Back to Journals » Cancer Management and Research » Volume 10
Incidence of bone metastasis and factors contributing to its development and prognosis in newly diagnosed renal cell carcinoma: a population-based study
Authors Guo Q , Zhang C , Guo X , Tao F , Xu Y, Feng G, Han X, Ren Z, Zhang H, Zhang P, Wang X , Wang G
Received 4 April 2018
Accepted for publication 29 May 2018
Published 28 August 2018 Volume 2018:10 Pages 2935—2944
DOI https://doi.org/10.2147/CMAR.S170083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Qi Guo,1,2,* Chao Zhang,1,* Xu Guo,1,3,* Fang Tao,1 Yao Xu,1 Guowei Feng,4 Xiuxin Han,1 Zhiwu Ren,1 Hui Zhang,2 Pingfang Zhang,2 Xin Wang,5 Guowen Wang1
1Department of Bone and Soft Tissue Tumors, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin, China; 2Department of Orthopedics, General Hospital of North China Petroleum Administration, Renqiu, China; 3Department of Orthopedics, Cangzhou Central Hospital, Cangzhou, China; 4Department of Urology, Tianjin Key Laboratory of Breast Cancer Prevention and Therapy, Ministry of Education, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China; 5Department of Epidemiology and Biostatistics, First Affiliated Hospital, Army Medical University, Chongqing 400038, China
* These authors contributed equally to this work
Purpose: The purpose of this study was to assess the incidence of and the risk factors and prognostic factors for bone metastasis (BM) in initial metastatic renal cell carcinoma (RCC) based on a large population analysis.
Patients and methods: Data were obtained for a total of 45,824 RCC patients recorded in the database of the Surveillance, Epidemiology, and End Results program of the National Cancer Institute between 2010 and 2014. Multivariate logistic and Cox regression analyses were used to identify the risk factors and prognostic factors associated with BM in RCC patients. Kaplan–Meier analysis was used to estimate the overall survival of RCC patients, and the difference between the survival curves was tested by log-rank tests.
Results: A total of 1,509 (3.29%) patients were diagnosed with bone metastases at initial diagnosis. Male gender, higher T stage, lymph node involvement, poor tumor grade, presence of lung, liver, and brain metastases, and the collecting duct type of RCC were positively associated with BM occurrence. The median survival time for RCC patients with bone metastases was 12.0 (95% confidence interval [CI]: 10.69–13.31) months, and the survival time for those with collecting duct, clear-cell, papillary, and chromophobe subtypes of RCC were 3 (95% CI: 0.23–5.77), 13 (95% CI: 11.60–14.40), 8 (95% CI: 5.09–10.91), and 11 (95% CI: 5.02–16.98) months; these differences were significantly different (P<0.01). Older age, higher T stage, lymph node involvement, poor tumor grade, the presence of lung, liver, and brain metastases, collecting duct RCC, and the absence of surgical treatments were the factors associated with worse prognoses.
Conclusion: BM was highly prevalent and significantly decreased the survival rate of RCC patients. A number of factors associated with the development and prognosis of BM were identified, and these insights provide preventive guidelines for screening and treatment of BM in RCC patients
Keywords: bone metastases, risk factor, initial renal cell carcinoma, prognostic factor, SEER
Introduction
Renal cell cancer (RCC) represents 3% of all malignant tumors and accounts for 80%–85% of primary renal neoplasms.1 The 5-year survival rate of RCC is reported to be <10%, and surgical treatment is an accepted therapy for improving disease-free survival and patients’ quality of life.2,3 Around one-third of RCC patients experience synchronous metastatic disease, while 20%–30% of the remaining RCC patients experience metachronous metastatic disease.2 Bone metastasis (BM) has been reported to occur in 20% of metastatic RCC. BM, which leads to the occurrence of skeletal-related events (SREs), can significantly increase morbidity and decrease patients’ quality of life.2,4
Among metastatic RCC patients, bone is involved in 20%–35% of patients. It was reported that only 2% of RCC patients with BM required surgery.5 Early recognition and evaluation have provided better quality of life and prevented complications.2 However, assessment of BM is not performed routinely in RCC patients. Thus, a reliable predictive system for BM in RCC is warranted.
A multicentric report based on the analysis of 470 patients suggested that age, Eastern Cooperative Oncology Group status, histology, Memorial Sloan Kettering Cancer Center score, time from nephrectomy to BM, and the presence of concomitant metastases are factors significantly associated with prognosis.6 After analyzing 94 patients, Kume et al identified another five risk factors for predicting the survival of RCC patients with BM, including RCC sarcomatoid differentiation, spinal involvement, extra-osseous metastases, alkaline phosphatase level >1.5 times the normal level, and C-reactive protein level >0.3 mg/dL.7 To summarize the clinical characteristics of metastatic RCC and to integrate the symptom-based survival predictive system, studies evaluating the risk factors and prognostic factors for BM in RCC patients are needed.
In our study, the Surveillance, Epidemiology, and End Results (SEER) database was used to assess the incidence and the risk factors for BM in initial RCC. At the same time, for patients who had developed BM during the time of RCC diagnosis, survival estimates were performed, and prognostic factors were identified.
Methods
Patient data were obtained from the SEER program database between 2010 and 2014, as the sites of metastases were not collected until 2010. The extracted data were restricted to those of patients with a primary site labeled as “C64.9 Kidney, ‘not otherwise specified’ (NOS) and one of four clinically relevant RCC-specific histologic subtype statuses: 1) clear cell (8310/3: clear-cell adenocarcinoma, NOS; 8322/3: water clear-cell adenocarcinoma; 8313/3: clear-cell adenocarcinofibroma), 2) papillary (8260/3: papillary adenocarcinoma, NOS), 3) chromophobe (8317/3: renal cell carcinoma, chromophobe type; 8270/3: chromophobe carcinoma), or 4) collecting duct (8319/3: collecting duct carcinoma). The statuses of age and laterality were not restricted. Patients diagnosed with carcinoma in situ and benign tumor were excluded. Patients diagnosed at autopsy or through death certificate and who had an unknown BM or follow-up status were removed. From the SEER database, 45,824 patients who were diagnosed with RCC from January 1, 2010, to December 31, 2014, and whose information also included definite details about BM were selected for analysis to identify the risk factors for bone metastases. Among those patients, 1,182 patients with bone metastases who were initially diagnosed between 2010 and 2013 (and thus had at least 1 year of follow-up) were selected for survival analysis to investigate the factors contributing to the prognosis of BM (Figure 1).
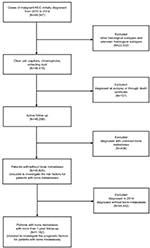  | Figure 1 Flowchart of the patient selection for analyzing the risk factors for the morbidity and prognosis of bone metastases in RCC patients. Abbreviation: RCC, renal cell carcinoma. |
The SEER is an open database, and the data released from the SEER database do not require patients’ consent because cancer is a reportable disease in every state of the United States. The present study complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and the Research Ethics Board of the Tianjin Medical University Cancer Institute and Hospital approved the study.
Statistical analysis
The patient-related variables included age (≤40, 41–69, and ≥70 years); gender (female and male); race (white, black, American Indian/Alaska Native [AI], and Asian or Pacific Islander [API]); marital status (married and unmarried); insurance status (insured and uninsured); laterality (right, left, and bilateral); primary tumor (T) stage (T1, T2, T3, and T4); regional lymph node stage (N0, and N1); tumor grade (grade I, grade II, grade III, and grade IV); presence or absence of lung, liver, or brain metastases; and the four RCC-specific histologic subtypes. The risk factors for RCC patients with de novo BM were first analyzed by univariate logistic regression. Characteristics that were significant (with P<0.05) in the univariate logistic regression analysis were further analyzed with a multivariate logistic regression model. Survival curves were generated using the Kaplan–Meier method; the differences between the curves were tested by a log-rank test. When identifying factors associated with mortality, the surgical treatment method at the primary site (none, tumor destruction, and resection) was added to the aforementioned factors that were significant by the log-rank test, and these factors were analyzed together by multivariate Cox proportional hazards regression analysis.
All the data were obtained using SEER*Stat Software version 8.3.5. All statistical analyses were performed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA), and all survival curves were prepared by MedCalc 15.2.2. Two-sided P-values <0.05 were considered statistically significant.
Results
Incidence of bone metastases
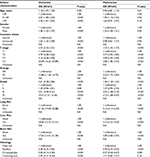
A total of 45,824 eligible patients were diagnosed with malignant RCC of one of the four RCC-specific histologic subtypes between 2010 and 2014 in this study; patients with the clear-cell, papillary, chromophobe, and collecting duct histologic subtypes accounted for 75.90% (34,780/45,824), 16.67% (7,640/45,824), 7.09% (3,248/45,824), and 0.25% (116/45,824) of the patients, respectively. Of the patients in the entire cohort, 3.29% (1,509/45,824) were diagnosed with bone metastases at initial diagnosis. The incidence of bone metastases with the clear-cell, papillary, chromophobe, and collecting duct histologic subtypes was 3.87%, 1.52%, 0.80%, and 18.10%, respectively. In the cohort, 1,182 patients who presented with bone metastases were followed up for >1 year (Table 1).
Risk factors for developing BM
Age (odds ratio [OR] =1.18, 95% confidence interval [CI]: 1.07–1.30; P=0.001); gender (OR =1.40, 95% CI: 1.25–1.57; P<0.001); insurance status (OR =1.62, 95% CI: 1.25–2.09; P<0.001); primary T stage (OR =2.37; 95% CI: 2.25–2.51; P<0.001); regional lymph node stage (OR =12.88, 95% CI: 11.26–14.72; P<0.001); tumor grade (OR =2.61, 95% CI: 2.38–2.86; P<0.001); presence of lung metastases (OR =21.30, 95% CI: 19.00–23.88; P<0.001), liver metastases (OR =18.05, 95% CI: 15.15–21.51; P<0.001), or brain metastases (OR =17.64, 95% CI: 14.41–21.59; P<0.001); and the four RCC-specific histologic subtypes (OR =0.72, 95% CI: 0.68–0.76; P<0.001) were all significantly associated with bone metastases in the univariate analysis (Table 2).
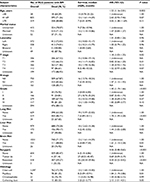
As shown in Table 2, male gender (OR =1.25, 95% CI: 1.04–1.51; P=0.02); higher T stage (OR =1.57, 95% CI: 1.42–1.73; P<0.001); lymph node involvement (OR =2.23, 95% CI: 1.75–2.84; P<0.001); poor tumor grade (OR =1.46, 95% CI: 1.30–1.63; P<0.001); presence of lung metastases (OR =5.15, 95% CI: 4.13–6.42; P<0.001), liver metastases (OR =3.14, 95% CI: 2.23–4.43; P<0.001), or brain metastases (OR =4.09, 95% CI: 2.82–5.92; P<0.001); and collecting duct subtype of RCC (OR =0.81, 95% CI: 0.74–0.88; P<0.001) were significantly associated with greater possibility of having developed bone metastases at diagnosis in the multivariate logistic regression analysis.
Survival and prognostic factors for BM
The median overall survival (OS) for all 1,182 patients was 12 months (95% CI: 10.69–13.31 months, Figure 2A). Compared with patients with other RCC subtypes, patients with collecting duct subtype had the highest risk of both metastases and mortality. The median survival of patients with collecting duct RCC (CDRCC) was only 3 months (95% CI: 0.23–5.77 months), whereas the median survival of patients with clear-cell, papillary, and chromophobe subtypes was 13 (95% CI: 11.60–14.40), 8 (95% CI: 5.09–10.91), and 11 (95% CI: 5.02–16.98) months, respectively (P<0.001).
By the time of the analysis, 871 (73.75%) of the patients had died. In the univariate analysis, age; marital status; laterality; T stage; lymph node involvement; tumor grade; the presence of lung, liver, or brain metastases; and the four RCC-specific histologic subtypes had a statistically significant influence on survival (P<0.05). In addition to the factors above, the type of surgical treatment at the primary site correlated with survival (P<0.001, Figure 2).
In the multivariate stepwise Cox regression analysis (Table 3), older age (hazard ratio [HR] =1.53, 95% CI: 1.16–2.01; P=0.002); higher T stage (HR =1.18, 95% CI: 1.03–1.35; P=0.02); lymph node involvement (HR =1.47, 95% CI: 1.12–1.93; P=0.01); poor tumor grade (HR =1.40, 95% CI: 1.19–1.66: P<0.001); presence of lung metastases (HR =1.78, 95% CI: 1.39–2.28: P<0.001), liver metastases (HR =1.44, 95% CI: 1.05–2.00; P=0.03), or brain metastases (HR =1.61, 95% CI: 1.13–2.28; P=0.01); the absence of surgery (HR =0.56, 95% CI: 0.48–0.65; P<0.001); and CDRCC (OR =1.13, 95% CI: 1.01–1.27; P=0.03) were significantly associated with a higher risk of mortality. As shown, surgical treatment at the primary site could noticeably prolong the median survival time. Patients with resection had the lowest risk of mortality in the Cox model (vs none: HR =0.30, 95% CI: 0.22–0.42, P<0.001).
Discussion
Bone metastases mainly leading to osteolytic lesions in RCC were reported to compromise skeletal integrity.2 The outcome of RCC patients can be negatively affected by BM. The present study, performed on a large population, determined the incidence of BM at the initial diagnosis of RCC, for the first time. In our series, 3.29% of the patients in the whole cohort had BM at initial diagnosis. It was reported that synchronous metastasis occurs in approximately one-third of newly diagnosed RCC patients.2 At the same time, based on an analysis of 947 patients, BM was found in 26.7% of RCC patients.8 BM was reported to occur in 30%–40% of advanced RCC patients.9,10 Thus, the incidence of BM may be underestimated at initial diagnosis. This underestimation could be due to the following causes: 1) asymptomatic patients were unable to be assessed at initial diagnosis, and 2) the patients who developed BM later in their disease course were not recorded in the SEER database. The current guidelines only recommend bone imaging in symptomatic patients or in those with an unusual alkaline phosphatase level.11,12 Thus, a study assessing the risk factors for BM in RCC patients is necessary.
Risk factors for BM occurrence at RCC diagnosis were determined. The RCC patients had significantly greater odds of BM when they presented with a series of factors, including male gender; higher T stage; lymph node involvement; poor tumor grade; presence of lung, liver, or brain metastases; and CDRCC. Accordingly, skeletal scanning could be considered for RCC patients with the aforementioned factors.
Prognostic factors for BM occurrence at RCC diagnosis were found. The RCC patients had a significantly higher risk of mortality when they presented with a series of factors, including older age (≥70 years); higher T stage (T4); lymph node involvement; poor tumor grade; presence of lung, liver, or brain metastases; absence of surgery; and CDRCC. Thus, a personalized treatment plan can be made based on these factors. Notably, we found that, rather than patients with grade I tumors, patients with grade II tumors had the longest median survival. One of the most important reasons for this finding may be the relative lack of patients with grade I tumors (N=26). Accordingly, these results should be evaluated with caution, and more studies should be conducted to confirm these results further.
In 1997, RCC was histologically classified into clear-cell, papillary, chromophobe, collecting duct, and unclassified RCC.13 CDRCC, a rare entity, was reported to occur in <2% of RCC patients.14 CDRCC develops from the collecting ducts in the renal medullary pyramid and usually results in poor prognosis of RCC patients.14 In the present study, CDRCC was associated with greater odds of BM occurrence and a dismal prognosis compared to the other RCC subtypes. This correlation was proven in two multi-institutional surgical case series.15,16 In our study, which included both surgical and non-surgical CDRCC patients from the United States, we provided further evidence to support the hypothesis that patients with CDRCC have a worse prognosis than those with other subtypes.
There are some limitations in the present study. First, the actual incidence rate of BM in patients with RCC might be underestimated because the asymptomatic patients were lost. Second, the SEER database had no records of patients who developed BM later in their disease course. Third, information on the number of bone lesions was unavailable in the public SEER dataset. Fourth, SREs, which are accepted as one of the important prognostic factors, were not recorded.
Conclusion
Based on the analysis of newly diagnosed initial RCC patients, the incidence and epidemiological characteristics of BM were determined. A series of risk factors for BM in RCC patients were identified; these factors can be potentially used for clinical surveillance. Survival estimates were conducted, and a list of prognostic factors for initial BM in RCC patients was found; these factors can be potentially used for making individualized treatment plans. CDRCC is associated with higher BM occurrence and poorer prognosis than the other RCC histological subtypes.
Acknowledgments
This study was sponsored by the Natural Science Foundation of China (81702161), the Natural Science Foundation of the Tianjin Science and Technology Committee of China (17JCQNJC11000), the Natural Science Foundation of Tianjin Medical University (2016KYZQ10), the China Postdoctoral Science Foundation Grant (2017M621091), and the Doctor Start-up Grant of Tianjin Medical University Cancer Institute and Hospital (B1612, B1711).
Author contributions
QG, CZ, and XW designed the study. FT and YX collected the data. XG and XW analyzed the data. QG, CZ, HZ, and XG organized the manuscript. GW, GF, PZ, and ZR reviewed the papers and revised the manuscript. All the authors have read and approved the final manuscript, contributed toward data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Rueckert J, Devitt K, Gardner JA. Renal cell carcinoma with monosomy 8: a case series and review of the literature. J Assoc Genet Technol. 2018;44(1):5–9. | ||
Shankar K, Kumar D, Kumar KV, Premlata C. Renal cell carcinoma with unusual skeletal metastasis to tibia and ankle: a case report and review of literature. J Clin Diagn Res. 2016;10(11):XD01–XD02. | ||
Yoshiyama A, Morii T, Susa M, et al. Preoperative evaluation of renal cell carcinoma patients with bone metastases on risks for blood loss, performance status and lethal event. J Orthop Sci. 2017; 22(5):924–930. | ||
Umer M, Mohib Y, Atif M, Nazim M. Skeletal metastasis in renal cell carcinoma: A review. Ann Med Surg (Lond). 2018;27:9–16. | ||
Kozlowski JM. Management of distant solitary recurrence in the patient with renal cancer: contralateral kidney and other sites. Urol Clin North Am. 1994;21(4):601–624. | ||
Santoni M, Conti A, Procopio G, et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J Exp Clin Cancer Res. 2015;34(1):10. | ||
Kume H, Kakutani S, Yamada Y, et al. Prognostic factors for renal cell carcinoma with bone metastasis: who are the long-term survivors? J Urol. 2011;185(5):1611–1614. | ||
Swanson DA, Orovan WL, Johnson DE, Giacco G. Osseous metastases secondary to renal cell carcinoma. Urology. 1981;18(6):556–561. | ||
Zekri J Ahmed N, Coleman RE, Hancock BW. The skeletal metastatic complications of renal cell carcinoma. Int J Oncol. 2001;19(2):379–382. | ||
Schlesinger-Raab A, Treiber U, Zaak D, Hölzel D, Engel J. Metastatic renal cell carcinoma: results of a population-based study with 25 years follow-up. Eur J Cancer. 2008;44(16):2485–2495. | ||
Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(6):804–834. | ||
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. | ||
Störkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997;180(5):987–989. | ||
Wright JL, Risk MC, Hotaling J, Lin DW. Effect of collecting duct histology on renal cell cancer outcome. J Urol. 2009;182(6):2595–2599. | ||
Tokuda N, Naito S, Matsuzaki O, et al. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176(1):40–43. | ||
Karakiewicz PI, Trinh QD, Rioux-Leclercq N, et al. Collecting duct renal cell carcinoma: a matched analysis of 41 cases. Eur Urol. 2007;52(4):1140–1145. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.