Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Incidence of Hepatotoxicity and Factors Associated During Highly Active Antiretroviral Therapy in People Living with HIV in Ethiopia: A Prospective Cohort Study
Authors Gebremicael G , Tola HH , Gebreegziaxier A, Kassa D
Received 1 October 2020
Accepted for publication 15 March 2021
Published 25 March 2021 Volume 2021:13 Pages 329—336
DOI https://doi.org/10.2147/HIV.S283076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Gebremedhin Gebremicael, Habteyes Hailu Tola, Atsbeha Gebreegziaxier, Desta Kassa
HIV and TB Diseases Research Directorate, Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia
Correspondence: Gebremedhin Gebremicael
Ethiopian Public Health Institute, P.O.Box: 1242, Addis Ababa, Ethiopia
Tel +251-91-3345 910
Email [email protected]
Introduction: Hepatotoxicity is one of the risk factors associated with treatment non-adherence, which is the main risk factor for drug resistance. Therefore, this study aimed to determine the incidence and risk factors of hepatotoxicity during highly active antiretroviral therapy (HAART) among people living with HIV in Ethiopia.
Methods: A prospective cohort study was conducted between April 2007 and January 2011 at Saint Peter Specialized Hospital, Akaki and Kality Health Centers, Addis Ababa, Ethiopia. A total of 316 HIV-infected adult individuals (70 participants were HIV and TB co-infected and 246 were infected with HIV alone) were included in this study. The study participants were followed for a total of 18 months with or without HAART. Socio-demographic data were collected using a structured questionnaire, and venous blood samples were collected for laboratory tests. Logistic regression and Poisson regression were used to determine the independent effect of each variable on hepatotoxicity at baseline and end of follow-up.
Results: Of 316 HIV-infected people, 72 (22.8%) participants had an elevated ALT/AST which was 100% mild-to moderate hepatotoxicity at baseline. Baseline CD4 T-cell count (p = 0.027) and HIV co-infection with TB (p < 0.001) were independently associated with hepatotoxicity at baseline. The overall incidence rate of hepatotoxicity in participants on HAART (21.8 per 100 person-years) was lower than participants who were HAART naïve (33.3 per 100 person-years) (p = 0.009).
Conclusion: High incidence of mild-to-moderate hepatotoxicity and low severe hepatotoxicity were observed in HIV-infected individuals who were on HAART or were HAART naïve. HAART may minimize the occurrence of hepatotoxicity. Although HAART could minimize hepatotoxicity among HIV-infected people, to manage mild and moderate hepatotoxicity liver function test monitoring is required.
Keywords: hepatotoxicity, HAART
Introduction
An estimated 36.9 million people were living with human immunodeficiency virus (HIV)/AIDS at the end of 2017 with significant national and regional variation.1 In 2017, about 53% of people living with HIV worldwide resided in the Sub-Saharan Africa region.1 After the introduction of highly active antiretroviral therapy (HAART), HIV-associated morbidity and mortality have been dramatically reduced and the burden of opportunistic infections (OIs) is considerably decreased.2
Despite the excellent tolerability of ART regimens, HAART has been associated with adverse drug events including drug-induced liver injury (hepatotoxicity).3 Hepatotoxicity is a potential risk factor of poor adherence to HAART, which is the main risk for drug-resistant viral strain development. Moreover, poor treatment adherence is the main risk of virologic and immunological failures which can cause a complex situation in medical decision making.4 Virologic and immunological failures might occur as a result of drug resistance, leading to treatment shifting from first line to second line regimens, which are more expensive and less likely to be accessed by patients in resource-limited countries.5 The WHO recommendation is to test and treat by monitoring ART using viral load tests.6 This increases a risk for developing toxicity due to lifelong treatment. Therefore, management of HAART-related toxicity becomes a major obstacle in the care and treatment of HIV/AIDS.
Previous studies demonstrated that adverse drug reactions, low CD4 count, chronic hepatitis B and C infections, anti-tuberculosis treatment and immune reconstitution inflammatory syndrome (IRIS) are the risk factors for hepatotoxicity in individuals infected with HIV and on HAART.7–11 Moreover, evidence indicated that high baseline levels of alanine aminotransferase (ALT), cirrhosis, alcohol consumption, age and sex are the risk factors associated with hepatotoxicity in individuals infected with HIV and on HAART.7–11 However, there is contradictory evidence in the literature on the effect of adverse drug reactions or drug class.12 Moreover, studies also fail to demonstrate a consistent magnitude of severe hepatotoxicity occurring in HAART-experienced and naive individuals.13 Although many people living with HIV are on HAART in Ethiopia, there is limited evidence on the incidence and risk factors for hepatotoxicity in people living with HIV to support patients’ care and treatment management. Thus, this study aimed to determine the incidence and risk factors for hepatotoxicity in people living with HIV and on HAART in Ethiopia.
Materials and Methods
Study Design and Study Population
A prospective cohort study was conducted between April 2007 and January 2011 in Saint Peter Specialized Hospital, Akaki and Kality Health Centers, Addis Ababa, Ethiopia. A total of 316 HIV-infected individuals (70 HIV-TB co-infected (HIV+TB+) and 246 HIV-only infected (HIV+TB-) adult participants were included from a previous study.14 At recruitment, all participants were interviewed using a structured questionnaire to collect demographic and clinical characteristics. Demographic and clinical characteristics for this study were extracted from the previous study’s database. Pregnant women, individuals who had co-morbidity with diabetes mellitus or chronic bronchitis, individuals receiving steroid therapy and receiving TB and/or HAART treatment at recruitment or previously, and individuals who had a history of alcohol or drug abuse were excluded. In addition, four individuals co-infected with Hepatitis B virus (HBV) and Hepatitis C virus (HCV) were excluded after enzyme linked immunosorbent assay was done. All active TB cases confirmed at enrollment were treated according to the national TB treatment guidelines.15 All participants were followed for 18 months with or without HAART. Of 316 study participants, 166 (52.5%) started HAART at the beginning of the study and were on HAART until the end of the follow-up. However, 76 (24.1%) were naïve for HAART up to the end of the follow-up. Furthermore, 74 (23.4%) participants were lost to follow-up or their HAART status was not known. In this study, baseline was defined as participants who were enrolled at month zero of the study.
CD4 count was determined by flow cytometry using a FACS Calibur Flow cytometer (Becton Dickinson, San Jose, USA) while plasma HIV RNA viral load was determined using the NucliSensEasyQ NASBA diagnostic kit (OrganonTeknica, the Netherlands: detection range 50–3,000,000 copies/mL). Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) levels were measured at baseline and end of the follow-up using a Cobas® 6000(Roche Diagnostics Corporation, USA) automated immunochemistry analyser. However, of the 316 total participants, liver function tests of 74 participants were not performed at the end of follow-up due to either small volume of sample or participants being lost to follow-up.
Hepatotoxicity has five grades: grades 0, I, II, III and IV. Grade 0 is an increase in aminotransferase level <1.25 times the upper limit of normal range (ULN), and grade I is an increase in aminotransferase level 1.25–2.5 times the ULN range for those who had normal aminotransferase levels at baseline.16 Grade II is an increase in aminotransferase level 2.6–5.0 times ULN range, while grade III is an increase in aminotransferase level 5.1–10.0 times ULN range, and grade IV is an increase in aminotransferase level >10 times ULN range for those who had normal aminotransferase levels at baseline.16 The ULN ranges in the laboratory of the Ethiopian Public Health Institute are 41, 38 and 104 U/L (international units per liter) for ALT, AST and ALP respectively. For patients with abnormal aminotransferase levels or ALP at baseline, hepatotoxicity was defined as a two-fold increase from baseline levels.16 Hepatotoxicity was defined as elevation of the aminotransferases above the ULN range, but ALP was not considered in the definition of hepatotoxicity because it is non-specific to liver injury.
Statistical Analysis
All statistical analyses were performed using STATA version 12.0 (College Station, Texas, USA). Categorical variables were compared by Fisher’s exact test or the Chi-square test. Non-categorical variables were compared by Mann–Whitney U-test. Potential risk factors with a p-value of ≤ 0.2 and biologically/clinically important were included in the multivariate analysis. Logistic regression model was used to determine factors associated with hepatotoxicity. All comparisons were two-tailed and a p-value < 0.05 was considered as significant.
Results
Baseline Characteristics of the Participants
Among the 316 HIV-infected participants, 192 (61%) were female. The median age at baseline was 32 years, with an age range of 15–65 years, and the median CD4 T-cell count was 203 cells/μL.
There was no significant difference in baseline concentration of ALT and AST between the patients who completed follow-up or were lost to follow-up. However, the baseline concentration of ALP was significantly higher in those patients who completed the follow-up compared to those lost to follow-up (Table 1).
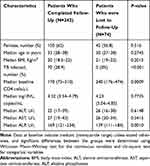 |
Table 1 Baseline Characteristics of the Study Participants |
Baseline Proportion of Hepatotoxicity
Overall proportion of hepatotoxicity was 22.8% with 12.34% grade I and 10.44% grade II. Of 70 HIV+TB+ participants, 34 (48.6%) had evidence of hepatotoxicity (impaired liver function tests [LFTs]) with 100% mild-to-moderate toxicity (Grades I and II). Of 70 HIV+TB+ participants, 20 (28.6%) had elevated ALP without aminotransferases (ALT and/or AST) elevation.
Of 246 HIV+TB-, 38 (15.4%) participants had evidence of mild-to-moderate hepatotoxicity (Table 2). HIV+TB+ participants had a high proportion of hepatotoxicity compared with those HIV+TB- (p < 0.001). However, there was no significant difference between participants eligible (21.7%) and ineligible (29%) for HAART at baseline (p = 0.219).
 |
Table 2 Baseline Proportion of Hepatotoxicity Among the Study Population |
Multiple Risk Factors for Hepatotoxicity at Baseline
Baseline CD4 T-cell count (OR = 1.57; 95% confidence interval [CI] (1.05–2.35), p = 0.027) and being TB/HIV co-infected (OR = 4.74; 95% CI (2.53–8.88), p < 0.001) were independently associated with hepatotoxicity prevalence (Table 3).
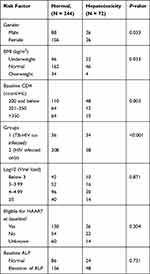 |
Table 3 Associated Factors/Predictors of Hepatotoxicity Proportion at Baseline |
Incidence of Hepatotoxicity After the Initiation of HAART
The mean length of follow-up period was 665 days (SD: 132 days). Of 316 study participants, 166 (52.5%) started HAART at the beginning of the study and were on HAART up to the end of follow-up. However, 76 (24.1%) were naïve for HAART up to the end of follow-up, whereas 74 (23.4%) were lost to follow-up or their HAART status was not known. At the end of follow-up, the ALT, AST and ALP concentrations of the study participants (both on HAART and HAART naïve) were significantly increased from baseline (Table 4).
 |
Table 4 Characteristics of the Study Participants at End of Follow-Up Compared with Baseline |
At the end of follow-up, there was no significant difference in the concentrations of ALT, AST and ALP between those participants on HAART and HAART naïve (p = 0.0509, 0.0971 and 0.7481, respectively). Moreover, there was no significant difference in the concentration of ALT (p = 0.082), AST (p = 0.683) and ALP (p = 0.501) between HIV+TB+ and HIV+TB- participants who were HAART naïve. However the concentrations of AST (p = 0.001) and ALP (p = 0.028) were significantly different between HIV+TB+ and HIV+TB- participants who were on HAART.
At the end of follow-up, 112 (46.3%) participants had greater than 1.25 times ULN range of ALT and/or AST levels. However, 20 (17.9%) participants had been with abnormal ALT and/or AST at baseline and they were not two-fold or greater increased from baseline levels at end of follow-up. These patients were not classified as incidence of hepatotoxicity according to the definition. Thirty-two (13%) of the participants had an elevated ALP without an elevated aminotransferase. Overall 92 (38%) participants including those with two-fold or greater increase from baseline levels developed hepatotoxicity. Of those who developed hepatotoxicity 34.7% had mild-to-moderate hepatotoxicity, and 3.3% had severe hepatotoxicity (Table 5). There was a statistically significant difference in the incidence of hepatotoxicity between the participants on HAART and those not on HAART (p = 0.009) (Table 5).
 |
Table 5 Incidence of Hepatotoxicity Between the HAART Received and Naïve at the End of Follow-Up |
Risk Factors for Hepatotoxicity
All variables included in the Poisson regression model were not significantly associated with hepatotoxicity (Table 6). However, after pooling participants on HAART and naïve, having a high baseline ALP concentration (incidence rate ratio (IRR) = 0.6; 95% CI (0.4–0.9), p = 0.017) was significantly associated with hepatotoxicity.
 |
Table 6 Risk Factors/Predictors of Hepatotoxicity Incidence |
Discussion
HAART is important to decrease HIV/AIDS related morbidity and mortality among people living with HIV.2 Nonetheless, the use of HAART is often complicated by adverse drug reactions. This study shown the overall prevalence of hepatotoxicity at baseline was 22.8% and this was 100% with mild-to-moderate toxicity. At the end of follow-up (1.5 years) the incidence rate of hepatotoxicity was 21.8 per 100 person-years with 20.9 per 100 person-years of mild-to-moderate hepatotoxicity and 0.8 per 100 person-years of severe hepatotoxicity among participants on HAART. The incidence rate of hepatotoxicity of those HAART naïve (33.3 per 100 person-years) was higher than the rate of hepatotoxicity in those who received HAART. Age, gender, baseline CD4 T-cell count, HAART plus Anti TB treatment, baseline toxicity, baseline viral load and BMI were not significantly associated with hepatotoxicity.
The baseline prevalence of hepatotoxicity was consistent with studies in Ethiopia, Cameroon and South Africa.17–19 However, the current study result is contradictory with a similar study reported in Nigeria.13 The high prevalence of toxicity in Nigeria might have resulted from including a high number of participants who were using traditional/herbal medicines, which were excluded from the current study. A study reported from Uganda also showed lower hepatotoxicity than the current studyat the baseline among patients initiating HAART.20 This difference is probably due to most of the study participants in the previous study receiving cotrimoxazole prophylaxis, which was excluded from our study at enrollment.21
In this study, the finding of high hepatotoxicity prevalence at baseline was associated with lower CD4 T-cell count. This is consistent with previous study findings reported from Nigeria and Ethiopia.13,18 In contrast to our finding, previous investigators have reported lower hepatotoxicity at baseline,22,23 which might be related to the fact that involvement of the immune system during infection could result in damage to liver cells (hepatocellular) by autoimmune diseases. In contrast, enhanced immunity could also decrease opportunistic infections at the beginning of HIV infection, contributing to the reduction of liver enzymes.
High hepatotoxicity at baseline was associated with TB diseases. The mechanism and the direction of development of liver enzyme elevation and TB diseases is not clear. Patients with liver cirrhosis are at a higher risk of developing both pulmonary and extra-pulmonary tuberculosis,24 with dysfunction in most of the reticuloendothelial cells that are central to clearing bacteria. On the other hand, liver disease can also occur due to tuberculosis.25 Our results showed the CD4 T-cell count in HIV patients co-infected with TB was significantly lower than HIV mono infection at baseline (data not shown). This was similar with the previous study. This is most probably due to T-cell expression associated genes being lower in HIV-TB co-infected patients than in HIV mono infected patients.26 Moreover, TB disease aggravates the HIV-related cell depletion mechanism and HIV-infection progression,27 which might contribute to the autoimmune diseases that lead to increase in liver enzymes.
The overall incidence of hepatotoxicity was consistent with previous studies reported from elsewhere.16 The incidence rate of severe hepatotoxicity in the present study differed from previous studies.28–30 The possible reason for the difference could be that HIV co-infection might be contributing to the higher liver enzyme elevation29 and low exposure to opportunistic infection could be contributing to the lower liver enzyme elevation (Sub Saharan vs America and Europe).28,30
In the present study, the incidence of hepatotoxicity in participants on HAART was lower than participants who were HAART naïve. Lucien et al. demonstrated that HAART was found to be associated with low levels of hepatotoxicity, regardless of drug class or combination,17 which is similar with our finding. The probable reason is not clear, but it might be related to the fact that HAART is important to restore the immune system and decreases opportunistic infection during HIV infection, which contributes to the reduction of liver enzymes levels. Our result showed that CD4 T-cell count in participants on HAART was significantly increased from baseline to the end of follow-up. Whereas, CD4 T-cell count in participants who were HAART naïve was not significantly increased from baseline to the end of follow-up. However, CD4 T-cell count and other independent variables such as age, gender, baseline, HAART plus Anti TB treatment, baseline toxicity, baseline viral load and BMI were not significantly associated with hepatotoxicity. This indicated the presence of other internal factors which are related to the presence of hepatotoxicity.
This study design was a prospective cohort study, so may have some limitations. Almost one fourth of the study participants were lost to follow-up, and this may have an impact on the result. However, there was no significant difference in proportion of hepatotoxicity between those participants lost to follow-up and those not lost to follow-up at baseline.
In conclusion, the overall prevalence of hepatotoxicity at baseline was 22.8% and this was 100% with mild-to-moderate hepatotoxicity. HIV co-infection with TB and low CD4 T-cell count were independent predictors of high prevalence of hepatotoxicity at baseline. At the end of follow-up (1.5 years), high incidence of mild-to-moderate toxicity and low severe hepatotoxicity were observed in HIV-infected participants on HAART or HAART naïve. HAART may be reducing the incidence of hepatotoxicity. Age, gender, baseline CD4 T-cell count, HAART plus Anti TB treatment, baseline toxicity, baseline viral load and BMI were not significantly associated with hepatotoxicity. Monitoring and management of the liver function test in people living with HIV is recommended.
Data Sharing Statement
All necessary data generated or analyzed during this study are included in this article.
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki. All study participants provided written informed consent and assent for those under the age of 18 from a parent or legal guardian on their behalf at enrollment. The previous study obtained ethical clearance from the Scientific and Ethics Research Office of Ethiopian Public Health Institute (Ref. EPHI 6.13/268), and the Health Research Ethics Review Committee of the Ministry of Ethiopian Science and Technology (Ref. RDHE/54-75/2002). The current study obtained ethical clearance from the Ethiopian Public Health Institute Sciences Ethics Review Committee (Ref. EPHI 7.15/2019) to use the stored sample.
Acknowledgments
The authors would like to acknowledge all participants who took part in this cohort study and all the nurses who collected data for their tolerance and strength.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Ethiopian Public Health Institute.
Disclosure
The authors declare that there is no conflicts of interest.
References
1. UNAIDS. Global HIV & AIDS statistics — 2018 fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet.
2. CDC. National Center for HIV/AIDS, Viral hepatitis, STD, and TB prevention. Division of AIDS prevention. Mortality Slide Series. https://www.cdc.gov/hiv/pdf/statistics_surveillance_hiv_mortality.pdf.
3. Sanne I, Mommeja-Marin H, Hinkle J, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191(6):825–829. doi:10.1086/428093
4. Zhang S, Rust G, Cardarelli K, Felizzola J, Fransua M, Stringer HG
5. Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24(6):915–919. doi:10.1097/QAD.0b013e3283360976
6. World Health Organization. Consolidated Guidelines on the Use Of Antiretroviral Drugs for Treating and Preventing HIV Infection. World Health Organization; 2016.
7. Sulkowski MS. Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S90–97. doi:10.1086/381444
8. Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377(9772):1198–1209. doi:10.1016/S0140-6736(10)62001-6
9. Kontorinis N, Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev. 2003;5(1):36–43.
10. den Brinker M, Wit FW, Wertheim-van Dillen PM, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14(18):2895–2902. doi:10.1097/00002030-200012220-00011
11. Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283(1):74–80. doi:10.1001/jama.283.1.74
12. Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186(1):23–31. doi:10.1086/341084
13. Hamza M, Adamu S, Maifada Y, et al. Prevalence and risk factors for hepatotoxicity among patients with HIV/AIDS on highly active antiretroviral therapy in North-Western Nigeria. Sub-Saharan Afr J Med. 2014;1(4):175–184. doi:10.4103/2384-5147.144727
14. Kassa D, de Jager W, Gebremichael G, et al. The effect of HIV coinfection, HAART and TB treatment on cytokine/chemokine responses to Mycobacterium tuberculosis (Mtb) antigens in active TB patients and latently Mtb infected individuals. Tuberculosis. 2016;96:131–140. doi:10.1016/j.tube.2015.05.015
15. WHO. Tuberculosis, Leprosy and TB/HIV Prevention and Control Programme Manual.
16. Tseng Y-T, Yang C-J, Chang S-Y, et al. Incidence and risk factors of skin rashes and hepatotoxicity in HIV-infected patients receiving nevirapine-containing combination antiretroviral therapy in Taiwan. Int J Infect Dis. 2019;29:12–17. doi:10.1016/j.ijid.2014.08.012
17. Lucien K, Clement A, Fon N, Weledji P, Ndikvu C. The effects of antiretroviral treatment on liver function enzymes among HIV-Infected out-patients attending the central hospital of Yaounde, Cameroon. Afr J Clin Exp Microbiol. 2010;11(3):1595–1689.
18. Shiferaw MB, Tulu KT, Zegeye AM, Wubante AA. Liver enzymes abnormalities among Highly Active Antiretroviral Therapy Experienced and HAART naive HIV-1 infected patients at Debre Tabor Hospital, North West Ethiopia: a Comparative Cross-Sectional Study. AIDS Res Treat. 2016;2016:7. doi:10.1155/2016/1985452
19. Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21(10):1301–1308. doi:10.1097/QAD.0b013e32814e6b08
20. Kalyesubula R, Kagimu M, Opio KC, et al. Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting. Afr Health Sci. 2011;11(1):16–23.
21. Pol S, Lebra P, Vallet PA. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Inf Dis. 2004;38(Supplement_2):S65–S72. doi:10.1086/381499
22. Nelson DR, Marousis CG, Davis GL, et al. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158(3):1473–1481.
23. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101(1):76–82. doi:10.1111/j.1572-0241.2005.00341.x
24. Saigal S, Nandeesh HP, Agarwal SR, Misra A, Jain SK, Sarin SK. High prevalence and profile of tuberculosis in chronic liver disease patients. Gastroenterology. 1998;114:A38. doi:10.1016/S0016-5085(98)80156-X
25. Sonika U, Kar P. Tuberculosis and liver disease: management issues. Trop Gastroenterol. 2012;33(2):102–106. doi:10.7869/tg.2012.25
26. Gebremicael G, Kassa D, Quinten E, et al. Host gene expression kinetics during treatment of tuberculosis in HIV-coinfected individuals is independent of HAART therapy. J Infect Dis. 2018;218(11):1833–1846. doi:10.1093/infdis/jiy404
27. Elston JW, Thaker HK. Co-infection with human immunodeficiency virus and tuberculosis. Indian J Dermatol Venereol Leprol. 2008;74(3):194–199. doi:10.4103/0378-6323.41362
28. Wood S, Byrne M, Deiss R, et al. the incidence and risk factors associated with chronic liver enzyme elevation (cLEE) in HIV-monoinfected persons. Open Forum Infect Dis. 2017;4(suppl_1):S220–S221. doi:10.1093/ofid/ofx163.453
29. Torti C, Lapadula G, Casari S, et al. Incidence and risk factors for liver enzyme elevation during highly active antiretroviral therapy in HIV-HCV co-infected patients: results from the Italian EPOKA-MASTER Cohort. BMC Infect Dis. 2005;5(1):58. doi:10.1186/1471-2334-5-58
30. de Castro N, Braun J, Charreau I, et al. Incidence and risk factors for liver enzymes elevations in highly treatment-experienced patients switching from enfuvirtide to raltegravir: a sub-study of the ANRS-138 EASIER trial. AIDS Res Ther. 2016;13(1):17. doi:10.1186/s12981-016-0101-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
