Back to Journals » Clinical Ophthalmology » Volume 14
Incidence, Risk Factors and Treatment Outcomes of Intraocular Hypertension and/or Glaucoma Post-Penetrating Keratoplasty: A 5-Year Lebanese Retrospective Descriptive Study
Authors Chanbour W , Ayoub MH, Towair E, Darwish M , Fakhoury H, Warhekar P , Jarade E
Received 28 May 2020
Accepted for publication 21 July 2020
Published 26 August 2020 Volume 2020:14 Pages 2497—2505
DOI https://doi.org/10.2147/OPTH.S263459
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wassef Chanbour,1,2 Mohammad Hussein Ayoub,2 Evelyne Towair,2 Mohamad Darwish,2 Henry Fakhoury,1,2 Pramod Warhekar,3 Elias Jarade1– 3
1Department of Ophthalmology, Beirut Eye and ENT Specialist Hospital, Beirut, Lebanon; 2Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon; 3Mediclinic Dubai Mall, Dubai, United Arab Emirates
Correspondence: Elias Jarade Email [email protected]
Purpose: Glaucoma is one of the most common complications post-penetrating keratoplasty (PK). In this study, we report the Incidence, risk factors and treatment outcomes of intraocular hypertension (IOH) or/and glaucoma post-penetrating keratoplasty (PKG).
Methods: A 5-year descriptive retrospective study, Lebanese patients who underwent PK at Beirut Eye & ENT Specialist Hospital, between 2012 and 2017, were included. Patients with history of glaucoma were excluded. IOH/PKG cases that necessitate treatment were identified and analyzed for the incidence, risk factors and treatment outcomes.
Results: A total of 189 eyes of 159 patients were included, with male/female ratio 1.6 and the mean age 47.2± 21.3 years. Bullous keratopathy (BK) presented with a high mean age: 70.3 years while ectasia patients were the youngest: 36.5 years. 34.9% of eyes developed high IOP within a mean of 25 months of follow-up distributed between sub-groups of patient with corneal ectasia (22.5%), redo-PK (51.2%), bullous keratopathy (BK) (50%), keratitis (24.9%), and others (dystrophy, trauma …) (21.4%). High IOP developed in 67.4% of the diabetic patients. Visual acuity was less likely to improve in cases developing elevated IOP while postoperative complications were significantly high. In those refractory to medical treatment, trabeculectomy as a glaucoma surgery was effective in lowering the IOP. Combining procedures with PK was not a risk factor for glaucoma. Interrupted sutures and higher number of suturing were associated with increased IOP levels.
Conclusion: IOH developed in one out of three patients who underwent penetrating keratoplasty. DM, bullous keratopathy, infectious keratitis and redo-PK were highly associated with PKG, whereas high IOP was less likely to develop in cases with keratoconus. Glaucoma is considered a poor prognostic factor in patients post-PK.
Keywords: penetrating keratoplasty, glaucoma, intraocular hypertension
Introduction
Corneal diseases are the fifth causative agents for blindness internationally.1 The cause of corneal disease is vastly related to the geographic distribution, with keratoconus and anterior corneal pathologies (keratitis) being the leading cause for corneal transplant in developing countries, while endothelial pathologies dominate the developed countries.1 Corneal transplantation or Keratoplasty remains the main method for vision restoration once the corneal clarity has been distorted by the disease.2
Post-Penetrating Keratoplasty Glaucoma (PKG) remains the most serious complication of penetrating keratoplasty (PK)3 due to its drastic effect on vision and the damage it does to the optic nerve. It has a variable incidence that differs between the early and late postoperative phases. It can range between 9% and 35%, during the early and late postoperative phase.3,4 Certain conditions are considered as important risk factors for PKG, such as; a previous history of glaucoma, a failed keratoplasty, a history of perforation, aphakic bullous keratopathy, combining PK with cataract extraction, steroid use and iridocorneal synechiae3. A study done by Stewart et al showed a higher incidence of PKG among patients with Pseudophakic/Aphakic Bullous keratopathy in comparison to those with keratoconus and Fuch’s dystrophy with an incidence of 41% to 18% respectively, whereas diseases such as keratoconus and corneal dystrophy were seen as low risk.5 As for the surgical factors that might cause an elevation in IOP, the use of close-fitting and shallow sutures and a larger graft will increase the risk of PKG.6
The diagnosis of PKG represents a challenge to ophthalmologists due to variation in the IOP reading during the early postoperative period caused by the changes in the thickness of the cornea or presence of scars or from corneal distortion.3,4 Glaucoma following keratoplasty can be defined as an increase in the intraocular pressure (IOP) above 21mmHG with or without associated alternation in vision or optic nerve modifications keratoplasty (ocular hypertension and glaucoma can be used interchangeably in this case)7,8 and that necessitates treatment.
This study will tackle the different indications of penetrating keratoplasty in the Lebanese population and will highlight the rate of post-op Glaucoma and the probable predisposing risk factors and treatment’s outcome. It should be noted that, in this study, ocular hypertension and/or PKG were combined and were defined as any increase in IOP that necessitate a treatment (surgical or medical) regardless of the IOP measurements with or without progressive optic nerve head damage and visual field defect.
Methods
Study Design
This is a retrospective, Analytical, and descriptive study. The approval was obtained from the institutional review board (IRB) at the Beirut Eye and ENT Specialist Hospital, with adherence to the Declaration of Helsinki, IRB approval number 2019–03. Patient’s informed consent was not required since it is a retrospective study and patients' names, addresses, and other confidential information were not collected nor shared throughout the study.
Study Population
Of a total of 243 eyes of 205 patients who underwent PK in the Beirut Eye Specialist Hospital between 2012 and 2017 were reviewed. One hundred and eighty-nine eyes of 159 patients were included.
Inclusion Criteria
- Patients who underwent PK between 2012 and 2017 at the Beirut Eye Specialist Hospital.
- Minimum of 1 year follow-up – Age>10 years
Exclusion Criteria
- All patients who did not sustain regular follow up for more than 2 months.
- Pre-existing glaucoma, weather it was surgically and/or medically treated or not.
- Children less than 10 years of age were excluded since they have different response to PK than normal population (high failure).
Data Collection
The data were obtained by reviewing all the charts including the complete assessment of the medical and surgical history, pre- and postoperative CDVA, clinical examination, and indication for surgery, intraocular pressures, operative methods and surgical techniques. All the post-operative regiments, follow up, complications and infections were analyzed and included. IOP was measured with Goldmann Applanation Tonometry.
High IOP was identified as having a post-operative IOP more than 21mmHG on two post-op visits and/or any intra-ocular tension status that necessitate a treatment (surgical or medical) regardless of the IOP measurements.
Surgical Technique
A standard surgical technique was used. All procedures were performed by the same experienced surgeon (E. J.). The basic surgical technique for PK starts on the host’s cornea where first the visual axis needs to be marked. Then, the diseased central cornea is trephined and removed; to be replaced by a full-thickness donor corneal tissue; the mean diameter of the recipient bed was 0.25 mm smaller than the donor corneal bed. Next to be sutured into place using interrupted or combined with continuous 10-0 nylon sutures. After suturing, the new seal between the graft and the host needs to be checked. End of the procedure, all patients received 4 mg of subconjunctival Dexamethasone.
Postoperative Follow-Up
Topical antibiotic eye drops four times/day for one month and topical prednisolone phosphate (0.5%) eye drops six times/day for one month, with gradually tapering doses over 6 months, were routinely applied in all cases.
Topical carbonic anhydrase inhibitors, prostaglandin analogs, alpha-2 agonists and/or beta-blockers were initiated in high intraocular pressure cases, where trabeculectomy was applied in cases refractory to medical treatment.
All patients were followed up postoperatively with routine ophthalmic examinations on the first day, first week, first month and on monthly basis up to the sixth month postoperatively.
For data analysis in the study, visual acuity measurements were documented as logMAR and the equivalent logMAR of count fingers (CF) (CF at 1, 2, 3 and 4 meters are equivalent to 2, 1.6, 1.8, 1.5 LogMAR, respectively), hand motion (HM= 3 LogMAR), perception of light (PL=3.3LogMAR), and no perception of light (NPL= 4 LogMAR) were used. Eyes were evaluated regarding the incidence and risk factors for developing high IOP.
Data Analysis
Statistical analyses were performed using SPSS version 18.ANOVA and independent t-tests were used to compare means for months of follow-up, ages at surgery, dates for suture removal and the size of donors’ graft concerning PKG and other risk factors. Odds ratios, CHI 2 test (Exact Fisher test) with 95% confidence intervals were calculated to determine the probability of developing post-PK high IOP, and using Kaplan–Meier survival study with post-PK high IOP as the dependent variable, and the risk factors as independent variables, Log rank test of equality of survival were used. Paired samples t-test was used for the assessment of pre- and postoperative CDVA and IOP. Graft rejection, failure, infections and type of sutures were compared in patients with and without high IOP using Chi X2 test. P-value < 0.05 was required for statistical significance.
Results
A total of 189 eyes of 159 patients were included in our study. High IOP developed in 66 (34.9%) of these eyes within 25±19 (6–44) months of follow-up. IOP returned to normal in 12/66 eyes (transient high IOP) and remained elevated in 54/66 eyes (28.5%).
Patients Classification and Demographic Data
Patients were divided into 5 groups according to Previous Ophthalmic History:
1st group: Ectasia (keratoconus 82.5%, keratoglobus 4.8%, post-keratorefractive ectasia 12.7%)
2nd group: History of corneal transplant, penetrating keratoplasty (PK) surgery for any previous cause.
3rd group: History of phacoemulsification (cataract surgery) all presenting with Bullous keratopathy (BK)
4th group: Active Infectious keratitis (herpetic, bacterial, fungal, abscess … )
5th group including trauma, dystrophy (Fuchs … ), pemphigoid, sarcoidosis, silicone oil keratopathy, Steven Johnson.
Among the 5 groups, ectasia was the most common indication (37.6%) of PK in our population (Table 1). Results show that groups with a history of Bullous Keratopathy procedures had the surgery performed at an older age (70.3 ± 12.6 years) compared to groups presenting with ectasia (36.5 ± 14.5 years). (ANOVA test for comparing means P-value=0.001) (Table 1). No significant difference was found concerning which eye or what gender received the surgery when data were compared between groups (Chi X2 P≤0.05) (Table 1).
 |
Table 1 Baseline Characteristics and Patient Grouping According to the Penetrating Keratoplasty Indication |
Combined Procedures
PK and Phacoemulsification with intraocular lens implantation were performed in 18 eyes, 4 of the latter developed high IOP (Table 2). A total of 18 (39.1%) out of 46 patients who underwent any combined surgeries (vitrectomy, pupilloplasty …) developed high IOP, compared to 48 (33.6%) out of 143 patients with isolated PK surgeries had high IOP. (Chi X2 P=0.491>0.05; odds ratio: 1.2).(Table 2).
 |
Table 2 Additional Procedures Performed in Combination with the Keratoplasty in Each of the Groups |
Redo-PK done for a history of corneal transplant (PK) was combined with additional ophthalmic surgeries (pupilloplasty, vitrectomy, phacoemulsification …) in eleven cases, nine of which developed high IOP. This group was the only one to show a significant increase in the risk of developing high IOP compared to other sub-groups (Chi X2 P=0.018<0.05; odds ratio: 6.8), other clinical differences had no statistical significances (Table 3)
 |
Table 3 Incidence of High IOP Between the Groups Divided by Surgical Indication That Underwent Additional Surgeries or Not |
High IOP Development and Risk Factors
The survival of the eyes post-PK was defined as the time (months) for an eye before developing high IOP. Data comparing mean times until the development of high IOP with respect to PK indication, and previous risk factors (HTN, DM) were shown in Kaplan–Meier survival studies (Figure 1). A significant relation was found between the indication of PK and developing high IOP study 1 (Log rank: P=0.011 < 0.05) (Figure 1A) with early incidence rate (3.7 months) in cases such as bullous keratopathy (BK) while Ectasia patients had a later mean incidence of high IOP (6.7 months) (Table 4). Also, 29 (67.4%) of the 43 eyes of DM type 2 patients developed high IOP, with a mean of 1.6 months post PK versus 36 (25%) of the 144 eyes of nondiabetic patients with a mean of 5.2 months post PK, results were highly significant with an odds ratio of 6.2. Study 2 (Log rank: P=0.0001<0.05) (Figure 1B). While there was no significant difference in high IOP incidence between having a history of arterial Hypertension (5/19 (26.3%) eyes diagnosed at 4.4 months) or not (59/166 (35.5%) diagnosed at 4.6 months). Study 3, Log rank: P=0.5>0.05 (Figure 1C).
 |
Table 4 The Mean Time to Develop High IOP (Months) in Groups Ophthalmic Clinical History |
The mean diameter of the donor corneal button was 8.04±0.66 cm in patients diagnosed with high IOP, compared with 7.89±0.51cm in those with normal pressure, nonsignificant difference was found relating corneal size to PKG t(189)= 1.5, p=0.136>0.05.
Visual Acuities
Patients with normal IOP had a very high significant improvement in the CDVA as it was detected comparing preoperative mean logMAR (1.37± 0.85) and 1 year postoperative mean logMAR (0.84± 0.96), (t(65)= 4.4, p=0.0001). While in Patients with high IOP the clinical improvement in visual acuity was statistically non-significant comparing preoperative mean logMAR (1.30±0.83) and 1 year postoperative mean logMAR (1.02±0.94), (t(35)= 1.2, p= 0.2). The difference of pre- and postoperative means between the two groups (with and without high IOP was not significant), t(100)= 0.792, p=0.43>0.05.
Glaucoma Management
All patients with temporary high IOP were well controlled with medical therapy (topical treatment) until spontaneous resolution (12/66: 18%). Pressure remained high in 54 patients and treatment with anti-glaucoma eye drops proved effectiveness in controlling IOP in the majority of these patients (42/66: 64%). Surgical intervention (Trabeculectomy) was done to control IOP when medical treatment failed (12/66: 18%). Treatment options were effective in controlling the disease; all P-values were strictly below 0.05 (Table 5).
 |
Table 5 Treatment of Post Keratoplasty Glaucoma in Each of the Analyzed Groups According to PK Indication |
Complications Associated with High IOP
Postoperative complications were significantly found in higher rates in the eyes developing high IOP (Chi X2, P=0.003<0.05). Corneal graft rejection occurred in five out of 66 eyes (7.6%) with high IOP and 2 out of 123 eyes (1.6%) with normal pressure (Table 6). There was a significantly higher risk of corneal epithelial defect and graft rejections in patients with high IOP compared to the nonglaucoma group. (Chi X2, Exact test: P=0.014<0.05). Regraft was performed in twelve of the complicated eyes of the total eyes of our group (Table 6).
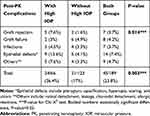 |
Table 6 The Incidence of Other Post-PK Complications in Patients with and without High IOP |
Sutures Effect on IOP
In the majority of cases, the donor’s cornea was fixed with 16 interrupted sutures (153 eyes of which 55 (35.9%) developed high IOP), 20 eyes were combined with running suture, 4 (20%) of which developed high IOP. Having more than 16 interrupted sutures were highly associated with high IOP in 7 (85%) out of 8 cases (Chi X2, P=0.003<0.05). On average, sutures were removed after 12.5±9.3 months in patients. In 12 patients with high IOP, pressure was measured before and after total or subtotal suture removal, drop of IOP was detected in most of these patients (5 ±7.5 mmHg).
Discussion
Despite the presence of multiple articles regarding keratoplasty in Lebanon, to the best of our knowledge, the aforementioned results would be the first to report the incidence of glaucoma post-PK in the Lebanese population and to establish a protocol for glaucoma treatment post-PK. Post PK, glaucoma is a challenging condition with an incidence of 10–37%9 and it imposes a high risk to jeopardize the outcome of the surgery. In our study, post-operative ocular hypertension developed in 66 cases (34.9%) out of 189 patients within 25 months of follow up; most of them were detected in the first 6 months, placing our results in the upper limits. This difference can be postulated either to the possible higher incidence of glaucoma in the Lebanese population compared to other populations or to the surgical technique and postoperative management performed by a single surgeon. However, analysis of the surgeon’s technique and post op regimens did not reveal any peculiarity compared to other published techniques and post op regimens. Also, the characteristics of the studied population in a tertiary referral center may have played a role compared to the other patients’ populations in the general eye care center (Beirut Eye and ENT Specialist hospital mostly receive complicated cases referred from other centers in addition to its patients. Also, complicated cases after PK are sent back for management which may relatively accumulate more complicated cases), more over having higher rates of Redo-PK surgeries as an indication (21.7%) of our population predisposing by itself to having higher risk of PKG. Worth mentioning, after eliminating the patients with transient increase in IOP that have had spontaneous resolution after short term of medical treatment, the incidence of PKG in the total population of our study has dropped from 34.9% to 28.5% which makes our results similar to other published data.
It has been reported that the incidence of PKG is associated with the patients’ ophthalmic history and the indications for PK.10 In our population patients with Bullous keratopathy and those with previous graft rejection were shown to be at high risk for high IOP (50% and 51.2%) the same results were reported by Nilgun Yildirim et al (43% and 45%).11 The lowest incidence of high IOP in our study was in the patients presenting with Ectasia (Keratoconus, Keratoglubous …) (22.5%) and that is similar to what was previously reported (20%).9
In our study, early cases with IOP elevation (3 months) were detected in cases with bullous keratopathy (BK), and a later cases were reported in patients presenting for a redo-PK or active keratitis (6 months).
Most of the studies reported glaucoma history as the riskiest factor for developing PKG with 59.4% incidence.9 In our study, we excluded patients with previous glaucoma history so to consider other risk factors independently. Having a history of DM type II was highly associated with elevated IOP while HTN had no significant relation.
Combined surgeries did not show an increase in the risk of high IOP, this was supported by some studies with similar results and rejected in others and was stated to be controversial.11,12 However, a subgroup analysis showed that a combined procedure in the subgroup of Redo-PK was statistically significantly higher than the non-combined procedures.
Major improvement in the postoperative visual acuity was detected in patients post PK, while specifically, eyes with high IOP had clinically significant improvement with no statistical significance in visual acuity measurement. As listed in the literature, the use of topical medications to control IOP is still the first-line treatment of PKG.10 All patients with temporary high intraocular pressure were well controlled with medical therapy (topical treatment) until spontaneous resolution. Treatment with anti-glaucoma eye drops proved effectiveness in controlling IOP in the majority of our patients. Surgical intervention (Trabeculectomy) was done to control IOP when medical treatment failed.
Steroids usage is considered as one of the main causes of post-PK glaucoma.10 In our study, to prevent graft rejection, the use of topical prednisolone phosphate (10%) eye drops six times/day for one month was initiated evenly in every post-PK eye, eliminating the difference of its effect on our study groups.
Temporary IOP elevations can be due to 1) inflammatory processes that can occur in the early postoperative period, 2) retained materials in the anterior chamber or 3) temporary response to heavy steroid use in the early postoperative period and this can interfere with the diagnosis of PKG.13 The average period between surgery and the first documented IOP elevation was 5±6 months.
Our observation in small set of patients, total or subtotal removal of sutures in 12 patients resulted in immediate and significant drop of IOP measurement of 5 mmHg. We postulate that this drop is attributed either to drastic anatomical changes in the anterior chamber angle after removal of sutures, which may lead to the increase of aqueous humor outflow, or it is attributed to normalizing of IOP measurement after removal of sutures that may have had induced significant surface tension and whop stress changes at the level of the corneal surface that may by its turn have affected the IOP measurement and not the actual manometric IOP inside the anterior chamber. This theory may have a significant impact on the IOP measurement after PK and any corneal surgery that may require relatively extensive corneal suturing; thus, we do suggest a further evaluation of the effect of corneal suturing on the IOP measurement by a large cohort of patients. Further studies and closer follow-up are required to confirm this theory.
Glaucoma is possibly the most devastating complication following PK. There was a significantly higher risk for developing other post-PK complications especially corneal epithelial defect and graft rejections in patients with high IOP (36.4%) compared to those with normal pressures (17%). Similar results were controversial in the previous literature and studies.14 The incidence of post-keratoplasty infections was 3.7% in the present study. It was reported to range from 2% to 12% of eyes undergoing PK.15 In our study, the high percentage of eyes undergoing PK due to non-inflammatory conditions such as ectasia may explain the low incidence of post-keratoplasty infections.
The survival of grafts was previously reported to be 98%, 90%, 81%, and 74% at 1, 2, 3, and 4 years, respectively.14 The highest survival rate was documented in grafts for eyes with non-inflammatory conditions and the lowest in grafts for eyes with BK, being 70% at five years.15 In this study including 189 eyes that underwent PK for the first (78.3%) and second time (21.7%), the high number of eyes with non-inflammatory conditions was a possible explanation for the low incidence of graft failure (4.2%) after a mean follow-up of 25 months (2 years).
There are some limitations to the current study. First of all our study was only in one referral tertiary center as we hope this study’s results can resemble the Lebanese population and be representative of their data. The diagnosis of post-keratoplasty Glaucoma is a challenging process due to difficulties in the measurement of IOP in the corneal graft. We used the Goldmann’s applanation Tonometry as it showed no difference when comparing it with Rebound Tonometer.16 Of note, it was reported that the accuracy of tonometry is reduced in certain situations, such as corneal edema, scars, blood staining, pain, tenderness, or any other condition that thickens or alters the corneal elasticity.10
The number of eyes that underwent PK due to non-inflammatory conditions such as ectasia 37.6% (keratoconus) was more than the eyes that underwent PK due to other pathologies. BK and active keratitis comprised 14.8% and 11.1% of the study group, respectively. Dystrophy and trauma comprised small groups, where they needed to be combined with other minority groups to achieve better statistical and analytical results, minimizing the ability to generalize our results to included groups.
In conclusion, high IOP developed in one out of three patients who underwent penetrating keratoplasty. Diabetes mellitus type II, bullous keratopathy, active keratitis, and Redo-corneal transplants were highly associated with high IOP, whereas high intraocular pressure was less likely to develop in cases with keratoconus. Combined procedures were not a risk factor for glaucoma. Visual acuity was less likely to improve in cases developing high IOP. Medical therapy is effective in both temporary and persistent high IOP cases. In those refractory to medical treatment, trabeculectomy as a glaucoma surgery was effective in lowering the IOP. Corneal epithelial defect and graft rejection occurred more frequently in patients with elevated IOP. Higher values of IOP post-PK directly correlate with a higher number of sutures used to fix the cornea.
Abbreviations
CDVA, corrected distance visual acuity; DM, diabetes mellitus; HTN, arterial hypertension; IOP, intraocular pressure; KC, keratoconus; LogMAR, logarithm of the minimum angle of resolution; BK, bullous keratopathy; PKG, post keratoplasty glaucoma; PK, penetrating keratoplasty.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
1. Mathews PM, Lindsley K, Aldave AJ, Akpek EK. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37(9):1198–1203. doi:10.1097/ICO.0000000000001666
2. Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi:10.1016/S0140-6736(12)60437-1
3. Ayyala RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol. 2000;45(2):91–105. doi:10.1016/S0039-6257(00)00141-7
4. Zemba M, Stamate AC. Glaucoma after penetrating keratoplasty. Rom J Ophthalmol. 2017;61(3):159. doi:10.22336/rjo.2017.30
5. Sharma A, Sharma S, Pandav SS, Mohan K. Post penetrating keratoplasty glaucoma: cumulative effect of quantifiable risk factors. Indian J Ophthalmol. 2014;62(5):590. doi:10.4103/0301-4738.129790
6. Zimmerman T, Olson R, Waltman S, Kaufman H. Transplant size and elevated intraocular pressure: postkeratoplasty. Arch Ophthalmol. 1978;96(12):2231–2233. doi:10.1001/archopht.1978.03910060533012
7. Raj H, Bhanushree G, Hulinaykar RM, Vijayanath V. Preoperative risk factors and incidence of glaucoma after penetrating keratoplasty. Int J Opthalmol. 2014;1(2):55.
8. Dada T, Aggarwal A, Minudath KB, et al. Post-penetrating keratoplasty glaucoma. Indian J Ophthalmol. 2008;56(4):269. doi:10.4103/0301-4738.41410
9. Haddadin RI, Chodosh J. Corneal transplantation and glaucoma. Semin Ophthalmol. 2014;29(5–6):380–396. doi:10.3109/08820538.2014.959201
10. Karadag O, Kugu S, Erdogan G, Kandemir B, Ozdil SE, Dogan OK. Incidence of and risk factors for increased intraocular pressure after penetrating keratoplasty. Cornea. 2010;29(3):278–282. doi:10.1097/ICO.0b013e3181b6eb9e
11. Yildirim N, Gursoy H, Sahin A, Ozer A, Colak E. Glaucoma after penetrating keratoplasty: incidence, risk factors, and management. J Ophthalmol. 2011;2011:951294. doi:10.1155/2011/951294
12. Sihota R, Sharma N, Panda A, et al. Post-penetrating management and keratoplasty glaucoma: risk factors, visual outcome. Aust N Z J Ophthalmol. 1998;26:305–309. doi:10.1111/j.1442-9071.1998.tb01334.x
13. Waked N, Fayad AM, Fadlallah A, El HR. Keratoconus screening in a Lebanese students’ population. J Fr Ophtalmol. 2012;35(1):23–29. doi:10.1016/j.jfo.2011.03.016
14. Moreno-Montañés J, García N, Fernández-Hortelano A, García-Layana A. Rebound tonometer compared with Goldmann tonometer in normal and pathologic corneas. Cornea. 2007;26(4):427–430. doi:10.1097/ICO.0b013e318030df6e
15. Huang SC, Wu SC, Wu WC, Hong HL. Microbial keratitis—a late complication of penetrating keratoplasty. Trans R Soc Trop Med Hyg. 2000;94(3):315–317. doi:10.1016/S0035-9203(00)90338-9
16. Thompson RW
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

