Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Incidence rate and risk factors of early repolarization in patients with growth hormone-secreting pituitary adenoma: a cohort study
Authors Chen Z, Hu B, Feng Y, Wang Z, Jiang X , Cheng Y, He D, Zhu D, Xiao Z, Wang H, Mao Z
Received 30 August 2018
Accepted for publication 4 December 2018
Published 28 December 2018 Volume 2019:15 Pages 65—72
DOI https://doi.org/10.2147/TCRM.S185929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Zhiyong Chen,1,2,* Bin Hu,1,* Yajuan Feng,3 Zongming Wang,1 Xiaobing Jiang,4 Yunjiu Cheng,5 Dongsheng He,1 Dimin Zhu,1 Zheng Xiao,1 Haijun Wang,1 Zhigang Mao1
1Department of Neurosurgery and Pituitary Tumor Center, The First Affiliated Hospital, SunYat-sen University, Guangzhou, People’s Republic of China; 2Department of Neurosurgery, The First Affiliated Hospital, Jinan University, Guangzhou, People’s Republic of China; 3Department of Histology and Embryology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, People’s Republic of China; 4Department of Neurosurgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 5Department of Cardiology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Purpose: To investigate the incidence and risk factors for early repolarization (ER) in patients with growth hormone (GH)-secreting pituitary adenomas.
Methods: From August 2014 to August 2016, patients with GH-secreting pituitary adenomas and non-functioning pituitary adenomas admitted to the First Affiliated Hospital, Sun Yat-sen University, were prospectively enrolled. Logistic regression analysis was used to investigate risk factors for ER development.
Results: A total of 118 patients with GH-secreting pituitary adenomas (41 with concomitant ER) and 103 patients with non-functioning pituitary adenomas were included. Compared with the non-functioning adenoma group GH and IGF-1 levels, left ventricular mass index (LVMI), and incidence of ER were significantly higher in the GH-secreting pituitary adenoma group (all P<0.05). LVMI was an independent risk factor for ER. Bivariate correlation analysis showed that course of disease, GH, IGF-1, and diabetes were correlated with LVMI. Course of disease and IGF-1 were directly correlated with LVMI. Two-year follow-up of patients who underwent transsphenoidal resection showed that incidence of ER was significantly decreased in patients with normal GH and IGF-1 levels.
Conclusion: Compared with non-functioning pituitary adenoma patients, patients with GH-secreting pituitary adenomas have a significantly higher incidence of ER. Elevation of serum GH and IGF-1 had positive correlations with cardiac muscle cell hypertrophy and increased LVMI.
Keywords: pituitary tumors, growth hormone-secreting pituitary adenoma, insulin-like growth factor, left ventricle mass index, early repolarization
Introduction
Cardiogenic sudden death can occur in patients with growth hormone (GH)-secreting pituitary adenomas, and is an important cause of death in these patients.1,2 However, the reasons and mechanism of cardiogenic sudden death are currently unknown. Early repolarization (ER) refers to the appearance of ST interval elevation on electrocardiography in healthy, asymptomatic individuals. It is characterized by upward concavity on the ST interval or upslope elevation, and can lead to sudden ventricular arrhythmia, ultimately resulting in cardiogenic sudden death.3,4 Development of ER is related to cardiac muscle cell hypertrophy, valve disease, myocardial ischemia, and other factors.5–7 However, the relationship between GH-secreting pituitary adenoma and ER is currently unclear. This study aimed to investigate the incidence of ER in patients with GH-secreting pituitary adenoma and related risk factors, thereby providing clinical information for research on cardiogenic sudden death due to GH-secreting pituitary adenomas.
Materials and methods
Patient selection
From August 2014 to August 2016, 118 patients with GH-secreting pituitary adenomas (GH-secreting pituitary adenoma group) and 103 patients with non-functioning pituitary adenomas (non-functioning adenoma group) admitted to the Neurosurgery Department of the First Affiliated Hospital, Sun Yat-sen University, were included in this prospective study. Inclusion criteria for the GH-secreting pituitary adenoma group included:8,9 1) serum GH levels >7.5×10−3 IU/L; 2) IGF-1 levels exceeding the upper limit of the normal range in the corresponding age group; 3) nadir GH of glucose tolerance test >5×10−3 IU/L; and 4) GH-secreting pituitary adenoma indicated by postoperative pathology results. Inclusion criteria for the non-functioning adenoma group included: 1) no abnormally elevated pituitary hormones; 2) normal serum IGF-1 levels; 3) non-functioning pituitary adenoma indicated by postoperative pathology results. The exclusion criteria were as follows: 1) pregnancy, lactation, or psychiatric disorders; 2) arrhythmia; 3) underwent treatment for cardiac disease; 4) patients with history of smoking or alcoholism; 5) patients with other malignant tumors; and 6) patients over the age of 70 years or under the age of 18 years. GH-secreting pituitary adenoma patients were further divided into an ER group (41 patients) or a non-ER group (77 patients), depending on whether ER was present. Inclusion of patients needs to exclude patients with hypopituitarism and patients who have received hormone replacement therapy. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the ethics committee of the First Affiliated Hospital of the Sun Yat-sen University. Written informed consent was obtained from all individual participants included in the study.
Clinical parameter collection
The sex, height, weight, age, course of disease (time to appearance of clinical symptoms and time to treatment), body mass index (BMI), blood pressure, fasting blood sugar (FBS), serum GH, serum IGF-1, maximum diameter of pituitary adenoma, left ventricular mass index (LVMI), presence or absence of heart valve disease, presence or absence of myocardial ischemia, and myocardial ER status of the subjects were collected. Systolic pressure ≥140 mmHg (1 mmHg =0.133 kPa) or diastolic pressure ≥90 mmHg was diagnosed as essential hypertension. All medical histories and physical examinations were performed by the same physician.
Endocrine examination and MRI examination
Venous blood was collected from all subjects after fasting for 8–10 hours. GH, IGF-1, and other pituitary endocrine hormones were measured using a chemiluminescent immunoassay system. Diabetes was diagnosed if an FBS >6.1 mmol/L was measured for 3 consecutive days using an automated biochemical analyzer. Conventional and enhanced MRI scanning of the pituitary gland was performed on all patients, and pituitary adenomas were classified as microadenomas, macroadenomas, and giant adenomas based on their maximum diameter.10
Cardiac examinations
Echocardiography and electrocardiography were used to measure relevant parameters. The Devereux adjusted equation for ventricular mass was used to calculate the LVMI, which served as the reference index for the diagnosis of ventricular hypertrophy. The degree of heart valve insufficiency and changes in heart valve structure were classified as minimal, mild, moderate, or severe based on the criteria of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Minimal changes were not included as heart valve disease.11 The appearance of ST depression, or T-wave flattening or inversion on any lead on electrocardiography was diagnosed as myocardial ischemia. A diagnosis of ER by electrocardiography was made if all of the following conditions were fulfilled:12 1) presence of slurring of the end of the QRS complex or R-wave downslope notching – slurring must be completely above baseline, and notching must at least begin above baseline; 2) notching of the end of the QRS complex on ≥2 adjacent leads or J-point elevation ≥0.1 mV (not including chest leads 1–3); and 3) QRS complex duration <120 ms. All echocardiography and electrocardiography results were read by the same two cardiovascular medicine physicians and a consensus was reached.
Treatment and follow-up
After each patient was admitted and all relevant examinations performed, transsphenoidal pituitary adenoma resection was performed and follow-up was conducted. Patients who underwent other procedures for treatment of pituitary adenoma, patients who took medication for heart disease, and patients lost to follow-up were excluded. After follow-up for 2 years, clinically relevant data of the patients were measured (six patients in total).
Statistical analysis
SPSS 19.0 software was used for statistical analysis. Normally distributed data were shown as mean ± SD. Homoscedastic normally distributed data between the two groups were analyzed using the independent t-test. The non-parametric rank sum test was used for heteroscedastic data. Non-normally distributed count data were shown as median (interquartile range) and analyzed using the rank sum test. Categorical data were described using percentage (%), and the chi-squared test was used to compare the groups. Bivariate correlation analysis and multivariate linear regression analysis were used for continuous variables. Logistic regression analysis was used for binary variables. Differences with P<0.05 were considered statistically significant.
Results
Clinical characteristics
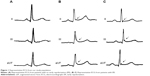
Characteristics of ER electrocardiograph manifested as elevation of the QRS–ST junction (J-point) and QRS notching or slurring in multiple leads (Figure 1). Table 1 shows the comparison of clinical characteristics between the GH-secreting pituitary adenoma group and the non-functioning adenoma group. There were statistically significant differences in course of disease, BMI, serum GH levels, serum IGF-1 levels, presence or absence of diabetes, pituitary adenoma size, LVMI, presence or absence of valve disease, and presence or absence of ER between the two groups (all P<0.05). The incidence rates of ER in the GH-secreting pituitary adenoma group and the non-functioning adenoma group were 34.7% (41/118) and 13.6% (14/103), respectively. There were no statistically significant differences in age, sex, presence or absence of essential hypertension, or presence or absence of myocardial ischemia between the two groups (all P>0.05).
Table 2 shows the comparison of clinical characteristics between the ER group and the non-ER group in patients with GH-secreting pituitary adenoma. Compared with the non-ER group, serum GH levels and LVMI in the ER group were both significantly elevated (all P<0.05), whereas there were no statistically significant differences in age, sex, course of disease, BMI, serum IGF-1 levels, presence or absence of essential hypertension or diabetes, pituitary adenoma size, presence or absence of valve disease, or presence or absence of myocardial ischemia between the two groups (all P>0.05).
Analysis of risk factors
The analysis of risk factors for ER is shown in Table 3. Univariate logistic regression analysis showed that course of disease, serum GH levels, serum IGF-1 levels, presence or absence of diabetes, and LVMI can be factors correlated with development of ER (all P<0.05). When binary variable multifactorial logistic regression analysis was performed using these ER risk factors, the results indicated that only LVMI was an independent risk factor for the development of ER (P<0.05, Table 4).
Correlation analysis showed that course of disease (r=0.287, P<0.01), BMI (r=0.289, P<0.01), serum GH levels (r=0.310, P<0.01), serum IGF-1 levels (r=0.282, P<0.01), presence or absence of diabetes (r=0.247, P<0.01), and pituitary adenoma size (r=0.312, P<0.01) were correlated with LVMI. Bivariate correlation analysis results (Table 5) showed that course of disease, BMI, serum IGF-1 levels, and presence or absence of diabetes were risk factors correlated with LVMI. Multifactorial linear regression analysis results (Table 5) showed that course of disease and IGF-1 were independent risk factors directly correlated with LVMI.
Follow-up
A total of six patients underwent transsphenoidal pituitary adenoma resection and were observed over the following 2 years. It was found that ER incidence was significantly decreased in patients whose GH and IGF-1 levels returned to normal. The LVMI level had a certain degree of decline and five of six ER patients recovered (Table 6).
  | Table 6 Follow-up results in patients with GH-secreting pituitary adenoma |
Discussion
It is reported in the literature that the incidence of ER among normal individuals is 3.4%–12.8%, and that the incidence is even higher in young people and athletes.13,14 ER can cause severe cardiac disorders, such as ventricular arrhythmia and cardiogenic sudden death.15,16 Studies have shown that patients with GH-secreting pituitary adenoma have a significantly higher incidence of cardiogenic sudden death,3,4 but whether this is related to the development of ER remains unclear. At present, there have been limited studies reporting on the correlation between GH-secreting pituitary adenoma and ER. In the present study, the incidence rates of ER in the GH-secreting pituitary adenoma group and the non-functioning adenoma group were 35.0% (36/103) and 13.1% (13/99), respectively, suggesting that the incidence of ER was significantly higher in GH-secreting pituitary adenoma patients. The incidence of ER in non-functional adenoma patients is similar to that of the general population, whereas the incidence of ER in the GH-secreting pituitary adenoma group was far higher than that in the general population.13,14 This result suggests that GH-secreting pituitary adenomas may be correlated with ER. Additionally, course of disease, BMI, serum GH levels, serum IGF-1 levels, presence or absence of diabetes, pituitary adenoma size, LVMI, and presence or absence of valve disease had significantly changed between the GH-secreting pituitary adenoma group and the non-functioning adenoma group, suggesting that these characteristics may be risk factors for ER in patients with GH-secreting pituitary adenomas.
Studies have shown that cardiac muscle hypertrophy and cardiac structural changes lead to cardiac electrophysiological instability, of which ER is a manifestation, suggesting that the development of ER is closely correlated with cardiac muscle hypertrophy, myocardial ischemia, valve disease, and other conditions.5–7 The results of the present study showed that the course of disease and LVMI of patients with GH-secreting pituitary adenomas with concomitant ER was significantly higher than those of patients with GH-secreting pituitary adenomas without ER. Additionally, we found that course of disease, serum GH levels, serum IGF-1 levels, presence or absence of diabetes, and LVMI can be factors correlated with development of ER, and LVMI is an independent risk factor for the development of ER in patients with GH-secreting pituitary adenomas. Cardiac hypertrophy can stimulate the NF-κB signaling pathway and transient outward K+ current, thereby promoting ER development.5–7 Thus, we suggest that increased LVMI and cardiac muscle hypertrophy can directly lead to ER development, and is not correlated with myocardial ischemia or valve disease.
LVMI is an accepted reference index for the diagnosis of ventricular hypertrophy. Studies have shown that IGF-1 induction and excessive synthesis of the IGF-1 receptor can lead to cardiac muscle cell hypertrophy.17,18 In normal adult rats, administration of exogenous GH and IGF-1 can induce cardiac hypertrophy. In addition, the effect is more prolonged as IGF-1 concentration and LVMI increase, and the symptoms of cardiac hypertrophy become more apparent.19 GH/IGF-I excess in young adult patients with gigantism and in patients with acromegaly is associated with morphologic and functional cardiac abnormalities including left ventricular hypertrophy.20 In GH-secreting pituitary adenoma patients, excessive IGF-1 secretion can induce a significant increase in LVMI. The primary reason for this is that both GH and IGF-1 can directly or indirectly act on cardiac muscle cells, increasing protein synthesis and the volume of cardiac muscle cells, thereby leading to cardiac muscle hypertrophy.21 In addition, excessive IGF-1 secretion can also cause the degeneration and necrosis of cardiac muscle cells, muscle fiber disorganization, lymphocyte and monocyte infiltration, and interstitial collagen deposition and fibrosis, further leading to ventricular expansion, ventricular wall thickening, and aggravation of left ventricular remodeling, thereby affecting cardiac function.22 In this study we found that course of disease, serum GH levels, serum IGF-1 levels, and presence or absence of diabetes were correlated with LVMI. Additionally, we found that the course of disease, serum IGF-1 levels, BMI, and the presence or absence of diabetes were dependent risk factors for increased LVMI, of which the course of disease and serum IGF-1 levels were independent risk factors leading to an increased LVMI in patients with GH-secreting pituitary adenomas. The results of this study and previous studies indicate that the high GH and IGF-1 levels of patients with GH-secreting pituitary adenomas have a long-term effect on the heart, causing gradual hypertrophy of cardiac muscle and changes in cardiac muscle structure, eventually leading to possible instability in cardiac muscle electrophysiology and ER, similar to previous results which showed that GH and IGF-1 could directly or indirectly lead to cardiac hypertrophy.23 Currently, there is no clear evidence that these factors can directly lead to the development of ER. Thus, we believe that long-term elevation of GH and IGF-1 levels may lead to cardiac hypertrophy, thereby indirectly leading to the development of ER.
When the six patients with GH-secreting pituitary adenomas with ER who underwent transsphenoidal pituitary adenoma resection were observed for 2 years, it was found that the incidence of ER was significantly decreased in patients with GH and IGF-1 levels that had returned to normal (proportion of ER was 1/6). This result shows that decreasing GH and IGF-1 levels could promote cardiac electrophysiological stability and reduce the development of LVMI and ER, again showing that high GH and IGF-1 levels could increase LVMI and lead to cardiac muscle cell hypertrophy, thereby inducing the development of ER.
There are several limitations to the data in this study. First, the ER prevalence was calculated based on all samples received from our hospital, and not based on the entire population of this region. In addition, no correlation between ER and myocardial ischemia or valve disease was found in patients with GH-secreting pituitary adenoma. Thus, whether myocardial ischemia and valve disease are risk factors affecting ER development requires further investigation with a larger sample size. Of note, our results indicated that diabetes was associated with the presence of ES by univariate logistic regression analysis. It is also possible that GH-secreting pituitary adenomas, causing hyperglycemia or diabetes, may also result in a downregulation of transient outward K+ current (Ito) of endocardium, and thus give rise to a transmural voltage gradient and J-wave on electrocardiography.24,25 Additionally, thyroid hormones which promote GH secretion were correlated with occurrence of ER.26 However, we did not study the effect of stated blood glucose and thyroid hormone level on ER occurrence. Finally, other limitations include not investigating lifestyle characteristics of the participants and the number of patients with GH-secreting pituitary adenomas who were followed-up was too low.
Conclusion
The present study showed that long-term stimulation with high concentrations of GH and IGF-1 is a risk factor for increased LVMI in patients with GH-secreting pituitary adenomas. Elevated LVMI is a risk factor for the development of ER in patients with GH-secreting pituitary adenomas. In summary, elevation of serum GH and IGF-1 in patients with GH-secreting pituitary adenomas had positive correlations with cardiac muscle cell hypertrophy and increased LVMI, which may be a cause of the increased incidence of ER. Increased follow-up for patients with GH-secreting pituitary adenomas should occur to monitor the development of ER, preventing the progression to malignant arrhythmia that can lead to cardiogenic sudden death.
Acknowledgments
This work was supported by grants from the Science and Technology Planning Project of Guangdong Province of China (no 2016A050502024).
Disclosure
The authors report no conflicts of interest in this work.
References
Matturri L, Varesi C, Nappo A, Cuttin MS, Rossi L. Sudden cardiac death in acromegaly. Anatomopathological observation of a case. Minerva Med. 1998;89(7–8):287–291. | ||
Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361(26):2529–2537. | ||
Kim SH, Kim DY, Kim HJ, et al. Early repolarization with horizontal ST segment may be associated with aborted sudden cardiac arrest: a retrospective case control study. BMC Cardiovasc Disord. 2012;12(1):1471–2261. | ||
Pieroni M, Bellocci F, Crea F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;359(7):761–762. | ||
Panama BK, Latour-Villamil D, Farman GP, et al. Nuclear factor kappaB downregulates the transient outward potassium current I(to,f) through control of KChIP2 expression. Circ Res. 2011;108(5):537–543. | ||
He Q, Feng Y, Wang Y. Transient outward potassium channel: a heart failure mediator. Heart Fail Rev. 2015;20(3):349–362. | ||
Seo J, Park J, Oh J, et al. High prevalence and clinical implication of myocardial bridging in patients with early repolarization. Yonsei Med J. 2017;58(1):67–74. | ||
Giustina A, Barkan A, Chanson P, et al. Guidelines for the treatment of growth hormone excess and growth hormone deficiency in adults. J Endocrinol Invest. 2008;31(9):820–838. | ||
Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(24):2558–2573. | ||
Grote E. Characteristics of giant pituitary adenomas. Acta Neurochir. 1982;60(3–4):141–153. | ||
Maione L, Garcia C, Bouchachi A, et al. No evidence of a detrimental effect of cabergoline therapy on cardiac valves in patients with acromegaly. J Clin Endocrinol Metab. 2012;97(9):E1714–E1719. | ||
Cheng YJ, Mei WY, Chen XM, et al. Long-term prognosis associated with early repolarisation pattern in Chinese population with atherosclerotic risk factors. Heart. 2017;103(12):910–916. | ||
Muramoto D, Yong CM, Singh N, et al. Patterns and prognosis of all components of the J-wave pattern in multiethnic athletes and ambulatory patients. Am Heart J. 2014;167(2):259–266. | ||
De Asmundis C, Conte G, Levinstein M, et al. Prevalence and electrocardiographic characteristics of early repolarization pattern in young teen athletes. Acta Cardiol. 2014;69(1):3–6. | ||
Boineau JP. The early repolarization variant – normal or a marker of heart disease in certain subjects. J Electrocardiol. 2007;40(1):3.e11–3.e13. | ||
Bartczak A, Lelonek M. Early repolarization variant in syncopal patients referred to tilt testing. Pacing Clin Electrophysiol. 2013;36(4):456–461. | ||
Horio T, Kamide K, Takiuchi S, et al. Association of insulin-like growth factor-1 receptor gene polymorphisms with left ventricular mass and geometry in essential hypertension. J Hum Hypertens. 2010;24(5):320–326. | ||
Halldin M, Brismar K, Fahlstadius P, Vikström M, de Faire U, Hellénius ML. The metabolic syndrome and ECG detected left ventricular hypertrophy – influences from IGF-1 and IGF-binding protein-1. PLoS One. 2014;9(12):e108872. | ||
Cittadini A, Strömer H, Katz SE, et al. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat. A combined in vivo and in vitro evaluation. Circulation. 1996;93(4):800–809. | ||
Bondanelli M, Bonadonna S, Ambrosio MR, et al. Cardiac and metabolic effects of chronic growth hormone and insulin-like growth factor I excess in young adults with pituitary gigantism. Metabolism. 2005;54(9):1174–1180. | ||
Colao A. Improvement of cardiac parameters in patients with acromegaly treated with medical therapies. Pituitary. 2012;15(1):50–58. | ||
Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102–152. | ||
Lu C, Schwartzbauer G, Sperling MA, et al. Demonstration of direct effects of growth hormone on neonatal cardiomyocytes. J Biol Chem. 2001;276(25):22892–22900. | ||
Sato T, Kobayashi T, Kuno A, et al. Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol Heart Circ Physiol. 2014;306(7):H1054–H1065. | ||
Gallego M, Alday A, Urrutia J, Casis O. Transient outward potassium channel regulation in healthy and diabetic hearts. Can J Physiol Pharmacol. 2009;87(2):77–83. | ||
Ueno A, Yamamoto T, Sato N, Tanaka K. Ventricular fibrillation associated with early repolarization in a patient with thyroid storm. J Interv Card Electrophysiol. 2010;29(2):93–96. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.