Back to Journals » Lung Cancer: Targets and Therapy » Volume 11
Incidence of ROS1-Rearranged Non-Small-Cell Lung Carcinoma in India and Efficacy of Crizotinib in Lung Adenocarcinoma Patients
Authors Mehta A , Saifi M, Batra U , Suryavanshi M, Gupta K
Received 31 December 2019
Accepted for publication 11 February 2020
Published 24 February 2020 Volume 2020:11 Pages 19—25
DOI https://doi.org/10.2147/LCTT.S244366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Anurag Mehta, 1 Mumtaz Saifi, 2 Ullas Batra, 3 M Suryavanshi, 2 Kush Gupta 4
1Laboratory Services, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India; 2Department of Molecular Pathology, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India; 3Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India; 4Catalyst Clinical Services, New Delhi, India
Correspondence: Anurag Mehta
Laboratory Services, Rajiv Gandhi Cancer Institute and Research Centre, Sector V, Rohini, New Delhi 110085 Tel +91 9868 020 371
Email [email protected]
Background: The ROS1 gene is a member of the “sevenless” subfamily of tyrosine-kinase insulin-receptor genes. ROS1-fusion rearrangement causes constitutive downstream signal transduction, with an oncogenic role in non-small-cell lung carcinoma (NSCLC). Fortunately, crizotinib, an ALK1 tyrosine-kinase inhibitor, provides long-term disease control. The objective of this molecular epidemiological study was to estimate the frequency of ROS1 rearrangements and evaluate treatment outcomes with crizotinib therapy.
Methods: Patients with stage IV NSCLC adenocarcinoma histology were considered for this study. The study was conducted according to the ethical principles stated in the latest version of the Declaration of Helsinki and the applicable guidelines for good clinical practice. Clinical characteristics and treatment details were collected from patients’ medical records.
Results: A total of 709 stage IV NSCLC adenocarcinoma patients were included in the study. There were 457 (64.46%) men and 252 (35.54%) women, with a median age of 60 years. ROS1-gene rearrangement was positive in 20 (2.82%) cases, 13 using Fluorescent In-Situ Hybridization (FISH), and two and five cases, respectively, using immunohistochemistry (IHC) and next-generation sequencing (NGS), followed by confirmation with FISH. Fourteen of the 20 patients with ROS1-gene rearrangement received crizotinib therapy, with an objective response rate of 64.28%. At a median follow-up of 6 months, the study had not achieved the end points of median progression free survival and overall survival.
Conclusion: ROS1-gene rearrangement was present at a relatively higher frequency of 2.8% in north Indian patients with lung adenocarcinoma and was successfully targeted by crizotinib therapy. Although the only US Food and Drug Administration and Conformité Européenne approved method for testing ROS1 rearrangement is NGS, FISH alone or IHC with D4D6 antibody as initial screen with subsequent confirmation of IHC-positive cases by FISH are cost-effective methods in institutions lacking NGS facilities.
Keywords: NSCLC, ROS1, crizotinib
Introduction
With an overall 5-year survival rate of just 15%, lung cancer is the leading cause of cancer-related mortality across the globe.1 According to a GLOBOCAN 2018 report, lung cancer was the largest contributor to new cases (2.09 million) and cancer-related deaths (1.76 million) among all cancers.2 More than 70% of lung carcinomas are detected in the advanced stage. The molecular characterization of advanced non-small-cell lung cancer (NSCLC) followed by treatment with a corresponding inhibitor has become a well-established treatment strategy. The benefit in progression-free survival (PFS) and better quality of life through genome-directed therapy has raised the notion of “give the maximum number of patients a chance at genetic alteration–directed therapy”. It has been asserted that 64% of NSCLC patients harbour at least one activated pathway, with approximately two-thirds of these are actionable using available approved or off-label targeted therapies.3–5 The frequency of individual driver mutations, however, is population-specific. KRAS is the commonest driver in the Western population, but EGFR takes this place in Asian populations. ALK-fusion rearrangement, on the other hand, has more uniform distribution.5–8 ROS1-fusion rearrangement, the third actionable genetic change, despite occurring far less commonly, has evoked considerable interest, due to excellent objective response rates (ORRs; 72%) and substantial PFS of 19.3 months to ALK tyrosine-kinase inhibitors.9–12 Such gratifying results in ROS1-rearranged NSCLC necessitate a closer look at its incidence and response to a first-generation ALK tyrosine-kinase inhibitor (crizotinib) in different populations. The frequency of ROS1 rearrangement has been reported in Western literature to be around 1%. However, ROS1 incidence has not been widely reported from the Indian subcontinent.
Registration trials for ROS1-fusion rearrangement detection utilized fluorescence in situ hybridization (FISH), which is considered the gold standard. However, no testing methodology, assay system, or assay platform was given US Food and Drug Administration (FDA) approval till recently, when Oncomine Target Dx, a next-generation sequencing (NGS)-based multigene panel was accorded approval for EGFR-sensitizing mutations, BRAFV600E mutations, and ROS1-fusion rearrangement.13–15 College of American Pathologists–International Association for the Study of Lung Cancer–Association for Molecular Pathology guidelines recognize immunohistochemistry (IHC) as a cost-effective screening tool, to be followed by FISH confirmation in positive cases. IHC with ROS1 (D4D6) rabbit monoclonal antibody (Cell Signaling Technology, Cambridge, UK) stained with Ventana benchmark XT immunostainer has sensitivity of 100% and specificity of 92%.16–18 However, the inadmissibly high false positivity rates necessitate FISH or NGS confirmation. We undertook this molecular epidemiological study to estimate the prevalence of ROS1 rearrangements and evaluate treatment outcomes with crizotinib therapy in Indian lung adenocarcinoma patients.
Methods
Patients with stage IV NSCLC adenocarcinoma histology for the period May 2012 to June 2019 were considered for this study. Permission was obtained from the Institutional Review Board of Rajiv Gandhi Cancer Institute and Research Centre. The informed-consent requirement was waived, as the research was conducted on anonymized patient samples/data. The study was conducted according to the ethical principles stated in the latest version of the Declaration of Helsinki and applicable guidelines for good clinical practice. Clinical characteristics and treatment details were collected from patients' medical records.
FISH alone was performed on 498 cases. FISH was assayed on 4μm formalin-fixed, paraffin-embedded tumor tissue using a dual-color break-apart probe (ZytoLight Spec ROS1; ZytoVision, Germany), according to the manufacturer’s instructions.15,17 The ZytoLight Spec ROS1 has been designed to detect translocations involving chromosomal region 6q22.1 harboring ROS1. It contains two directly labeled probes hybridizing to the 6q22.1 band. While the orange-fluorochrome directly labeled probe hybridizes distally, the green-fluorochrome directly labeled probe hybridizes proximally to the ROS1-breakpoint region of 6q22.1. FISH signal evaluation was performed using fluorescent microscopy (Leica DM6000 B) equipped with three filters (DAPI, green, red). FISH results were based on a minimum of 50 evaluable tumor cells. Fused, split, or isolated green/orange signals were detected and enumerated (Figure 1). The rearrangement-positive cell rate was defined as ([number of cells with a split pattern + number of cells with isolated 3ʹ] {green}] pattern/total number of cells evaluated) × 100. A cutoff of at least 15% break-apart and/or isolated green events was used as the threshold for ROS1 FISH positivity. The ROS1 tyrosine-kinase domain is encoded by the 3ʹ part of the gene. The unpaired 3ʹ signal indicates the relevant oncogenic fusion gene, whereas the unpaired 5ʹ signal represents a likely nonfunctional reciprocal fusion product. As such, isolated 5ʹ signals were not included in the total count.
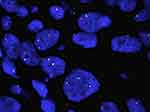 |
Figure 1 ROS1 fluorescence in situ hybridization. |
Formalin-fixed, paraffin-embedded section of 4 μm thickness following fixation for 6–48 hours in neutral buffered formalin and conventional tissue processing were stained by IHC for ROS1 protein expression using rabbit monoclonal antibody to ROS1 clone D4D6 (Cell Signaling Technology) on a Ventana benchmark XT immunostainer (Ventana Medical Systems, Tuscon, AZ, USA). Slides were pretreated with EDTA buffer (pH 8.3) for 48 minutes and incubated with the primary mAb at a dilution of 1:100 for 40 minutes at 37°C. Detection was performed using an OptiView DAB IHC detection kit (Ventana Medical Systems). Moderate–strong granular cytoplasmic staining was considered positive, and these cases proceeded to confirmation by FISH using the aforementioned method. In sum, 111 cases were tested using IHC as screening method.
NGS was performed using an Ion AmpliSeq RNA-fusion lung cancer research panel (Thermo Fisher Scientific), which targets 70 fusion transcriptsspecific for lung cancerbelonging to ALK, RET, ROS1, and NTRK1 genes. A total of 100 cases were tested by NGS. All positive cases were orthogonally validated using FISH as a reference method. Statistical analysis was performed using Pearson's χ2 or Fisher’s exact test, whichever was appropriate for categorical variables. Logistic regression was performed to compare the study groups. Two-sided p<0.05 was considered significant. Binary logistic regression with single independent variables was performed, and thus statistical correction was not applied to the p-values. Statistical analysis was performed using SAS version 9.4.
Results
A total of 709 stage IV NSCLC adenocarcinoma patients were included in the study. There were 457 (64.46%) men and 252 (35.54%) women, with a median age of 60 years. Of the 709 patients, 228 (32.16%) were smokers and 78 (11%) never-smokers. Baseline patient characteristics are presented in Table 1. Of the 709 cases, 498 were tested using FISH, whereas 111 and 100 each were tested using IHC or NGS, due either to the physician’s choice of test or restricted availability of tissue. Result of molecular testing are shown in Table 2. ROS1-gene rearrangement was positive in 20 (2.82%) cases. Thirteen of 20 positive cases of ROS1-gene arrangements were identified using FISH as the first definitive test. Two cases were recognized through IHC screening followed by confirmatory FISH and five cases by NGS with subsequent validation by FISH. The association of each individual factor with regard to ROS1-gene rearrangement is shown in Table 3. ROS1-gene rearrangement showed significant associations with age, cigarette smoking, IHC, and morphology (p<0.05). Table 4 presents the distribution of ROS1-gene rearrangement across the study population. Smoking density could not be calculated, as patients were reticent in fully disclosing their smoking history.
 |
Table 1 Summary of Patient Demographics and Tumour Characteristics (N=709) |
 |
Table 2 Results of Molecular Testing |
 |
Table 3 Association of Each Factor Vis-à-Vis ROS1-Gene Rearrangement |
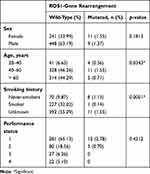 |
Table 4 Distribution of ROS1-Gene Rearrangement |
Fourteen of the 20 patients with ROS1-gene rearrangement received crizotinib therapy, whereas six patients could not afford the therapy due to financial constraint. Of the 14 patients who received crizotinib therapy, only five patients received it as first-line therapy and nine as second-line therapy. Among those who received crizotinib therapy, nine (64.28%) achieved partial response, three (21.43%) stable disease, and two (14.28%) progressive disease. There were four deaths, two each in patients who achieved partial response and progressive disease. Duration of response was 1.5 to14 months. At a median follow-up of 6 months, the study had not achieved the endpoints of median PFS (Figure 2) and overall survival (OS; Figure 3). Estimated 1-year PFS and OS were 56.2% and 36.9%, respectively.
 |
Figure 2 Progression-free survival with crizotinib therapy. |
 |
Figure 3 Overall survival with crizotinib therapy. |
Discussion
The use of molecular profiling–based targeted therapies has significantly improved median PFS outcomes in NSCLC. The excellent response rate of crizotinib in ROS1-rearranged NSCLC has gained significant attention in the recent past. Here, we report the frequency of ROS1 rearrangement and treatment outcomes in an Indian population. We found a 2.82% incidence of ROS1-gene rearrangement among 709 stage IV NSCLC adenocarcinoma patients. Two previous studies from India have reported the prevalence of ROS1 rearrangements to be 2.9%19 and 4.1%20 usingFISH. The prevalence of ROS1 rearrangements in Asian NSCLC populations has been reported to be 1.54%–2.59%.16,21,22 Similar prevalence of 1.7%–2.5% has been reported for Caucasian NSCLC populations.13,23 The prevalence (2.82%) of ROS1-gene rearrangement in our study is consistent with previously published reports for Indian, Asian, and Caucasian populations, with the exception of one study from India that reported much higher prevalence of 4.1%.20
In the present study, a higher ROS1 gene–rearrangement rate was observed in females than males (1.55% vs 1.27%), but the results were not statistically significant. In contrast to this observation, a previous study from India had all three female patients as higher,19 whereas another reported dominance for males (13 of 22).20 We observed a positive correlation of ROS1-gene rearrangement in the never-smoker group compared to smokers (1.13% vs 0.14%, p=0.0001). Although all age-groups were affected, there was a positive correlation for the age-group 40–60 years (1.55%, p=0.0343). There was no specific association with morphology type (papillary, acinar lepidic, solid), and thus it cannot be used as a selection criterion for testing. Previously published studies for Asian and Caucasian populations also reported a higher rate of ROS1-gene rearrangement in younger, never-smoker, female patients and adenocarcinoma histology.22,24–26
Although oncogenic drivers in NSCLC such as EGFR, ALK, and ROS1 rearrangements, are mutually exclusive, there have been few reports on concomitant existence of EGFR–ALK,27–29 EGFR–ROS1,16 and ALK–ROS1 mutations.13 Two cases in the present study also had concurrent EGFR mutation with ROS1-gene rearrangement. IHC serves as a rapid and cost-effective alternative to FISH, especially in low-resource settings. Although ROS1 IHC readouts may lead to false-positive results, due to aneuploidy, two cases in our study were recognized through IHC screening, and both were found to be ROS1-positive on subsequent confirmation by FISH.
The PROFILE 1001 study found a very high ORR of 72% with crizotinib therapy among 50 patients with ROS1-rearranged NSCLC.30 The study also demonstrated a very high disease-control rate of 90% and median PFS of 19.2 months, leading to the FDA approval of crizotinib for the treatment of advanced ROS1-rearranged NSCLC. Updated results of PROFILE 1001 showed similar ORR and median PFS among 53 patients.31 Three subsequent studies of crizotinib in ROS1-rearranged NSCLC showed shorter median PFS of 9–13.4 months.32–34 Updated results of the AcSé phase II trial showed a higher best ORR of 69.4% during treatment in 37 patients of an ROS1-translocation cohort.35 Similarly, the METROS phase II trial also showed a very high ORR of 65% and median PFS of 22.8 (95% CI 15.2–30.3 months with crizotinib therapy among 26 patients with ROS1-rearranged pretreated NSCLC.36 Two studies from India have reported a good response to crizotinib therapy.19,20 In one study, crizotinib therapy achieved an ORR of 93.8%, with 1- and 2-year OS of 72% and 54%, respectively.20 A third study from India also showed a good response rate of 80% with crizotinib therapy in ROS1-rearranged NSCLC patients.37 At a median follow-up of 9 months, median PFS and OS had not been reached. In our study, 14 (70%) of the 20 patients with ROS1-gene rearrangement received crizotinib therapy. With an ORR of 64.28% and clinical benefit rate of 85.71%, the results of our study show much lower ORR than previously published reports. At a median follow-up of 6 months, the study had not achieved the end points of median PFS and OS, and the same shall be presented in future publications.
There were four (28.57%) deaths in our study, two each in patients with partial response and progressive disease, and no grade 3/4 toxicities. Updated results of PROFILE 100131 showed progressive disease/deaths in 26 (49%) patients, with no grade 3/4 treatment-related adverse events. Similarly, two studies from India showed progressive disease on first assessment in one (6.25%)20 and two (66.66%)19 patients, along with no grade 3/4 treatment-related adverse events. More recently, entrectinib has shown clinical activity in patients with locally advanced or metastatic ROS1 fusion–positive NSCLC. Entrectinib is an ROS1 inhibitor that has been designed to penetrate effectively and remain in thecentral nervous system. In an integrated analysis of three phase I–II trials, 41 (77%) of 53 locally advanced or metastatic ROS1 fusion–positive NSCLC patients had objective response with entrectinib at a dose of at least 600 mg orally once per day. Median duration of response was 24.6 months with a manageable safety profile. However, these findings need confirmation in randomized controlled clinical trials with a much larger patient population.38
Conclusion
Our study reports data on ROS1-gene rearrangement for Indian patients with lung adenocarcinoma using IHC, NGS, and FISH techniques. The incidence of ROS1-gene rearrangement (2.82%) in this Indian population was consistent to that previously reported and supports the clinical utility of crizotinib therapy in this patient subgroup. The inclusion of IHC for initial screening of ROS1-gene rearrangement followed by confirmation using FISH seems justified in low-resource settings.
Disclosure
The authors report no conflicts of interest in this work. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi:10.3322/CA.2007.0010
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
3. Tsao AS, Papadimitrakopoulou VA. The future of NSCLC: molecular profiles guiding treatment decisions. Oncology. 2011;25:1–3.
4. Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small‐cell lung cancers into routine clinical practice. Ann Oncol. 2011;22:
5. Kris MG, Johnson BE, Kwiatkowski DJ. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI’s Lung Cancer Mutation Consortium (LCMC). J Clin Oncol. 2011;29:Abstr CRA7506. doi:10.1200/jco.2011.29.15_suppl.cra7506
6. Okamoto I, Mitsudomi T, Nakagawa K, Fukuoka M. The emerging role of epidermal growth factor receptor (EGFR) inhibitors in first-line treatment for patients with advanced non-small cell lung cancer positive for EGFR mutation. Ther Adv Med Oncol. 2010;2:301–307. doi:10.1177/1758834010370698
7. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small cell lung cancer. Nature. 2007;448:561–566. doi:10.1038/nature05945
8. Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK associated with development of lung adenocarcinomas lacking EGFR and K-RAS mutations is correlated with ALK expression. Mol Cancer. 2010;9:188. doi:10.1186/1476-4598-9-188
9. Moro-Sibilot D, Cozic N, Pérol M, et al. OA12.03 activity of crizotinib in MET or ROS1 positive (+) NSCLC: results of the AcSe trial. J Thorac Oncol. 2018;13(10):S348. doi:10.1016/j.jtho.2018.08.301
10. Solomon BJ, Kim DW, Wu YL, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36(22):2251–2258. doi:10.1200/JCO.2017.77.4794
11. Lim SM, Kim HR, Lee JS, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35(23):2613–2618. doi:10.1200/JCO.2016.71.3701
12. Doebele R, Ahn M, Siena S, et al. OA02.01 efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC). J Thorac Oncol. 2018;13(10):S321–S322. doi:10.1016/j.jtho.2018.08.239
13. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi:10.1200/JCO.2011.35.6345
14. Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi:10.1038/nm.2658
15. Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18:4570–4579. doi:10.1158/1078-0432.CCR-12-0550
16. Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18:4449–4457. doi:10.1158/1078-0432.CCR-11-3351
17. Bubendorf L, Büttner R, Al-Dayel F, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 2016;469:489–503. doi:10.1007/s00428-016-2000-3
18. Sholl LM, Sun H, Butaney M, et al. ROS1 immunohistochemistry for detection of ROS1-rearranged lung adenocarcinomas. Am J Surg Pathol. 2013;37:1441–1449. doi:10.1097/PAS.0b013e3182960fa7
19. Suryavanshi M, Panigrahi MK, Kumar D, et al. ROS1 rearrangement and response to crizotinib in stage IV non-small cell lung cancer. Lung India. 2017;34(5):411–414. doi:10.4103/lungindia.lungindia_116_17
20. Joshi A, Pande N, Noronha V, et al. ROS1 mutation non-small cell lung cancer-access to optimal treatment and outcomes. Ecancermedicalscience. 2019;13:900. doi:10.3332/ecancer.2019.900
21. Zeng L, Li Y, Xiao L, et al. Crizotinib presented with promising efficacy but for concomitant mutation in next-generation sequencing-identified ROS1-rearranged non-small-cell lung cancer. Onco Targets Ther. 2018;11:6937–6945. doi:10.2147/OTT
22. Zhang Q, Wu C, Ding W, et al. Prevalence of ROS1 fusion in Chinese patients with non-small cell lung cancer. Thorac Cancer. 2019;10(1):47–53. doi:10.1111/tca.2019.10.issue-1
23. Wiesweg M, Eberhardt WEE, Reis H, et al. High prevalence of concomitant oncogene mutations in prospectively identified patients with ROS1-positive metastatic lung cancer. J Thorac Oncol. 2017;12(1):54–64. doi:10.1016/j.jtho.2016.08.137
24. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi:10.1038/nature13385
25. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi:10.1038/nm.4333
26. Chen YF, Hsieh MS, Wu SG, et al. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol. 2014;9:1171–1179. doi:10.1097/JTO.0000000000000232
27. Tanaka H, Hayashi A, Morimoto T, et al. A case of lung adenocarcinoma harboring EGFR mutation and EML4-ALK fusion gene. BMC Cancer. 2012;12:558. doi:10.1186/1471-2407-12-558
28. Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer. 2012;77(2):460–463. doi:10.1016/j.lungcan.2012.04.012
29. Popat S, Vieira de Araújo A, Min T, et al. Lung adenocarcinoma with concurrent exon 19 EGFR mutation and ALK rearrangement responding to erlotinib. J Thorac Oncol. 2011;6(11):1962–1963. doi:10.1097/JTO.0b013e31822eec5e
30. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi:10.1056/NEJMoa1406766
31. Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–1126. doi:10.1093/annonc/mdz131
32. Moro-Sibilot D, Faivre L, Zalcman G, et al. Crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). Preliminary results of the ACSé phase II trial. J Clin Oncol. 2015;33(suppl):abstr 8065. doi:10.1200/jco.2015.33.15_suppl.8065
33. Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi:10.1200/JCO.2014.58.3302
34. Goto K, Yang JC, Kim DW, et al. Phase II study of crizotinib in East Asian patients (pts) with ROS1-positive advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(suppl):abstr 9022. doi:10.1200/JCO.2016.34.15_suppl.9022
35. Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol. 2019;30:1985–1991. doi:10.1093/annonc/mdz407
36. Landi L, Chiari R, Tiseo M, et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): a Phase II, prospective, multicenter, two-arms trial. Clin Cancer Res. 2019;25(24):7312–7319. doi:10.1158/1078-0432.CCR-19-0994
37. Noronha V, Chandrakanth MV, Joshi AP, et al. ROS1 rearranged nonsmall cell lung cancer and crizotinib: an Indian experience. Indian J Cancer. 2017;54(2):436–438. doi:10.4103/ijc.IJC_269_17
38. Drilon A, Siena S, Dziadziuszko R, et al. trial investigators. entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2019.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
