 priority TB and TB+HIV burden countries including Ethiopia, where sub-Saharan Africa and Asia comprised 88% of the global HIV+TB associated burden.1
Ethiopia is progressing towards HIV epidemic control to end AIDS by 2030. As part of this strategic move, the country committed to achieving the new global 95-95-95 target of UNAIDS by 2025.15 However, there is limited recent evidence of mortality among HIV positives receiving antiretroviral therapy with TB coinfection despite the high burden of HIV-associated TB in Ethiopia. Most previous studies on HIV+TB coinfection mortality were conducted before ART policy changes of “Test and Treat” and the scale-up of TB+HIV integrated services.10,16 Therefore, this retrospective cohort study aimed to compare mortality among coinfected (HIV positives with TB) and HIV positives without TB coinfection in a retrospective cohort study in Addis Ababa, Ethiopia.
Methods
Study Design and Population
A retrospective medical review of adults 15 years or older enrolled in ART care was conducted between 2011 to 2018 in 28 health facilities (11 hospitals and 17 health centers) in Addis Ababa. HIV positives with documented TB test results, regardless of whether the TB results were positives or negatives were included. Among HIV+TB test results, 553 cases (49.2%) were diagnosed clinically, 373 cases (33.3%) with Sputum Microscopy, and 197 cases (17.5%) were by Genexpert.
Addis Ababa, the capital city, constitutes one-fourth of the burden of PLHIV in Ethiopia. In 2020, 105,000 PLHIV received ART in 123 (22 hospitals and 101 health centers).4 Comprehensive HIV care, treatment, and prevention services including, HIV+TB integrated services, are provided following the national guidelines adopted from the WHO-2016 global guideline.17
The national guideline recommends immediate initiation of anti-TB treatment and co-trimoxazole preventive therapy (CPT), followed by ART within the first eight weeks of treatment. HIV services provided at TB clinics includes, counseling, HIV testing, education on HIV prevention, and CPT. The preferred first-line regimen for HIV+TB coinfected people was TDF + 3TC + DTG (Dolutegravir) or TDF + 3TC + EFV (FDC, Fixed Dose Combination). WHO recommends the dose of DTG to be increased by 50mg BID for HIV+TB coinfected people to reduce viral replication.18
In the study facilities, in accordance to the national guidelines, TB was clinically diagnosed among HIV positive person if an individual presents with symptoms or signs consistent with TB including cough (any duration in HIV positive), fever for more than 2 weeks, drenching night sweats, and unexplained weight loss (more than 1.5 kg in a month). Additionally, further laboratory test could be done as appropriate and available. The diagnostic tests include acid-fast bacilli (AFB) microscopy, GeneXpert, chest radiographs and fine needle aspirate.
Sample Size and Procedure
The sample size was determined using STATA11 software. The sample size for the exponential test was calculated using inputs from the previous study: survival rate for HIV without TB coinfection of survival rate of p1 (0.972, survival rate of p2 (0.83) HIV with TB coinfection, survival ratio (1.10),19 alpha of 5%, and 80% power were used. The proportion of exposed group (HIV coinfected with TB) from the total sample increased from 9% in the previous study to 20% in our study to increase the representation of coinfection in the total sample. Then, a minimum estimated sample size of 6076 (5063 HIV without TB coinfection, nonexposed and 1013 HIV with TB coinfection) was calculated and accounted for 15% missing data (missing data, n=911), which brings the total sample (n=6987) to meet the objective of the study. The required sample size was proportionally allocated to each hospital and health center based on their client load in previous months. Then, using the ART register as a sampling frame HIV positives were sampled after stratifying the medical record by year of entry with the country policy of ART initiation: Strata 1: January 2011-December 2013 (ART treatment initiated if CD4 count was below 200), Strata 2: January 2014- December 2014 (ART treatment initiated if CD4 count was below 350), Strata 3: January 2015- December 2016 (ART treatment initiated if CD4 count below <500), and Strata 4: January 2017-December, 2018 (test and treat policy implemented). The sample was allocated proportionally to each stratum. An electronic medical record was used to generate the random sample for each stratum. Finally, a total of 7192 samples from Addis Ababa health facilities were retrieved.
Data Collection Procedure
Data abstraction sheet prepared to extract relevant individual-level information from the medical records. Extracted data includes a) sociodemographic information: age, sex, marital status, education b) HIV care information at enrollment including date of confirmed HIV positivity, ART initiation date, and date outcomes occurred; c) HIV care at baseline and follow-up, including CD4 cells/mL at baseline, at six months, and 12 months; WHO clinical staging; functional status; hemoglobin; TB status at (baseline and during the follow-up period); and d) outcome variable of death. Six data abstractors with a qualification of MPH, BSc. Nurse, data managers, and a supervisor (MPH) were trained for a week by the principal investigator. The training topic includes the objective of the study, ART program procedures, how to abstract data ethically from medical records, the data abstraction sheet, and the use of tablets for data capturing and uploading to the Research Electronic Data Capture (REDCap) server. The data abstraction sheet was pretested in two centers and minor modifications were made. Data abstraction was done using a preloaded data form on a tablet. Each HIV positives folder was accessed to abstract the relevant information mentioned above. The study nurses abstracted the clinical and laboratory data from the records. Extracted data were uploaded daily to the REDCap server after a quality check by the supervisor.
Data Analysis
Clinical variables were compared using the chi-square test for categorical variables and the Kruskal–Wallis or Wilcoxon test for nonnormally distributed continuous variables, as appropriate. Statistical significance was set at p <0.05. The cumulative TB incidence rate was calculated per 1000 populations at risk. The population at risk includes only those HIV positives who were free of TB at ART initiation (HIV+Only).
The event of interest for this paper was death. Mortality is a death reported in the ART register and HIV positives’ follow-up cards at health facilities. In this study, TB is defined as those with TB disease. Death from TB among HIV-positive people is officially classified as deaths caused by HIV/AIDS in the International Classification of Diseases.20 Mortality was compared between HIV with TB coinfection (coinfected group) and HIV without TB coinfection (HIV+Only group). Date ART initiated and the last day of the study, transferred out, loss to follow-up, and stopped treatment were used as a right-censor to calculate the time to death. Mortality rates were calculated per 100,000 person-time-months by coinfection status.
A parametric regression model was used to adjust for potential confounders. The parametric regression model is less biased than the cox proportional model when the distribution of the parameter is known.21
Although the hazard ratio of the different models is approximately similar, the Akaike’s information criterion (AIC) values reported for each model showed that parametric models had a better fit and were more powerful than Cox’s regression model.22,23 AIC and the Log-likelihood test were used for regression model selection. The Gompertz parametric regression model with the lowest AIC and highest Log-likelihood value was selected in the final analysis (Supplementary Table 1). The Global test was used to check for Hazard proportionality for key covariates included in the analysis and obtained a Global test of Prob>chi2=0.52.
The hazard ratio was adjusted for selected covariates: TB (coinfection versus without coinfection), sex, age at ART initiation (15–29, 30–44, 45+), education (no education, primary, secondary, and tertiary), marital status, cotrimoxazole (taken versus not taken), BMI (<18, 18–25, >25), baseline CD4 cell count (<200 versus ≥200), and year of Strata (2011–2013, 2014, 2015–2016, 2016–2018). To account for the clustering effect type of health facility (hospital versus health center) was applied using the shared frailty model. To consider the influence of the potential covariates that might exist and the assumption of homogeneity realistic, we introduced a random variable that accounts for the neglected covariates (hidden heterogeneity or frailty covariates designated by theta) (Supplementary Table 2).
Multicollinearity was checked using Variance Inflation Factor (VIF) after stepwise linear regression and there was a strong correlation between TB and WHO staging and functional status. We excluded functional status and WHO staging from covariates because of the strong correlation with TB. Sensitivity analysis was carried out by excluding HIV positives with CD4 cell count<50 where HIV+only group (n=511) and coinfected (n=263) observations were excluded from the analysis (Supplementary Table 3).
Handling of Missing Data
At the analysis stage, the main exposure variable (TB documented status) had 2.14% missing information and was excluded from the analysis based on the principle of complete case analysis. However, a background characteristics check was made between the excluded variables versus those included for potential bias presence and did not find significant important differences. Of the total records analyzed (N= 7038) on average 98% of selected covariate and outcome variables were completed (Supplementary Table 4). We assumed the missing rate of 3% or less for the most important covariates is inconsequential. Viral load and hemoglobin data were excluded from covariate because (>65%) of the samples have missing values. The study was approved by the Institutional Review Board of the University of Gondar and Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia and we affirm that all methods were performed in accordance with the /relevant guidelines.
Result
Sociodemographic Characteristics
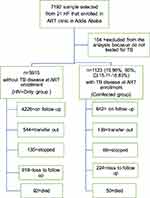
A total of 7192 adults 15 years and over age were included in the study. Of which, 154 (2.14%) were not tested for TB and excluded from the analysis. Of the 7038 eligible adults, 1123 (15.96%, with 95%, CI:15.11–16.83%)) had HIV and TB coinfection at ART enrollment (Figure 1). The median age of the study population was 35 years, and 59.46% were female. The proportion of males 587 (52.46%, p=0.001) and never married 357 (33.40%, p<0.0001) was higher in the coinfected group. The median follow-up time for the coinfected group was 3.3 years and 4.5 for HIV+Only group (Table 1).
 |
Table 1 Baseline Characteristics of HIV Positives Individuals Enrolled for ART Care Between 2011 and 2018 in Addis Ababa, Stratified by Tuberculosis Coinfection Status, n=7038 |
Clinical Characteristics at ART Enrollment
HIV positives in the coinfected group had a lower median CD4 count of 96 (IQR, 49.5–183) at enrollment compared to the median CD4 cell count of 199 (IQR, 109–310) in the HIV+Only group (p<0.05). Similarly, most (91.24%) of the coinfected group were in WHO III or IV clinical stages, at ART initiation versus 30.26% in the HIV+Only group (p=0.001). Regarding cotrimoxazole, 89.35% of the coinfected group and 73.68% of HIV+Only group received cotrimoxazole at enrollment. The proportion of HIV positives who switched drug from First-line regimen to Second-line regimen at 6, 12, and 60 months in coinfected group were 0.87% (n=9), 0.85% (n=7), 2.37% (n=9), respectively, versus 0.66% (n=38), 9=0.87% (n=44), and 2.73 (n=69) respectively in the HIV+Only group (Table 2).
 |
Table 2 Baseline Clinical and Health Factors of HIV Positives Initiating ART by Coinfection Status in Addis Ababa, Ethiopia (September 2011 to December 2018), n=7038 |
TB Incidence Rate
The overall TB incidence density was 2.65 per 1000 person-time-months (95% CI: 2.50–2.83). TB incidence density was 3.67 per 1000 person-time-months (95%: CI 3.41–3.95) in immune-compromised HIV positives with CD4 <200 compare to 1.37 per 1000 person -time-months (95%: CI 1.20–1.57) with HIV positives who had initiated CD4 cell count ≥200. Similarly, the overall TB incidence density was higher in male sex 3.51 per 1000 person-time-months (95% CI:3.22–3.83) compare to 2.10 per 1000 person-time-months (95% CI: 1.92–2.30) in female sexes (data not shown). The equality Log rank test shows that overall TB incidence was significantly high in immune-suppressed HIV positives with CD4 <200 and male HIV positives with chi2 (3)=182.97 and Pr>chi2=0.001 (Figure 2).
The Proportion of Death by Coinfection Status and Sex Distribution
During the entire follow-up time a total of 142 death occurred. The proportion of death at the 6, 12, and 60-month follow-up in coinfected group were 2.85% [95% CI:2.0–4.0], 3.65% [95% CI:2.69–4.92], and 4.39% [95% CI: 3.33–5.73] respectively, versus 0.71% [95% CI: 0.52–0.95%], 0.89%[95% CI: 0.68–1.17], and 1.38%[95% CI:1.12–1.72] respectively in HIV+Only group, respectively. Proportion of death at six months was higher (3.24%, 95% CI:2.07–5.03%) in the male coinfected subgroup than in female coinfected subgroup, (2.45%, 95% CI:1.4–4.1%, p-value=0.7036) (Supplementary Table 5).
Short and Long-Term Mortality Rates per 100,000 by Coinfection Status
The overall mortality rate in HIV-positive people enrolled on ART was 38.96 (95% CI: 33.0,45.9) per 100,000 person-time-months. Mortality was higher 99.89 (95% CI: 75.7–131.7) per 100,000 person-time-months in the coinfected group compared with 29.26 (95% CI: 23.8, 35.9) per 100,000, p= 0.0001) in HIV+Only group. In the coinfected group, 60% of death occurred in the first three months compared to 39% in the HIV+Only group. In both coinfected and the HIV+Only groups, mortality shows a declining trend as the follow-up period increases (Table 3).
 |
Table 3 Mortality per 100,000 Person-Time-Months in Adult’s HIV Positives Enrolled in ART Care by Coinfection Status in Addis Ababa, Between September 2011 to December 2018, n=7038 |
Adjusted Hazard Ratios of Mortality
The adjusted hazard ratio showed that PLHIV who had coinfection with TB at enrollment were more likely to die than those with HIV+Only group. HIV positives with TB coinfection had more than twice a higher overall mortality risk than HIV positives without TB coinfection (AHR: 2.53; 95% CI:1.63–3.91, p<0.001). Mortality risk at 6, 12, and 60 months after ART initiation, were higher in the coinfected group with an adjusted hazard ratio (AHR) of 3.03 (95% CI: 1.58–5.80 p<0.001), 3.20 (95% CI:1.83–5.61 p<0.001), and 2.83 (95% CI: 1.79–4.46, p<0.001) respectively (Table 4).
 |
Table 4 Adjusted and Unadjusted Hazard Ratio of Outcomes in Coinfection with Use of Parametric Gompertz Regression Model, in Addis Ababa, Ethiopia, (September 2011 to December 2018) |
Discussion
We compared the mortality of HIV-infected individuals with and without the TB using a retrospective cohort study in Addis Ababa, Ethiopia. Our finding revealed a significantly higher 99.89[75.7–131.7] per 100,000 overall mortality in HIV+TB coinfected group compared to 29.26[23.8–35.9] per 100,000 in HIV positives without TB group. Mortality was three times higher in the coinfected group in the first year of the follow-up period at 6 and 12 months.
Early mortality in the coinfected group may be due to delayed TB diagnoses in HIV positives, nearly 50% of the TB cases were diagnosed clinically after developing signs and symptoms. Early ART initiation significantly reduces all-cause mortality in HIV-positive with TB.24 In this study, three fourth of the coinfected HIV positives-initiated ART with CD4 less than 200 cell count and one-fourth of the TB coinfection had CD4 cell count less than 50 at enrollment, 75% of the coinfected group were at an advanced stage of AIDS as opposed to 30% in those without TB coinfection, and 91% of them were at clinical stage (III or IV). WHO defines advanced stage AIDS disease when CD4 cell count is below 200 cell counts.18 The fact that 31% of HIV positives in the coinfected group were bedridden at enrollment in our study may explain the higher early mortality in coinfected HIV positives. Being bedridden at enrollment is a risk factor for higher mortality in TB+HIV people on ART.25 In this study a significant proportion of HIV+TB people were underweight (36% <18 BMI). Poor nutritional status and lower BMI is risk factors for mortality in HIV positives.26 The overall high mortality rate in the coinfected group may be due to TB treatment, as concurrent TB treatment is associated with virologic failure.27 WHO recommended the increase of dose in Rifampicin (RF) by 50 with Dolutegravir (DTG) in order to reduce the viral load in HIV+TB coinfected people after TB treatments.13 The appropriate implementation of the WHO recommendation is warranted to reduce virological failure in HIV+TB coinfected people in the long-term ART provision in TB+HIV integrated services.
The high early mortality rate observed at 6 and 12 months in the coinfected group in our study is consistent with other studies in Ethiopia and elsewhere.7,16,28 Our finding of the overall mortality rate for the HIV+TB coinfected group was higher than the WHO global estimates of mortality rate, 2.2 (1.3–3.2) per 100,000 in HIV+TB coinfection for Ethiopia.3 Ethiopia targeted to reduce AIDS-related mortality to 6 per 100,000 at the national level and 24 per 100,000 for Addis Ababa by the end of 2025.4 As more than half of the deaths in this study are contributed by HIV+TB coinfected group and among advanced stage of AIDS diseases in HIV positives without TB group, a slow pace of reduction in mortality in the coinfected group could negatively affect the HIV mortality targets of Ethiopia in general and Addis Ababa in particular.
A similar finding was reported from a large national study in Tanzania, where 40% higher overall mortality was found in coinfected individuals,28 and also in Myanmar, India, and England.7,19,25 We found a lower overall mortality rate in HIV+TB coinfection compared with a previous study in Addis Ababa conducted in 2012. Some of the explanations for the difference can be the timing of the study and improved ART interventions. Death among HIV+TB has reduced since 2010.1 The scale-up of IPT has increased survival among HIV+TB.29 Ethiopia has adopted and scaled up the use of IPT as part of HIV+TB integrated services.18 The GenExpert TB diagnosis technology has increased early diagnosis of TB in HIV positives and increased survival in HIV+TB coinfection.30 Ethiopia adopted and scaled up GenExpert diagnosis technology since 2014.31
To our best knowledge, this study is among the few studies that compared mortality between HIV+TB coinfection and HIV without TB coinfection groups in large sample sizes, multicenter sites, account for CD4 initiation policy changes, and accounting for setting heterogeneity using the shared frailty model. We believe that our study findings provide recent evidence on TB mortality rates on ART between HIV+TB coinfection and without TB coinfection groups for Addis Ababa.
Strengthen and Limitation
To mention a few limitations of the study, there might be a differential misclassification bias because of missing diagnosis of TB among HIV positives with advanced stage of diseases. The differential misclassification may affect the result in the under-estimation of mortality in coinfected groups. The misclassification bias does not alter the direction of our finding. However, the magnitude of mortality in the coinfected group may be slightly greater than the one estimated in this study. This highlights the importance of early TB diagnosis using the Lipoarabinomannan (LAM) urine test in advanced stage AIDS diseases at the health facilities as recommended by WHO. Urine LAM testing is beneficial among HIV positives with functional impairment, elevated inflammatory markers, or greater immunosuppression.32
Though the interpretation of this finding needs to consider the above-mentioned limitation, the mortality rate obtained from this study can reasonably be used to estimate Addis Ababa mortality rate at a population level for both HIV+TB coinfection and without TB coinfection by adjusting for the underdiagnosis of TB among advanced stage of AIDS in the HIV+Only group.
Conclusions
This large retrospective cohort study reveals significantly higher mortality in HIV and TB coinfected groups. This suggests the need for the enhanced utility of integrated HIV and TB health services in sub-Saharan Africa where TB prevalence is highest.
Abbreviation
ART, Antiretroviral Therapy; PLHIV, People Living with HIV; WHO, World Health Organization; IQR, Inter-quartile Range; CI, Confidence Interval; TB, tuberculosis.
Data Sharing Statement
The corresponding author will share the data set used in this study upon reasonable request.
Ethical Consideration
This study was approved by the Institutional Review Board of the University of Gondar in Ethiopia and the Armauer Hansen Research Institute, Addis Ababa, Ethiopia. No consent was required from participants. No external persons accessed the HIV positives folder and no HIV positives identifier was included in our data set. The HIV positives folder that was taken from the health information system room for data abstraction was kept in a locked file cabinet until it was returned to the archive room to maintain participant’s confidentiality. We used routinely collected patient data that was set up for program monitoring and evaluation purposes, and there was no direct contact with patients. As this was a retrospective study, the Institutional Review Board University of Gondar waived the need for informed consent. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgments
We express our gratitude to the data collectors and supervisors at the 28 health facilities in Addis Ababa. The special thank also goes to the Federal HIV/AIDS Prevention and Control Program for covering the cost of data collection through AHRI. Finally, we express our thanks to the Addis Continental Institute of Public Health and the University of Gondar for giving us the opportunity.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We would like to thank the financial support from the Federal HIV Prevention and Control Office through AHRI for covering the cost of the data collection at the twenty-eight health facilities in Addis Ababa. The funding agencies had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. The authors had full access to all the data in the study and takes the responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2021. [Internet]. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.
2. UNAIDS. Global AIDS Update | 2021. Confronting Inequalities: lessons for pandemic responses from 40 years of AIDS [Internet]. 20 Avenue Appia 1211 Geneva 27 Switzerland: UNAIDS Joint United Nations Programme on HIV/AIDS; 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf.
3. World Health Organization. Tuberculosis profile: Ethiopia [Internet]; 2020. Available from: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22ET%22.
4. HIV_estimation_and_projection_for_Ethiopiawith-uncertainity_2019-PR.pdf [Internet]. [
5. Toossi Z, Johnson JL, Kanost RA, et al. Increased replication of HIV-1 at sites ofMycobacterium tuberculosisInfection: potential mechanisms of viral activation. JAIDS J Acquir Immune Defic Syndr. 2001;28(1):1–8. doi:10.1097/00042560-200109010-00001
6. Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet Lond Engl. 2001;358(9294):1687–1693. doi:10.1016/S0140-6736(01)06712-5
7. Narayanan S, Swaminathan S, Supply P, et al. Impact of HIV infection on the recurrence of tuberculosis in South India. J Infect Dis. 2010;201(5):691–703. doi:10.1086/650528
8. Xun J, Qi T, Zou L, et al. Mycobacterium tuberculosis co-infection is associated with increased surrogate marker of the HIV reservoir. AIDS Res Ther. 2020;17(1):63. doi:10.1186/s12981-020-00320-0
9. Day JH, Grant AD, Fielding KL, et al. Does tuberculosis increase HIV load? J Infect Dis. 2004;190(9):1677–1684. doi:10.1086/424851
10. Biset Ayalew M. Mortality and its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. AIDS Res Treat. 2017;30(2017):e5415298.
11. Azanaw MM, Derseh NM, Yetemegn GS, Angaw DA. Incidence and predictors of tuberculosis among HIV patients after initiation of antiretroviral treatment in Ethiopia: a systematic review and meta-analysis | tropical Medicine and Health | full Text [Internet]. [
12. Gesesew H, Tsehaineh B, Massa D, Tesfay A, Kahsay H, Mwanri L. The role of social determinants on tuberculosis/HIV co-infection mortality in southwest Ethiopia: a retrospective cohort study | BMC research notes | full Text [Internet]. [
13. World Health Organization. Consolidated guidelines on tuberculosis. Module 2: screening – systematic screening for tuberculosis disease. [Internet]. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://apps.who.int/iris/bitstream/handle/10665/340255/9789240022676-eng.pdf.
14. UNAIDS. 2025 AIDS target: ending the AIDS epidemic by 2030. United Nation Joint Program for AIDS.
15. Federal HIV/AIDS Prevention and Control Office. HIV/AIDS national strategic plan for Ethiopia: 2021–2025; 2020.
16. Jerene D, Abebe W, Taye K, et al. Tuberculosis along the continuum of HIV care in a cohort of adolescents living with HIV in Ethiopia. Int J Tuberc Lung Dis. 2017;21(1):32–37. doi:10.5588/ijtld.16.0105
17. National comprehensive HIV care guideline 2018.pdf [Internet]: [cited October 25, 2021]. Available from https://www.afro.who.int/sites/default/files/2019-04/National%20Comprehensive%20HIV%20Care%20%20Guideline%202018.pdf. Accessed March 3, 2022
18. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring recommendations for a public health approach [Internet], [cited 2021 March 3]. Available from: https://www.who.int/publications-detail--redirect/978924003.
19. Zenner D, Abubakar I, Conti S, et al. Impact of TB on the survival of people living with HIV infection in England, Wales and Northern Ireland. Thorax. 2015;70(6):566–573. doi:10.1136/thoraxjnl-2014-206452
20. ICD-10 version:2019 [Internet]. [cited October 30, 2021]. Available from: https://icd.who.int/browse10/2019/en#/A15-A19.
21. Comparison of cox regression and parametric models: application for assessment of survival of pediatric cases of acute Leukemia in Southern Iran [Internet]. [
22. Nardi A, Schemper M. Comparing Cox and parametric models in clinical studies. Stat Med. 2003;22(23):3597–3610. doi:10.1002/sim.1592
23. Teshnizi SH, Ayatollahi SMT. Comparison of cox regression and parametric models: application for assessment of survival of pediatric cases of acute Leukemia in Southern Iran. Asian Pac J CancerPrev. 2017;18–5(4):981.
24. Yan S, Chen L, Wu W, et al. Early versus delayed antiretroviral therapy for HIV and tuberculosis co-infected patients: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2015;10(5):e0127645. doi:10.1371/journal.pone.0127645
25. Aung ZZ, Saw YM, Saw TN, et al. Survival rate and mortality risk factors among TB–HIV co-infected patients at an HIV-specialist hospital in Myanmar: a 12-year retrospective follow-up study. Int J Infect Dis. 2019;80:10–15. doi:10.1016/j.ijid.2018.12.008
26. Naidoo K, Yende-Zuma N, Augustine S. A retrospective cohort study of body mass index and survival in HIV infected patients with and without TB co-infection. Infect Dis Poverty. 2018;7(1):35. doi:10.1186/s40249-018-0418-3
27. Braun A, Sekaggya-Wiltshire C, Scherrer A, et al. Early virological failure and HIV drug resistance in Ugandan adults co-infected with tuberculosis. AIDS Res Ther. 2017;14:453.
28. Mollel EW, Todd J, Mahande MJ, Msuya SE. Effect of tuberculosis infection on mortality of HIV-infected patients in Northern Tanzania. Trop Med Health. 2020;48(1):26. doi:10.1186/s41182-020-00212-z
29. Geremew D, Endalamaw A, Negash M, Eshetie S, Tessema B. The protective effect of isoniazid preventive therapy on tuberculosis incidence among HIV positive patients receiving ART in Ethiopian settings: a meta-analysis. BMC Infect Dis. 2019;19(1):405. doi:10.1186/s12879-019-4031-2
30. Sorsa A, Kaso M, Quinn F. Diagnostic performance of GeneXpert in tuberculosis–HIV co–infected patients at Asella Teaching and Referral Hospital, Southeastern Ethiopia: a cross sectional study. PLoS One. 2021;16(1):e0242205. doi:10.1371/journal.pone.0242205
31. Ministry of Health/Ethiopian Public Health Institute. Implementation Guideline for GeneXpert MTB/RIF Assay in Ethiopia, June, 2014 Addis Ababa. Available from: https://www.ephi.gov.et/publications/guideline
32. Drain PK, Gounder L, Sahid F, Moosa M-YS. Rapid urine LAM testing improves diagnosis of expectorated smear-negative pulmonary Tuberculosis in an HIV-endemic region. Sci Rep. 2016;11(6):19992. doi:10.1038/srep19992
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.