Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Incorporation of the 40-Gene Expression Profile (40-GEP) Test to Improve Treatment Decisions in High-Risk Cutaneous Squamous Cell Carcinoma (cSCC) Patients: Case Series and Algorithm
Authors Singh G, Tolkachjov SN , Farberg AS
Received 19 January 2023
Accepted for publication 25 March 2023
Published 5 April 2023 Volume 2023:16 Pages 925—935
DOI https://doi.org/10.2147/CCID.S403330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Gaurav Singh,1 Stanislav N Tolkachjov,2– 5 Aaron S Farberg5– 7
1Gaurav Singh MD, Milwaukee, WI, USA; 2Epiphany Dermatology, Dallas, TX, USA; 3University of Texas at Southwestern, Dallas, TX, USA; 4Baylor University Medical Center, Dallas, TX, USA; 5Texas A&M College of Medicine, Dallas, TX, USA; 6Bare Dermatology, Dallas, TX, USA; 7Baylor Scott & White Health System, Dallas, TX, USA
Correspondence: Aaron S Farberg, Bare Dermatology, 2110 Research Row, Dallas, TX, 75235, USA, Tel +1 847-721-2725, Email [email protected]
Abstract: Cutaneous squamous cell carcinoma (cSCC) has become a significant public health issue due to its rapidly rising incidence and an estimated 1.8 million newly diagnosed cases annually. As with other cancers, treatment decisions for patients with cSCC are based primarily on a patient’s risk for poor outcomes. There has been improvement in clinicopathologic factor-based risk assessment approaches, either through informal methods or ever evolving staging approaches. However, these approaches misidentify patients who will eventually have disease progression as low-risk and conversely, over classify patients as high-risk who do not experience relapse. To improve the accuracy of risk assessment for patients with cSCC, the 40-gene expression profile (40-GEP) test has been validated to show statistically significant stratification of a high-risk cSCC patient’s risk of nodal or distant metastasis, independent of currently available risk-assessment methods. The 40-GEP test allows for a more accurate classification of metastatic risk for high-risk cSCC patients, with the aim to influence appropriate allocation of clinician time and therapeutic resources to those patients who will most benefit. The objective of this article is to present a treatment algorithm in which clinicians can easily integrate the results of the 40-GEP test into their current treatment approaches to tailor patient care based on individual tumor biology. The following modalities were considered: surveillance imaging, sentinel lymph node biopsy (SLNB), adjuvant radiation therapy (ART), and clinical follow-up. The authors have contributed their own cases for discussion as to how they have seen the beneficial impact of 40-GEP test results in their own practice. Overall, clinicians can identify risk-aligned treatment pathway improvements with the use of the 40-GEP test for challenging to manage, high-risk cSCC patients.
Keywords: 40-gene expression profile, 40-GEP, cutaneous squamous cell carcinoma, cSCC, metastasis, patient management, clinician algorithm, prognostic, risk-stratification
Plain Language Summary
- There are 1.8 million annually diagnosed cSCC cases annually and while more than 95% are cured by surgery, an average of 5% progress to metastasis, with up to 2.1% dying from the disease.
- Broad guidelines and limitations of risk-stratification tools result in disparities in clinical practice and management, creating a diversity of patient outcomes among high-risk cSCC patients.
- The 40-GEP test was developed and validated to determine a personalized risk of nodal/distant metastasis for high-risk cSCC patients, with the intention of the test result to be used in combination with clinicopathologic factors and/or staging systems to guide risk-appropriate clinical decisions.
- With the objective to provide guidance to clinicians considering use of the 40-GEP the authors have merged their risk-aligned management approaches for three real-world, high-risk cSCC patients into a singular algorithm focused on how to incorporate the results of the 40-GEP test into common treatment modalities.
- This data provides a framework for stratifying patients with advanced cSCC by identifying their unique biological profile with the use of the 40-GEP and then treating them in a risk-aligned manner to enhance the prognostic capability of current cSCC risk assessment methods to guide decisions within current guidelines.
Introduction
Nonmelanoma skin cancer (NMSC) is the most common malignancy in the United States with predictions of a 20-fold increase in incidence by 2044.1 Basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) comprise 99% of NMSCs.2 Several studies have recently recognized a significant increase from the historically accepted 4:1 BCC:cSCC ratio for the general population to closer to 2:1, with an even higher estimated 1:1 ratio in the Medicare population.3,4 More than 95% of the 1.8 million annually diagnosed cSCC cases are cured by surgery; however, an average of 5% progress to metastasis, with up to 2.1% dying from the disease.3,5,6 Thus, while treatment decisions for the bulk of cSCC patients are straightforward, there are a substantial number of patients who will die from this cancer. A reduction of poor outcomes and optimization of the healthcare costs incurred in treating high-risk patients can be achieved with early detection, along with risk-aligned, personalized treatment plans. Unfortunately, broad guidelines and limitations of risk-stratification tools to encompass the heterogeneous cSCC population have resulted in disparities in clinical practice and management7 and thus a diversity of patient outcomes among high-risk cSCC patients.8
Given the unprecedented increase in the incidence of cSCC and broad guidelines for the treatment of high-risk patients, the 40-gene expression profile (40-GEP; DecisionDx-SCC, Castle Biosciences Inc.) test was developed and validated to determine a personalized risk of metastasis (nodal or distant), with the intention of the test result to be used in combination with clinicopathologic factors and/or staging systems to guide risk-appropriate clinical decisions. The test utilizes a neural network algorithm to classify patients into three molecular risk profiles: Class 1 as low, Class 2A as moderate, and Class 2B as at high risk of regional or distant metastasis three years post diagnosis.9 The statistically significant stratification of these risk groups was further evaluated in a clinical validation cohort demonstrating metastasis rates of 6.6%, 20.0% and 52.2% for Class 1, Class 2A and Class 2B, respectively, along with the 40-GEP providing independent and additive prognostic value when compared with current clinicopathologic factor-based risk assessment.10 Several clinical impact studies have shown that 40-GEP test results can be incorporated with national guidelines to accurately stratify high-risk cSCC patients and produce appropriate risk-aligned treatment plans.11–14 As demonstrated by publications assessing clinician evaluation of high-risk cSCC real-world cases and patient vignettes, each 40-GEP result can provide additional and actionable information for escalation or de-escalation of cSCC treatment interventions when compared to pre-40-GEP treatment approaches.12,15,16 With the objective to provide guidance to clinicians considering use of the 40-GEP, the authors have merged their risk-aligned management approaches for high-risk cSCC patients into a singular algorithm focused on how to incorporate the results of the 40-GEP test into the following treatment modalities: surveillance imaging, sentinel lymph node biopsy (SLNB), adjuvant radiation therapy (ART), and clinical follow-up.
Testing Criteria for Use of the 40-GEP
Prognostic testing through the 40-GEP is warranted for those cSCC patients whom the clinician is uncertain about their metastatic risk and would like additional prognostic information to guide clinical decisions. Specifically, 40-GEP testing is indicated for patients with diagnosed primary cSCC having one or more high-risk factors (Table 1). Based on the work by Hooper et al,16 clinicians are appropriately using the 40-GEP to guide risk-aligned management, as demonstrated by their responses to pre- and post-40-GEP assessment of real-world cases of high-risk cSCC. They are also testing the appropriate population of patients, with summary metrics from the first year of clinical testing demonstrating alignment of the clinically tested population with the intended use population via risk factor count per patient [average 2.8] and number of risk factors stratified among 40-GEP results. The authors of this manuscript are supportive of the baseline testing conditions for inclusion, as patients without the high-risk factors listed for 40-GEP testing criteria typically have a very low likelihood of poor outcomes and do not warrant additional information. However, for those patients where uncertainty remains in clinical decisions or where additional information is needed to support shared decision making with the patient and/or multi-disciplinary care team, the authors recommend utilizing the results of the 40-GEP. For the following study, the authors confirm that all participants provided informed written consent to participate in the study and for the sharing of accompanying images and data. Institutional approval was not required for the publication of these cases.
 |
Table 1 Use of the 40-GEP is Indicated for Patients Diagnosed with Primary Cutaneous Squamous Cell Carcinoma (cSCC) with One or More Risk Factors |
Treatment Modalities Most Impacted by Use of the 40-GEP and a Clinician-Derived Algorithm for Incorporation of Test Results
Surveillance Imaging
Medical imaging is a vital tool to determine the extent of local invasion, regional and/or distant metastasis and, in some cases, determine clear margins post-surgery. The National Comprehensive Cancer Network (NCCN) primarily states that the imaging modality and targeted area should be at the discretion of the treating clinician(s) but does offer general guidance for assessment of extent of local disease (suggested use of MRI), perineural disease or deep soft tissue involvement (MRI with contrast), or suspected bone disease or pathologic evidence of lymph node disease (CT with contrast). Accumulating evidence does support the utility of imaging to guide management plans for high-risk cSCC patients,17,18 yet there are studies showing conflicting results regarding accuracy, preference, and outcomes associated with an imaging modality and cadence.19,20 Thus, while national guidelines broadly define use of surveillance imaging for high-risk cSCC patients, the specifics of treatment remain in the hands of the clinician, whose options are variable and lack standardization. Therein lies the applicability of the 40-GEP test, whose results can readily be integrated into a treatment pathway to assist a clinician in making the most effective use of this intervention. Due to the low metastatic rate associated with a Class 1 result,9,10 it is recommended by the authors that patients with this test result may forgo imaging (Figure 1). However, for Class 2A and 2B results, imaging ― magnetic resonance imaging (MRI) for soft tissue, ultrasonography (US) due to its high sensitivity, high diagnostic odds ratio, and its easy use to guide the biopsy of palpable nodes,21,22 or computed tomography (CT) ― should be discussed or considered depending upon the combination and number of risk factors (which includes clinical, pathologic and 40-GEP results) influencing the clinicians’ perceived elevation of metastatic risk for the patient.
Sentinel Lymph Node Biopsy (SLNB)
SLNB can be used to identify regional metastasis and allow for early treatment, prognosis stratification, and patient selection criteria for adjuvant clinical trials. However, growing discordance among published studies on identifying which risk factors or combination of risk factors will encompass the majority of positive SLNBs has made decisions regarding performing SLNBs challenging.23,24 Unlike melanoma and breast cancer,25 where SLNB has been shown to help detect nodal micrometastases and is recommended by NCCN as standard of care for specific subsets of patients, the value of SLNB in cSCC patients has yet to be determined, likely owing to the above mentioned inconsistencies.26
Recently published studies do support SLNB for American Joint Committee on Cancer (8th Edition; AJCC827) Stage T3+ disease or Brigham and Women’s Hospital (BWH28) Stage T2b/T3 disease from a patient management29 and health economics outcomes research perspective.30 Also, NCCN guidelines31 encourage discussing and considering SLNB before surgery for very-high risk cSCC, those tumors with one of the following features: perineural invasion ≥0.1mm, desmoplastic subtype, poorly differentiated, diameter ≥4 cm, lymphovascular invasion, or invasion beyond the subcutaneous fat or >6mm. While the optimal cSCC patient clinicopathologic factors that would guide the decision to perform a SLNB procedure are still unknown, future studies involving 40-GEP, in combination with staging criteria and imaging modality, may be useful in stratifying patient populations at high risk of metastasis who may benefit from SLNB. Currently, in cases where there are concerning patient or tumor factors, yet nodal palpation is negative and BWH or AJCC8 stage is low, the authors have concluded that a SLNB procedure may not be advantageous, and the 40-GEP Class 1 result would further support the rationale to avoid SLNB (Figure 1). Although current recommendations support a SLNB for patients staged T2b/3 or T3+ by BWH or AJCC8 systems, respectively, there are limitations in the accuracy of these methods to appropriately predict poor outcomes.32,33 The 40-GEP test has demonstrated improved accuracy over staging systems in its prediction of metastasis9,10 and therefore, patients receiving a Class 2A or 2B (metastatic rates of 20.0% and 52.2%, respectively) 40-GEP result should be considered sufficiently at risk for metastasis to undergo nodal evaluation.
Adjuvant Radiation Therapy (ART)
After Mohs micrographic surgery, radiation therapy is the first-line adjuvant treatment for cSCC.31 The recent NCCN guidelines recommend radiation to treat local, low-risk and local, high-risk/very-high-risk cSCC but also consider ART for patients with PNI or extensive nerve involvement, even in patients with clear surgical margins. The American Society for Radiation Oncology (ASTRO)34 has acknowledged that the role of radiation for BCC and cSCC has been poorly defined owing to lack of high-quality evidence and that there are currently no evidence-based clinical practice guidelines to provide direction on the use of radiation for these diseases. Their commissioned task force formulated evidence-based recommendations for the use of definitive and postoperative radiation therapy (PORT) in patients with cSCC. These guidelines, similar to NCCN, offer broad inclusion criteria, in which selection among the various radiation techniques are reviewed regarding how to best enhance cosmetics with functional sparing. PORT is recommended for cSCC patients ranging from those having recurrence after margin-negative resection or positive margin that cannot be corrected with further surgery to those with desmoplastic histology, gross PNI, chronic immunosuppression or T3/T4 AJCC8 staged tumors. This wide inclusion criteria, along with limited high-quality evidence on improved survival, and the rarity of patients presenting with such risk factors, has made it difficult to define the role of ART in managing high-risk cSCC patients. However, a recent retrospective study by Ruiz et al identified a 50% reduction in locoregional recurrence for high-risk cSCCs treated with radiation in the adjuvant setting compared to untreated patients.35 Several studies have even reported the idea of using 40-GEP36–38 to filter and identify patients at higher risk of metastasis, thus treating those selective patients with radiation therapy to improve the disease sequelae. Current suggestions from the authors as how to best incorporate 40-GEP test results into the application of ART for their high-risk patients have originated from consideration of ASTRO guidelines and publications defining mid-to-high levels of metastasis as between 20–29% and 52–70%, respectively.9,10,32,34 Therefore, those patients receiving a Class 1 result should avoid ART based on the low likelihood of metastasis, and those with a Class 2A or Class 2B result should be considered or recommended for ART due to their moderate or high likelihood of metastasis, respectively (Figure 1).
Clinical Follow-Up
The majority of cSCCs can be cured by surgery alone; however, depending upon the type of surgery, 3–8% of cSCC patients have a risk of recurrence and/or metastasis with >90% of total recurrences occurring within the first two years post-surgery.39–41 Implementing the appropriate follow-up schedule and long-term surveillance plan is critical for early detection of progression. For example, there are frequent reports of higher risk of poor outcomes among the immunosuppressed cSCC population,42,43 along with the documented evidence that patients with prior cSCC are also at higher risk for developing cutaneous melanoma and basal cell carcinoma.44 Although there are no prospective studies on the survival benefit of the follow-up approach for cSCC patients, observational and retrospective studies have reported significant improvement in overall survival.26,45 From a clinician’s perspective, clinical follow-up serves the purpose of not only allowing for early detection of any disease progression, but offers the opportunity to educate the patient on risk and currently available treatment options. From a skin cancer patient’s perspective, most find clinical follow-up useful46 and, in particular, find value in the additional prognostic information provided by GEP testing.47 Therefore, taking into account the above perspectives, and given the high volume of cSCC cases and limited healthcare resources for some patients, the standardized risk-titrated clinical follow-up schedule composed by the authors can be used to guide appropriate clinical follow-up decisions for high-risk cSCC patients when incorporating the results of the 40-GEP test (Figure 1), all while staying within the broad national recommendations. Clinical follow-up with total-body skin examination (TSE) can be performed every 6–12 months for two years with a Class 1 result. Clinical follow-up and TSE frequency can be raised to every 3–6 months for a Class 2A result, while a Class 2B result would necessitate follow-up and TSE every 2–3 months for up to two years.
Patients and Methods
Application of a Clinician Composed Algorithm for Integration of 40-GEP into Clinical Practice as Demonstrated by Real-World High-Risk cSCC Cases
Case 1 Presentation
A 74-year-old white male patient presented with a primary cSCC on the left posterior scalp with pre-operative size of 2.2 cm × 2.2 cm. The patient required three stages of Mohs surgery and no additional high-risk pathological features were observed. The final defect size was 4.2 cm × 4.2 cm with a defect depth to adipose tissue (Figure 2). The excised cSCC was moderately differentiated which demonstrated moderate to marked nuclear pleomorphism with numerous mitotic figures. The AJCC8 and BWH T-stages were T2 and T2a, respectively. Having presented with two NCCN high-risk factors, and the clinicians concern with multiple cellular abnormalities along with a depth within the adipose tissue (albeit not a depth beyond subcutaneous tissue), the 40-GEP test was ordered.
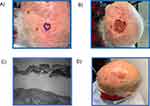 |
Figure 2 Various stages of treatment for Case 1. (A) Presentation; (B) Mohs surgery; (C) Histological diagnosis; (D) Post-procedure. |
Case 2 Presentation
A >90-year-old white male patient presented with a primary cSCC on the left lateral neck, measuring 3.1 cm × 2.9 cm (Figure 3). The patient required multiple stages of Mohs surgery. The final defect size was 4.4 cm × 4.1 cm. The clinical lymph node evaluation was negative for lymphadenopathy. The cSCC was moderately differentiated. The AJCC8 and BWH T-stages were T2 and T2a, respectively. Having presented with two NCCN high-risk factors, and the clinicians concern with having performed extensive Mohs surgery the 40-GEP test was ordered.
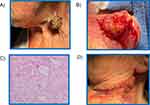 |
Figure 3 Various stages of treatment for Case 2. (A) Presentation; (B) Mohs surgery; (C) Histological diagnosis; (D) Post-procedure. |
Case 3 Presentation
A 63-year-old white male patient presented with a >2cm primary cSCC on the right posterior scalp (Figure 4). Mohs surgery requiring multiple stages was performed. Tumor invasion beyond the subcutaneous fat was found. The AJCC8 and BWH T-stages were T3 and T2b, respectively. Having presented with two NCCN high-risk and one very-high risk factors, and needing multiple stages of Mohs surgery, the 40-GEP test was ordered.
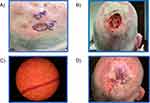 |
Figure 4 Various stages of treatment for Case 3. (A) Presentation; (B) Mohs surgery; (C) Histological diagnosis; (D) Post-procedure. |
Results
Risk-Aligned Management Strategy with 40-GEP Test Result: Case 1
The test result was Class 1 (low metastatic risk). Initially, CT, radiation, and follow-up of every 1-month were considered. In light of the Class 1 40-GEP test result, follow-up was scheduled for monthly wound check and nodal exams were scheduled for every 6 months. One-year post-treatment, the wound has healed with no evidence of recurrence or metastasis.
Risk-Aligned Management Strategy with 40-GEP Test Result: Case 2
The test result was Class 2A (moderate metastatic risk). Prior to ordering the 40-GEP, radiation, SLNB, and follow-up every 6 months were considered. Given the age of the patient and the 40-GEP result, the decision was to forgo SLNB and radiation with follow-up scheduled for every 3 months. Three months post-treatment, the wound has healed with no evidence of recurrence or metastasis.
Risk-Aligned Management Strategy with 40-GEP Test Result: Case 3
The test result was Class 2A (moderate metastatic risk). Prior to ordering the 40-GEP, imaging to evaluate for distant metastasis was considered. The Class 2A 40-GEP test result supported the rationale for the pre-test treatment pathway. Surveillance of lymph nodes with ultrasound imaging every 6 months for two years and clinical follow-up of every 3 months with clinical lymph node exam was recommended. Sixteen months post-treatment, the wound has healed with no evidence of disease.
Discussion
The proposed algorithm was composed by the authors as a mechanism to assist clinicians in best implementing 40-GEP test results into their current risk-assessment and treatment approaches, along with providing recommendations for personalized management of patients based on their risk for poor outcomes. Although the NCCN guidelines for cSCC provide a framework for stratifying patients, the heterogeneous nature of the disease has made accurate risk assessment by use of clinicopathologic factors alone challenging and incomplete.48,49 Furthermore, evaluating subjective clinicopathologic factors like “degree of differentiation” or “rapidly growing tumor”, having multiple measurements of depth of invasion (Breslow depth or Clark level) and even considering of the wide spectrum of patients deemed immunosuppressed, have further created uncertainty in the field.31,50–52 Currently, the use of multidisciplinary tumor boards (MDTBs) are known to improve outcomes in managing several malignancies.53 MDTBs provide a platform for discussing treatment alternatives, improving communication among disciplines, and helping to develop tailored evidence-based treatment plans for the patient. Unfortunately, MDTBs, while impactful, are not feasible for every patient with high-risk cSCC, and organization of MDTBs for high-risk cSCC is often complicated due to the variety of approaches to risk-assessment (ie, NCCN defined high-risk factors, patient history/other clinicopathologic risk factors, AJCC8 staging and BWH staging options). Consequently, these variations have caused inconsistency in treatment plans, potentially negatively influencing patient outcomes. A recent survey study comprised of dermatologists (n=50) and oncologists (n=54) reported that 72% of respondents felt the level of care coordination between these specialties was “very low” or “low”. This study highlights the gap and significant challenges in the multidisciplinary treatment of cSCC in community oncology clinics.54 A benefit to the personalized test results provided by the 40-GEP is that it can be utilized to provide a common platform to initiate MDTBs to guide risk-aligned treatment plans, regardless of the specialty or staging systems used.
The 40-GEP test has been shown to accurately categorize the risk of metastasis in patients with cSCC who have one or more risk factors and gives prognostic information independent of recognized high-risk factors or conventional staging systems.9,10 Compelling studies have further demonstrated how integration of clinicopathologic risk markers with 40-GEP test results can augment metastatic risk stratification and aid clinicians in administering risk-aligned therapy regimens.10,13 All three 40-GEP test results (Class 1, 2A, and 2B) have shown clinical utility,14,16 emphasizing the impact the test could have for any high-risk cSCC scenario. Class 1 40-GEP results can be utilized to identify patients with a biologically low-risk of metastasis who might benefit from a de-escalation of post-surgical management, surveillance, and clinical follow-up compared to their pre-40-GEP result treatment plan and therefore save valuable healthcare resources. Class 2 40-GEP test results can be utilized to identify patients with biologically high-risk malignancies who would benefit from moderate to higher intensity therapies and a more frequent follow-up schedule. A limitation of this study is that there is no representation of a Class 2B patient. Data from one year of clinical testing of the 40-GEP16 shows that only 2.9% of 2503 patients tested received a Class 2B result. Therefore, the lack of a Class 2B is not unexpected as the case series includes three patients.
The presented algorithm is not intended to challenge established guidelines but to enhance the prognostic capability of current cSCC risk assessment methods to guide decisions within current guidelines. The approach proposed here, based on usage and integration of 40-GEP test results within real-world patient cases, provides a framework for stratifying patients with advanced cSCC by identifying their unique biological profile with the use of the 40-GEP, and then treating them in a risk-aligned manner. The clinical utility of GEP profiling in breast, prostate, melanoma, and other cancer types is well-established,55–58 and the algorithm prepared herein is just one method whereby the potential of the 40-GEP for cSCC can be realized.
Conclusion
The 40-GEP test independently identifies cSCC patients who have tumors with high metastatic potential. Appropriate application of test results could lead to more accurate and effective implementation of treatment modalities in a risk-aligned manner. The broadness attributed to the classification of a high-risk cSCC patient allows for those at a low risk of poor outcomes to potentially be over-treated while those patients truly at a higher risk may be bypassed for appropriate adjuvant interventions. The impact of appropriate distribution of treatment modalities and follow-up schedules would greatly benefit patient care and assist in relieving a substantial burden on health care infrastructure. For high-risk cSCC patients, the provided algorithm demonstrates how incorporation of 40-GEP test results can assist clinicians in identifying risk-aligned treatment pathway improvements within their existing clinical practices, with the goal of ultimately improving patient outcomes.
Compliance with Ethics Guidelines
The authors confirm that all participants provided informed consent to participate in the study.
Medical Writing Assistance
The authors would like to acknowledge that editorial assistance in the preparation of this article was provided by CBI employees Anesh Prasai, PhD, and Alison L. Fitzgerald, PhD.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors did not receive any financial support for this manuscript. The Article Publishing Charge was funded by Castle Biosciences, Inc. (CBI).
Disclosure
GS is a consultant for Castle Biosciences Inc., during the conduct of the study and a consultant for Regeneron, outside the submitted work. ASF is an advisor for Castle Biosciences Inc. and Regeneron. SNT declares no relevant conflicts of interest in this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
1. Hu W, Fang L, Ni R, et al. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer. 2022;22:836.
2. Ciążyńska M, Kamińska-Winciorek G, Lange D, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11:4337.
3. Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (Keratinocyte carcinomas) in the US Population, 2012. JAMA Dermatol. 2015;151:1081–1086.
4. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192.
5. Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149:541–547.
6. Skin Cancer Foundation: Our New Approach to a Challenging Skin Cancer Statistic [Internet]. Skin cancer found, 2021. Available from: https://www.skincancer.org/blog/our-new-approach-to-A-challenging-skin-cancer-statistic/.
7. Reynolds KA, Schlessinger DI, Yanes AF, et al. Development of a core outcome set for cutaneous squamous cell carcinoma trials: identification of core domains and outcomes. Br J Dermatol. 2021;184:1113–1122.
8. Blomberg M, He SY, Harwood C, et al. Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225–1233.
9. Wysong A, Newman JG, Covington KR, et al. Validation of a 40-gene expression profile test to predict metastatic risk in localized high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2021;84:361–369.
10. Ibrahim SF, Kasprzak JM, Hall MA, et al. Enhanced metastatic risk assessment in cutaneous squamous cell carcinoma with the 40-gene expression profile test. Future Oncol. 2022;18:833–847.
11. Farberg AS, Hall MA, Douglas L, et al. Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: impact on patient management. Curr Med Res Opin. 2020;36:1301–1307.
12. Teplitz R, Giselle P, Litchman GH, et al. Impact of gene expression profile testing on the management of squamous cell carcinoma by dermatologists. J Drugs Dermatol. 2019;18:980–984.
13. Arron ST, Blalock TW, Guenther JM, et al. Clinical considerations for integrating gene expression profiling into cutaneous squamous cell carcinoma management. J Drugs Dermatol. 2021;20:5s–s11.
14. Litchman GH, Fitzgerald AL, Kurley SJ, et al. Impact of a prognostic 40-gene expression profiling test on clinical management decisions for high-risk cutaneous squamous cell carcinoma. Curr Med Res Opin. 2020;36:1295–1300.
15. Au JH, Hooper PB, Fitzgerald AL, et al. Clinical utility of the 40-Gene Expression Profile (40-GEP) test for improved patient management decisions and disease-related outcomes when combined with current clinicopathological risk factors for cutaneous Squamous Cell Carcinoma (cSCC): case series. Dermatol Ther. 2022;12:591–597.
16. Hooper PB, Farberg AS, Fitzgerald AL, et al. Real-world evidence shows clinicians appropriately use the prognostic 40-Gene Expression Profile (40-GEP) test for high-risk cutaneous Squamous Cell Carcinoma (cSCC) Patients. Cancer Invest. 2022;2022;1–12.
17. Ruiz ES, Karia PS, Morgan FC, et al. The positive impact of radiologic imaging on high-stage cutaneous squamous cell carcinoma management. J Am Acad Dermatol. 2017;76:217–225.
18. Maher JM, Schmults CD, Murad F, et al. Detection of subclinical disease with baseline and surveillance imaging in high-risk cutaneous squamous cell carcinomas. J Am Acad Dermatol. 2020;82:920–926.
19. Gandhi MR, Panizza B, Kennedy D. Detecting and defining the anatomic extent of large nerve perineural spread of malignancy: comparing “targeted” MRI with the histologic findings following surgery. Head Neck. 2011;33:469–475.
20. Nemzek WR, Hecht S, Gandour-Edwards R, et al. Perineural spread of head and neck tumors: how accurate is MR imaging? AJNR Am J Neuroradiol. 1998;19:701–706.
21. Bondt RBJ, Nelemans PJ, Hofman PAM, et al. Detection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radiol. 2007;64:266–272.
22. Humphreys TR, Shah K, Wysong A, et al. The role of imaging in the management of patients with nonmelanoma skin cancer. J Am Acad Dermatol. 2017;76:591–607.
23. Costantino A, Canali L, Festa BM, et al. Sentinel lymph node biopsy in high-risk cutaneous squamous cell carcinoma of the head and neck: systematic review and meta-analysis. Head Neck. 2022;44:2288.
24. Ilmonen S, Sollamo E, Juteau S, et al. Sentinel lymph node biopsy in high-risk cutaneous squamous cell carcinoma of the head and neck. J Plast Reconstr Aesthet Surg. 2022;75:210–216.
25. Lyman GH, Giuliano AE, Somerfield MR, et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720.
26. Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560–578.
27. Amin MB, Edge S, Greene F, et al., eds. AJCC Cancer Staging Manual.
28. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402.
29. Tejera-Vaquerizo A, Cañueto J, Llombart B, et al. Predictive value of sentinel lymph node biopsy in cutaneous squamous cell carcinoma based on the AJCC-8 and Brigham and women’s hospital staging criteria. Dermatol Surg. 2020;46:857–862.
30. Quinn PL, Kim JK, Prasath V, et al. Sentinel lymph node biopsy for head and neck cutaneous squamous cell carcinoma using the Brigham and women’s staging system: a cost analysis. Arch Dermatol Res. 2022;2022:1–8.
31. National Comprehensive Cancer Network: Squamous cell skin cancer, NCCN guidelines version 2.2022, in NCCN clinical practice guidelines in oncology [Internet]; 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf.
32. Ruiz ES, Karia PS, Besaw R, et al. Performance of the American joint committee on cancer staging manual, 8th edition vs the Brigham and women’s hospital tumor classification system for cutaneous squamous cell carcinoma. JAMA Dermatol. 2019;155:819.
33. Cañueto J, Burguillo J, Moyano-Bueno D, et al. Comparing the eighth and the seventh editions of the American joint committee on cancer staging system and the Brigham and women’s hospital alternative staging system for cutaneous squamous cell carcinoma: implications for clinical practice. J Am Acad Dermatol. 2019;80:106–113.e2.
34. Likhacheva A, Awan M, Barker CA, et al. Definitive and postoperative radiation therapy for basal and squamous cell cancers of the skin: executive summary of an American society for radiation oncology clinical practice guideline. Pract Radiat Oncol. 2020;10:8–20.
35. Ruiz ES, Kus KJB, Smile TD, et al. Adjuvant radiation following clear margin resection of high T-stage cutaneous squamous cell carcinoma halves the risk of local and locoregional recurrence: a dual center retrospective study. J Am Acad Dermatol. 2022;87(1):87–94.
36. Ibrahim SF, Arron ST, Somani A-K, et al. 25726 prospective adjuvant therapy trial design using a prognostic 40-gene expression profile (40-GEP) test for high-risk cutaneous squamous cell carcinoma (cSCC) and BWH staging-based risk assessment. J Am Acad Dermatol. 2021;85:AB67.
37. Koyfman SA, Wysong A, Arron S, et al. Improved risk stratification in an adjuvant radiation therapy (ART) eligible cutaneous squamous cell carcinoma (cSCC) patient population by integration of the 40-gene expression profile prognostic test (40-GEP). J Clin Oncol. 2021;39:e21589–e21589.
38. Schmults C, Covington KR, Kurley SJ, et al. Implications of a prognostic 40-gene expression profile (40-GEP) test for high-risk cutaneous squamous cell carcinoma (cSCC) on staging-based risk assessment and adjuvant therapy trial design. J Clin Oncol. 2020;38:e22091–e22091.
39. van Lee CB, Roorda BM, Wakkee M, et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol. 2019;181:338–343.
40. Khan K, Mykula R, Kerstein R, et al. A 5-year follow-up study of 633 cutaneous SCC excisions: rates of local recurrence and lymph node metastasis. J Plast Reconstr Aesthet Surg. 2018;71:1153–1158.
41. Tschetter AJ, Campoli MR, Zitelli JA, et al. Long-term clinical outcomes of patients with invasive cutaneous squamous cell carcinoma treated with mohs micrographic surgery: a 5-year, multicenter, prospective cohort study. J Am Acad Dermatol. 2020;82:139–148.
42. Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a delphi consensus statement. JAMA Dermatol. 2021;157:1219–1226.
43. Elghouche AN, Pflum ZE, Schmalbach CE. Immunosuppression impact on head and neck cutaneous squamous cell carcinoma: a systemic review with meta-analysis. Otolaryngol Head Neck Surg. 2019;160:439–446.
44. Flohil SC, van der Leest RJT, Arends LR, et al. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2365–2375.
45. Madani S, Marwaha S, Dusendang JR, et al. Ten-year follow-up of persons with sun-damaged skin associated with subsequent development of cutaneous squamous cell carcinoma. JAMA Dermatol. 2021;157:559–565.
46. Themstrup L, Jemec GE, Lock-Andersen J. Patients highly value routine follow-up of skin cancer and cutaneous melanoma. Dan Med J. 2013;60:A4713.
47. Ahmed K, Siegel JJ, Morgan‐Linnell SK, et al. Attitudes of patients with cutaneous melanoma toward prognostic testing using the 31‐gene expression profile test. Cancer Med. 2022;12:2008–2015.
48. Zheng Y, Chi S, Li C. Identification of potential gene drivers of cutaneous squamous cell carcinoma: analysis of microarray data. Medicine. 2020;99:e22257.
49. Thai AA, Lim AM, Solomon BJ, et al. Biology and treatment advances in cutaneous squamous cell carcinoma. Cancers. 2021;13:5645.
50. Prezzano JC, Scott GA, Lambert Smith F, et al. Concordance of squamous cell carcinoma histologic grading among dermatopathologists and mohs surgeons. Dermatol Surg. 2021;47:1433–1437.
51. Yanik EL, Pfeiffer RM, Freedman DM, et al. Spectrum of immune-related conditions associated with risk of keratinocyte cancers among elderly adults in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:998–1007.
52. Farberg AS, Fitzgerald AL, Ibrahim SF, et al. Current methods and caveats to risk factor assessment in Cutaneous Squamous Cell Carcinoma (cSCC): a narrative review. Dermatol Ther. 2022;12:267–284.
53. El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American society of clinical oncology international survey. J Glob Oncol. 2015;1:57–64.
54. Kirkwood JM, Krakowski AC, Carter JD, et al. Real-world practice patterns in multidisciplinary squamous cell carcinoma care at community oncology centers. J Clin Oncol. 2022;40:261.
55. Scope A, Essat M, Pandor A, et al. Gene expression profiling and expanded immunohistochemistry tests to guide selection of chemotherapy regimens in breast cancer management: a systematic review. Int J Technol Assess Health Care. 2017;33:32–45.
56. Kristiansen G. Markers of clinical utility in the differential diagnosis and prognosis of prostate cancer. Mod Pathol. 2018;31:S143–S155.
57. Berger AC, Davidson RS, Poitras JK, et al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr Med Res Opin. 2016;32:1599–1604.
58. Dillon LD, Gadzia JE, Davidson RS, et al. Prospective, multicenter clinical impact evaluation of a 31-gene expression profile test for management of melanoma patients. SKIN J Cutan Med. 2018;2:111–121.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

