Back to Journals » Clinical Ophthalmology » Volume 17
Incidence, Pathophysiology, Complications, and Management of Positive Vitreous Pressure During Penetrating Keratoplasty: A Literature Review
Authors Alkharashi M, AlAbdulhadi HA, Otaif W , Alahmadi AS, Alanazi B, Al Habash A , Aldayel A, Aljindan M, Almulhim A, Bin Helayel H
Received 15 July 2022
Accepted for publication 20 December 2022
Published 14 February 2023 Volume 2023:17 Pages 583—590
DOI https://doi.org/10.2147/OPTH.S382502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Majed Alkharashi,1 Halla A AlAbdulhadi,2 Wael Otaif,1 Adel Salah Alahmadi,3,4 Bader Alanazi,5 Ahmed Al Habash,6 Ahmed Aldayel,2 Mohanna Aljindan,6 Abdulmohsen Almulhim,5 Halah Bin Helayel2
1Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 2Anterior Segment Division, King Khalid Eye Specialist Hospital, Riyadh, Saudi Arabia; 3Vitreoretinal Division, King Khalid Eye Specialist Hospital, Riyadh, Saudi Arabia; 4Department of Ophthalmology, MOH, Madinah, Saudi Arabia; 5Department of Ophthalmology, College of Medicine, Jouf University, Sakakah, Saudi Arabia; 6Department of Ophthalmology, College of Medicine, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Correspondence: Wael Otaif, Department of Ophthalmology, King Saud University, Riyadh, Saudi Arabia, Tel +966 549778998, Email [email protected]
Abstract: Positive vitreous pressure (PVP) is common during open anterior segment surgery and penetrating keratoplasty (PKP) has a reported incidence rate of 40– 50%. Despite adequate perioperative preventive precautions, positive pressure occurs during surgery and optimum management of PVP is required to avoid serious complications. Many pharmacological and mechanical approaches can be employed either preoperatively or intraoperatively to decrease vitreous pressure. Surgical techniques such as graft-over-host technique, the modified graft-over-host technique, techniques employed mattress sutures and needle, or Vitreous aspiration or vitrectomy can be effectively used to manage intraoperative PVP during PPK. This article reviews the incidence, risk factors, prevention, and different approaches to the management of positive vitreous pressure during PKP to analyze the available evidence in order to improve the safety profile of PKP and prevent sight-threatening complications.
Keywords: positive vitreous pressure, penetrating keratoplasty, complications, management, prevention
Introduction
Penetrating keratoplasty (PKP) is an effective procedure in low-risk corneal diseases with a success rate of over 90%.1 Despite the rapid growth of selective lamellar keratoplasty during the past decade, PKP remains an essential corneal transplantation procedure and is the optimal treatment for full-thickness corneal disease.2 As an open-sky surgical procedure, there is a significant risk of intraoperative complications in PKP due to positive vitreous pressure (PVP). This may lead to major intraoperative and postoperative sight-threatening complications such as vitreous loss, expulsion of the lens, and suprachoroidal or expulsive hemorrhage. These complications can preclude favorable outcomes following corneal transplantation.3–5 Prevention is the preferred approach for PVP management in intraocular surgery, particularly for PKP. Pharmacological and mechanical methods may be used either preoperatively or intraoperatively to decrease vitreous pressure. Despite adequate preventive measures, positive pressure often occurs during surgery.6 The management of intraoperative PVP during the majority of anterior segment procedures is usually effectively achieved through rapid wound closure, viscoelastic substance use, and reassessment. However, rapid wound closure during PKP is technically challenging, and other methods to manage PVP during PKP may be required.5,6 This article reviews the incidence, risk factors, prevention, and different approaches to the management of positive vitreous pressure during PKP to analyze the available evidence in order to improve the safety profile of PKP and prevent sight-threatening complications.
Incidence of PVP During PKP
A 40–50% incidence rate of PVP has been reported during penetrating keratoplasty (PKP).7–9 This rate may be higher in eyes with iris atrophy, pseudophakic eyes with a posterior capsular tear, shallow anterior chamber, weak zonules, or eyes that have been treated for acute angle-closure glaucoma.6,10
Pathophysiology of PVP
The main cause for intraoperative PVP during PKP is acute hypotony from open-sky surgery.6 Following the start of open anterior chamber surgery, there is a gradual increase in the vitreous cavity pressure relative to the decrease in vitreous cavity volume, which may be attributed to the external compression in the globe or intraoperative acute intraocular intumescence. A pressure difference is created between the vitreous cavity and the anterior chamber when there is simultaneous aqueous loss and hypotony. The pressure in the vitreous cavity will be considerably larger than that in the anterior chamber; thus, the content of the vitreous cavity will be pushed forward.6,10 In addition, acute and prolonged operative hypotony during PVP leads to an increase in transmural pressure and vessel rupture, resulting in suprachoroidal hemorrhage, which is sight-threatening.11–13 Purcell et al identified several risk factors for choroidal hemorrhage during penetrating keratoplasty, including hypertension, glaucoma, previous ocular trauma, inflammation or surgery, Valsalva maneuvers during surgery, operating under open sky technique, and history of previous ocular trauma.14 Advanced age, cardiovascular disease, long-term steroid use, and anticoagulant therapy are risk factors for choroidal hemorrhage.15 Moreover, poor choroidal vascular fragility in high myopia has been implicated in choroidal hemorrhage.13
Risk Factors of PVP
Ocular-Related Risk Factors
The incidence of PVP is increased in the presence of ocular disorders such as iris pathology, presence of zonular instability, shallow anterior chamber, or acute angle-closure glaucoma.6,16 Ruptured capsule or abnormal location of the Intraocular lens (IOL) outside the bag pose additional risks in patients with pseudophakia.16 Also, the presence of IOL or iris prolapse is associated with a 100% incidence of positive vitreous pressure in pseudophakic or aphakic eyes.17 In addition to poor rigidity, as in keratoconus, high myopia and buphthalmos contribute to the elevation of vitreous pressure.17 High axial length of the eye can cause a decreased orbital volume, leading to increased pressure on the globe. In addition, Short axial length and large crystalline lens are also risk factors.18 Orbital-related risk factors include reduction of the vitreous cavity from external compression during surgery from a handheld instrument pressing on the sclera, strong blink reflex,17 or lid speculum pressure on the eye will also predispose to increased vitreous pressure. Further orbital-related risk factors include an anatomically small orbit17 or orbital deformity; increased orbital tissue pressure, which may occur due to increased tissue or fluid volume within the orbit, such as retrobulbar or intraorbital hemorrhage; orbital congestion from inflammation or edema as in thyroid-related ophthalmopathy and orbital inflammatory syndromes; orbital tumors; and increased orbital fat in occurring in patients with marked obesity. A history of infectious keratitis, ocular inflammation, or glaucoma is also a risk factor for PVP during PKP. However, the underlying mechanism remains unknown.8
Anesthesia-Related Risk Factors
The rate of PVP is higher under peribulbar anesthesia than in general anesthesia.1 Further anesthesia-related risk factors include incomplete akinesia by local or general anesthesia or bucking under general anesthesia. Increased orbital pressure due to the large volume of local anesthesia contributes to PVP.16 Pillar et al reported 2 cases of increased PVP caused by the use of positive end-expiratory pressure (PEEP) during general anesthesia.20
Patient-Related Risk Factors
Increased systemic venous pressure due to a variety of causes including positioning of the head below the heart, congestive heart failure, pulmonary edema, obstructive sleep apnea, chronic obstructive pulmonary disease,20 Valsalva maneuver, severe obesity, a bull-necked patient poorly positioned so that venous pressure in the head is elevated, and rarely an arteriovenous fistula are predisposing factors for PVP.6 Younger age is also a predisposing factor for increased intraoperative positive vitreous pressure.8 Infants have less rigidity and increased elasticity of the cornea and sclera, which can contribute to the forward movement of the iris-lens diaphragm. Further risk factors include blood dyscrasias or coagulation defects, diabetes mellitus, a history of liver disease, and preoperative use of digoxin.19 Furthermore, reports has found that patients with anxiety are at a higher risk of increased PVP.17
Surgeon-Related
It has been suggested that the length of time the globe is open is a risk factor predisposing to elevated vitreous pressure.8 In addition, the external compression during surgery from a handheld instrument pressing on the sclera will result in PVP. The risk factors for PVP are summarized in Table 1.
 |
Table 1 Risk Factors of PVP |
Complications
Several serious complications can result from a positive vitreous pressure during penetrating keratoplasty.3–5 These complications include capsular rupture, vitreous loss, peripheral anterior synechiae, subsequent secondary angle-closure glaucoma, iris sphincter trauma, cataract formation, zonular damage, lens loss, endothelial trauma, and difficulty in suturing the corneal graft due to contact with the iris or lens. If keratoplasty is combined with extracapsular cataract extraction, difficult cortical removal and posterior capsular rupture with vitreous loss are more likely.3–5 Suprachoroidal hemorrhage is one of the most serious complications of PVP during PKP.12 Extrusion of intraocular content is further possible serious complication.21
Prevention
The initial step in preventing PVP during PKP depends on identifying and controlling modified risk factors.6 Several preoperative and intraoperative measures have been described to decrease PVP.
Preoperative measures can be used, including pharmacological agents such as intravenous mannitol 20% 1–1.5 mg/kg or oral glycerin to shrink and dehydrate the vitreous volume.22 Moreover, ensuring adequate akinesia with the peribulbar or retrobulbar deep block using the least volume with supplementary hyaluronidase and external ocular pressure for 30 minutes using Honan’s balloon, rubber ball, or external ocular digital pressure may also be helpful.4,22 At the beginning of surgery, avoiding positioning the patient, particularly obese patients, in the Trendelenburg position to avoid increased thoracic and intraorbital pressure.20
Additionally, appropriate placement of the eyelid speculum is important and lateral canthotomy/cantholysis may also be considered in a tight orbit.22 Intra-operative measures include injection of high molecular weight ophthalmic viscoelastic device (OVD) through paracentesis to preserve the anterior chamber.3 If OVD fails to maintain anterior chamber depth, aspiration of anterior vitreous through pars plana before complete trephination has been described as an effective and safe technique in controlling PVP during PKP.10,23 Additionally, scleral support using Flieringa ring, particularly in the aphakic eyes, vitrectomized eyes, or low scleral rigidity (eg, infant and children), has been described as a useful method to maintain the globe during PKP.24 The use of the Flieringa ring before trephination is also helpful for expanding the sclera and preventing globe collapse and subsequent expulsion of intraocular contents. The Flieringa ring is often secured to the globe with an interrupted suture through radially oriented short bites into the conjunctiva, episcleral, and superficial sclera, and positioned directly below the ring. Although rare, reported complications with the Flieringa ring include scleral perforation, irregular graft recipient bed, and high astigmatism.24
Anesthesia
Optimal anesthesia for penetrating keratoplasty should provide adequate analgesia and complete akinesia, and prevent intraocular pressure (IOP) fluctuations, eyelid squeezing, and intraoperative coughing and movement. It is also important to avoid iatrogenic injury, abate the oculocardiac reflex, and avoid postoperative nausea and vomiting (PONV). The choice of anesthetic technique is influenced by the patient factors, surgeon preference, and anesthesiologist expertise.25
Local anesthesia is generally preferred because it is a simple and low-cost procedure, suppresses the oculocardiac reflex, and is associated with less PONV compared to general anesthesia. However, the drawbacks associated with local anesthesia are lack of patient full cooperation and difficulty remaining immobile for over 2 hours, anxiety, and the limited time available to treat coexisting anterior chamber pathology, which negatively affect the success rate of PKP.21,25 The volume and technique used during local anesthesia influence IOP. IOP is required to be stable within the normal or low range perioperatively. The larger the volume of anesthetic, the higher the increase in IOP.25 Evidence indicates that IOP elevation is the highest with peribulbar blocks (5–22 mmHg), followed by retrobulbar blocks (4–6 mmHg), while sub-Tenon blocks had no considerable effects on IOP. Eyelid block can be very helpful in providing akinesia of the orbicularis oculi muscle to prevent eyelid squeezing, which may elevate IOP by > 50 mmHg.25
In contrast, general anesthesia is suitable for children and adults who cannot lie flat, or have tremor or claustrophobia. A further advantage is the lack of time restrictions on surgery. However, general anesthesia carries the risk of PONV. The type of anesthetic used also influences IOP. Induction of anesthesia with thiopentone, volatiles, opioids, and propofol decreases IOP, while ketamine elevates IOP by 2–3 mmHg and should be avoided in PK. Maintaining the airway with a laryngeal mask provides smooth induction and emergence with fewer IOP fluctuations and a lower incidence of postoperative coughing. However, it is associated with a higher likelihood of PONV and has a time limit of 2 hours, as the airway is not completely secured. Endotracheal intubation can elevate IOP and cause coughing at emergence, although it has a lower incidence of PONV.25 The reported incidence of expulsive suprachoroidal hemorrhage was similar after local and general anesthesia; however, cases undergoing local anesthesia involved shorter procedures and were less complex than general anesthesia.26 Topical anesthesia is rarely used and may be suitable for repeated PK, as the corneal sensation is already compromised.25,27
Management
Graft-Over-Host Technique
Numerous techniques to manage PVP during PKP have been described in literature. In 1998, the graft-over-host technique was first introduced and named after the main surgeon, Price.4 In this procedure, as in a standard PKP, 80–90% of the host cornea is trephined, and the anterior chamber is subsequently entered using a 15-degree blade. Castroviejo corneal scissors is used to complete trephination. If any evidence of significant positive pressure is encountered, the cornea is incised and immediately closed using one or two interrupted 10–0 nylon sutures. Once a complete trephination of the host cornea is ensured, and adequate sutures are placed, a copious amount of viscoelastic is placed over the host cornea, over which the donor cornea is sutured at the 12, 3, and 6 o’clock positions, leaving the 9 o’clock position unsutured to facilitate host cornea removal afterward. Immediately after cutting and removing each suture stabilizing the host cornea, another suture is placed over it in the donor cornea to keep it in place. The host cornea is carefully removed and a 10–0 nylon suture is placed immediately. Interrupted or continuous sutures were placed to complete surgery. This technique was performed in 33 eyes with different diagnoses including keratoconus, Fuchs’ dystrophy, and herpes simplex virus (HSV)/herpes zoster virus (HZV) keratitis. No incidence of primary or secondary graft failure was identified during the follow-up period (mean= 14.63 months). The authors suggested that the absence of graft failure in their study was most likely due to the generous use of modern viscoelastic.
The Modified Graft-Over-Host Technique
In 2014, a modified graft-over-host technique was described by Dekaris et al1 In this technique, following signs of positive pressure such as iris prolapse from one quadrant, the host cornea is covered with a generous amount of methylcellulose viscoelastic. The donor cornea is then sutured with four interrupted 9–0 nylon sutures at the 12, 3, 6, and 9 o’clock positions. A continuous 10–0 nylon suture is used to close the donor cornea after incising the host cornea quadrant by quadrant, starting with the quadrant where a full-thickness incision was made. Once the third quadrant is sutured, the fourth quadrant is carefully incised and the host cornea is removed. The remaining quadrant is immediately sutured. All surgical procedures described by the authors were uneventful. This technique was performed in eight patients with identified intraoperative PVP out of 220 eyes, 6 of whom had keratoconus. In addition, the authors prospectively examined graft success rate, endothelial cell loss, and corneal astigmatism. There was no statistically significant difference in the success rates between the conventional PKP and modified graft-over-host (MGOH) techniques 2 years after surgery. The reduction in endothelial cell count was similar in both groups in the first postoperative month, and at 1 year and 2 years. Corneal astigmatism was significantly higher in the group operated on using the MGOH technique, and the best-corrected visual acuity was slightly lower than that in patients undergoing the conventional PKP technique. This difference was statistically insignificant once sutured and the eyes were fitted with contact lenses.
Techniques Employed Mattress Sutures and Needle
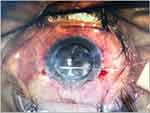
Another technique for managing positive pressure involves placing a temporary retaining suture across the anterior chamber. This technique was introduced in 1979, when Simcoe28 described the use of triangular or quadrangular sutures to prevent IOL from touching the endothelium. In 2020, Cheung et al17 described a modified technique of placing the sutures called “Basket” mattress sutures performed in 15 eyes. The variations of the technique depend on the lens status and timing of quadrangular suture placement (ie, before or after trephination). A technique similar to Simcoe’s is performed in aphakic or pseudophakic eyes. Once partial full-thickness trephination is performed, and signs of positive pressure are encountered, viscoelastic material is injected into the anterior chamber to push the iris/IOL complex back. A 10–0 nylon suture is then passed at the limbus level from one side of the pupil and externalized from the other side of the pupil at the limbus level. The second pass is made parallel to the first, externalized, and tied. The assistant may help secure the IOL back into place while passing through the suture. The distance between the two entry points is not as important as in phakic eyes. A second mattress suture is placed perpendicular to the first suture. This technique addresses iris prolapse but not vitreous prolapse. A closed system is ensured in phakic eyes by placing sutures to close the PKP wound. Intracameral Miochol can be injected, so the iris holds the lens back and minimizes the likelihood of the suture chafing the lens. Viscoelastic material is injected into the anterior chamber to maintain it. Then, the entry sites of the mattress sutures are marked 1–2 mm on either side of the pupil. A double-armed 9–0 or 10–0 polypropylene suture with long curved needles is inserted on either side of the pupil, just above and parallel to the iris. A bent 27-gauge needle can be inserted halfway through the anterior chamber, where the long-curved needle can be docked and externalized through the opposite sides and tied. An alternative method is to create four paracenteses at similar sites as in the former technique. A 27-gauge cannula is inserted across the anterior chamber, where a 10–0 nylon needle is docked after flattening. While the cannula protects the lens and iris, the needle is brought across the anterior chamber and externalized from the opposite side. A similar procedure can be performed using the remaining two paracenteses and the tied suture. Another mattress suture is placed perpendicular to the first suture to create a safe basket configuration. If the risk factors for positive vitreous pressure are identified preoperatively, a mattress suture can be placed prophylactically (ie, before trephination). The corneal button can then be removed, and the donor cornea can be sutured in place after placing a generous amount of viscoelastic material over the suture. In this case series, PKP was successful in all cases without damage to the intraocular structures by sutures. There was no acceleration of cataract formation in any of the cases, and the grafts were clear without primary graft failure, except in one case with persistent corneal edema, corneal epithelial defect, and stem cell deficiency. One patient experienced graft rejection that was successfully treated with frequent topical corticosteroid drops. The mean follow-up duration in this case series was 8.1 months (range 1–20 months), limiting the long-term identification of possible sequelae. In addition, the endothelial cell count was not measured pre-or postoperatively to determine the possible impact of the sutures on the endothelium. Moreover, in pseudophakic eyes, the intraocular lens can be held back in position by passing the straight long needle of the 10–0 prolene suture (STC, Ethicon) across the anterior chamber.3 (Figure 1)
Vitreous Aspiration or Vitrectomy
Another method to reduce intraocular pressure is vitreous aspiration or vitrectomy. Different techniques, such as vitreous aspiration through the pupil or pars plana and using a needle or automated vitrector, have been investigated.5,10,29 However, vitreous tap is a complex procedure and more challenging in an open sky. One way to decompress the vitreous in a closed system is to perform a vitreous tap after partial trephination of the host cornea (before full-thickness penetration). Vongthongsri et al10 first published a prospective case series (65 patients) using this technique in a triple procedure. A 23-gauge needle in a 5 mL syringe was used to aspirate the vitreous and inserted 3.5 mm away from the limbus. The tip of the needle was observed in the anterior to middle vitreous humor. The intended volume for aspiration was 1 mL (range 0.3–1.5 mL).10,23,30 If aspiration of the vitreous was difficult, the needle was moved carefully under the microscope until a pocket of liquid was found. The triple procedure was then performed regularly. The authors found no evidence of significant PVP in any of the patients. The rate of vitreous loss was reduced from 30% to 0%, and vitreous tapping-related complications were not observed. Although the authors claimed that the postoperative complications and adverse effects were similar to those in previous reports without PVP, the study did not illustrate the signs or risk factors of PVP in the patients before conducting the vitreous tap. In another prospective randomized study with a control group (total = 32 patients), core vitrectomy was performed where sclerotomy was performed, and a vitreous cutter was used to excise 0.3 mL.7 The sclerotomy site was closed using an 8–0 nylon suture before the start of the triple procedure. There was no statistically significant difference in any of the parameters between the two groups, although a higher success rate of IOL implantation in the capsular bag was slightly higher in the core vitrectomy group.7 Preoperative measures, such as proper ocular massage and systemic medications to decrease intraocular pressure, may have been sufficient and negated any added effect of the core vitrectomy.7
Conclusion
Penetrating keratoplasty plays an important role in treating full-thickness corneal diseases where selective lamellar keratoplasty cannot be accomplished. PVP during PKP remains challenging despite adequate perioperative preventive precautions. Preoperative identification of the risk factors, and different approaches to the management of positive vitreous pressure during PKP is crucial for planning the surgery, improve the safety profile, and prevent sight-threatening complications.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dekaris I, Gabric N, Pauk M, Drača N. Positive pressure during penetrating keratoplasty can be solved with a modified graft-over-host technique. Acta Ophthalmol. 2014;92(3):282–285. doi:10.1111/AOS.12085
2. Tan DTH, Dart JKG, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi:10.1016/S0140-6736(12)60437-1
3. McCartney DL, Gottsch JD, Stark WJ. Managing posterior pressure during pseudophakic keratoplasty. Arch Ophthalmol. 1989;107(9):1384. doi:10.1001/archopht.1989.01070020454054
4. Loden JC, Price J. Price graft-over-host technique to manage positive pressure during penetrating keratoplasty. J Cataract Refract Surg. 1998;24(6):736–738. doi:10.1016/S0886-3350(98)80123-5
5. Gross RH, Shaw EL. Management of increased vitreous pressure during penetrating keratoplasty using pars plana anterior vitreous aspiration: author’s reply. Cornea. 2002;21(4):435–436. doi:10.1097/00003226-200205000-00027
6. Chronopoulos A, Thumann G, Schutz J. Positive vitreous pressure: pathophysiology, complications, prevention, and management. Surv Ophthalmol. 2017;62(2):127–133. doi:10.1016/J.SURVOPHTHAL.2016.10.002
7. Konomi K, Shimazaki J, Shimmura S, Akabane N, Goto E, Tsubota K. Efficacy of core vitrectomy preceding triple corneal procedure. Br J Ophthalmol. 2004;88(8):1023–1025. doi:10.1136/bjo.2003.033902
8. Shimomura Y, Hosotani H, Kiritoshi A, Watanabe H, Tano Y. Core vitrectomy preceding triple corneal procedure in patients at high risk for increased posterior chamber pressure. Jpn J Ophthalmol. 1997;41(4):251–254. doi:10.1016/S0021-5155(97)00046-4
9. Baratz KH, Pulido JS. Vitreous tapping for positive pressure. Ophthalmology. 2006;113(3):501–502. doi:10.1016/J.OPHTHA.2005.11.014
10. Vongthongsri A, Jakpaiwong W, Preechanon A, Lekhanont K, Chuck RS. Anterior vitreous tapping to manage positive vitreous pressure during triple procedures. Ophthalmology. 2005;112(5):875–878. doi:10.1016/j.ophtha.2004.12.027
11. Chu TG, Green RL. Suprachoroidal hemorrhage. Surv Ophthalmol. 1999;43(6):471–486. doi:10.1016/S0039-6257(99)00037-5
12. Bandivadekar P, Gupta S, Sharma N. Intraoperative suprachoroidal hemorrhage after penetrating keratoplasty: case series and review of literature. Eye Contact Lens. 2016;42(3):206–210. doi:10.1097/ICL.0000000000000164
13. Glazer LC, Williams GA. Management of expulsive choroidal hemorrhage. Semin Ophthalmol. 1993;8(2):109–113. doi:10.3109/08820539309060218
14. Purcell JJ, Krachmer JH, Doughman DJ, Bourne WM. Expulsive hemorrhage in penetrating keratoplasty. Ophthalmology. 1982;89(1):41–43. doi:10.1016/S0161-6420(82)34859-9
15. Park Y, Kim MH, Won JY, Kim HS, Park YH. Vitreoretinal complications after penetrating keratoplasty. Retina. 2016;36(11):2110–2115. doi:10.1097/IAE.0000000000001049
16. Huang X, Zhou Q, Wang S, Zhang J, Niu G, Bi Y. Stepwise decreasing of vitreous pressure by anterior vitrectomy: a novel method for preventing positive vitreous pressure during penetrating keratoplasty. Adv Ther. 2020;37(1):617–629. doi:10.1007/S12325-019-01139-6
17. Cheung AY, Davis AR, Denny MR, et al. “Basket” mattress suture to manage positive vitreous pressure during penetrating keratoplasty. Can J Ophthalmol. 2020;55(6):509–517. doi:10.1016/J.JCJO.2020.06.012
18. Chalam KV, Shah VA. Successful management of cataract surgery associated vitreous loss with sutureless small-gauge pars plana vitrectomy. Am J Ophthalmol. 2004;138(1):79–84. doi:10.1016/j.ajo.2004.02.018
19. Lavinsky F, Moisseiev J, Levkovitch-Verbin H. The surgical management of massive intraoperative and postoperative suprachoroidal hemorrhage: anatomic and functional outcomes. Arq Bras Oftalmol. 2013;76(4):212–214. doi:10.1590/S0004-27492013000400003
20. Pillar A, Jeng BH, Munir WM. Positive end-expiratory pressure as a risk factor for severe positive vitreous pressure during combined penetrating keratoplasty and cataract extraction. Cornea. 2016;35(11):1491–1494. doi:10.1097/ICO.0000000000000945
21. Wang X, Dang GF, Li YM, Li WF, Wu XY. General anesthesia versus local anesthesia for penetrating keratoplasty: a prospective study. Int J Ophthalmol. 2014;7(2):278–282. doi:10.3980/j.issn.2222-3959.2014.02.15
22. Casey TA, Mayer DJ. Corneal Grafting: Principles & Practice. Saunders; 1984:352.
23. Gross RH, Shaw EL. Management of increased vitreous pressure during penetrating keratoplasty using pars plana anterior vitreous aspiration. Cornea. 2001;20(3):251–254. doi:10.1097/00003226-200104000-00003
24. Young AL, Leung GYS, Cheng LL, Cheng ACK, Lam DSC. Modification of Flieringa ring fixation. Eye. 2004;19(5):608. doi:10.1038/sj.eye.6701650
25. Chua AWY, Chua MJ, Kam PCA. Recent advances and anaesthetic considerations in corneal transplantation. Anaesth Intensive Care. 2018;46(2):162–170. doi:10.1177/0310057X1804600204
26. Price FW, Whitson WE, Ahad KA, Tavakkoli H. Suprachoroidal hemorrhage in penetrating keratoplasty. Ophthalmic Surg. 1994;25(8):521–525. doi:10.3928/1542-8877-19940801-09
27. Segev F, Voineskos AN, Hui G, et al. Combined topical and intracameral anesthesia in penetrating keratoplasty. Cornea. 2004;23(4):372–376. doi:10.1097/00003226-200405000-00011
28. Simcoe CW. Retaining devices for protection of corneal endothelium. J Am Intraocul Implant Soc. 1979;5(3):234–236. doi:10.1016/s0146-2776(79)80124-x
29. Schanzlin DJ, Smith RE. Management of an intact vitreous face in penetrating keratoplasty. Ophthalmic Surg. 1983;14(5):427–428.
30. Nossair AA, Ewais WA, Ali LS. Retrospective study of vitreous tap technique using needle aspiration for management of shallow anterior chamber during phacoemulsification. J Ophthalmol. 2017;2017. doi:10.1155/2017/2801025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.