Back to Journals » Vascular Health and Risk Management » Volume 17
Incidence and Risk Factors for Venous Thromboembolism Following 2462 Major Abdomino-Pelvic Surgeries in Tertiary Hospital
Authors Taengsakul N, Saiwongse T, Sakornwattananon O, Kreesaeng P, Kantathavorn N
Received 28 January 2021
Accepted for publication 18 March 2021
Published 8 April 2021 Volume 2021:17 Pages 135—143
DOI https://doi.org/10.2147/VHRM.S304187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Nawaphan Taengsakul,1 Thaweechai Saiwongse,1 Orattha Sakornwattananon,1 Pattraporn Kreesaeng,1 Nuttavut Kantathavorn2,3
1Department of Surgery, Chulabhorn Hospital, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand; 2Department of Obstetrics and Gynecology, Chulabhorn Hospital, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand; 3Faculty of Medicine and Public Health, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand
Correspondence: Nawaphan Taengsakul
Department of Surgery, Chulabhorn Hospital, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, 906 Kamphaeng Phet 6 Road, Talat Bang khen, Lak Si, Bangkok, 10210, Thailand
Tel/Fax +66-2-576-6791
Email [email protected]
Purpose: To determine the incidence and risk factor of postoperative venous thromboembolism (VTE) in Thai populations and to evaluate morbidity, mortality, bleeding complications and the benefit of thromboprophylaxis in real-world practice.
Patients and Methods: We performed a retrospective, single-center, cohort study of patients from all age groups who underwent elective open or laparoscopic major abdomino-pelvic surgery between January 2008 and December 2018 at Chulabhorn Hospital, Bangkok, Thailand. We collected general medical information and specific data based on items from the Caprini risk scoring system.
Results: A total of 2462 major abdomino-pelvic surgeries were included. The study population consisted of 742 males (30.1%) and 1720 females (69.9%) aged 54.59 ± 13.27 years. The incidence of VTE in Thai patients that underwent major abdominal surgery was 0.48%. The most frequent influencing factor for VTE was a history of pulmonary embolism, which increased the risk of VTE 98.28-fold, whereas a history of deep vein thrombosis increased the risk of VTE by 12.34-fold. Other factors influencing VTE development were obesity, anticoagulant use, postoperative chemotherapy, preoperative chemotherapy, endometrium cancer, tumor-node-metastasis (TNM) stage 4 and American College of Chest Physicians (ACCP) class 4. Protective factors included no history of VTE, laparoscopic surgery, TNM stage 0 and benign disease and BMI< 30. VTE significantly increased mortality whereas following ACCP guideline reduced mortality.
Conclusion: Post-operative VTE incidence in Thai patients undergoing major abdomino-pelvic surgery was lower compared with Western patients. Factors influencing for VTE were history of VTE, anticoagulant use, postoperative chemotherapy, preoperative chemotherapy, endometrium cancer, TNM stage 4 and ACCP class 4. Following ACCP guideline reduced the incidence of mortality.
Keywords: postoperative venous thromboembolism, deep vein thrombosis, thromboprophylaxis, abdomino-pelvic surgery, Thailand
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major healthcare problem that has resulted in a significant increase in mortality, morbidity, and medical expenses.1–3 Prevention of VTE is mandatory, but it seems insufficient in surgical practice. Surgical procedure, malignancy, immobilization, aging, and obesity are risk factors for VTE. Without thrombotic prophylaxis, the incidence of VTE varies from 10% to 40% in medicine and non-orthopedic surgery populations.4 Thromboprophylaxis could improve outcomes for patients who are at risk of VTE. Evidence-based clinical practice guidelines from the American College of Chest Physicians (ACCP) recommend thromboprophylaxis approach in non-orthopedic surgical patients by assessing the risk of VTE and bleeding complications.5
The ACCP proposed guideline based on a scoring system to aid the assessment of risk factors and implement appropriate VTE prophylaxis. This international guideline was first published in 1986 and was subsequently updated to the ninth edition in 2012. Abdomino-pelvic and general surgery were categorized into four categories by Caprini score as follow: very-low-risk (Caprini score 0), low risk (Caprini score 1,2), moderate risk (Caprini score 3,4) and high risk (Caprini score ≥5). In the very-low-risk group, the recommendation is early ambulation. In the low-risk group, the recommendation is mechanical prophylaxis, preferably intermittent pneumatic compression. In the moderate-risk group, recommends include pharmacological prophylaxis, especially low-molecular-weight heparin for patient with low risk of major bleeding, and mechanical prophylaxis for patients at high risk of major bleeding. In the high-risk group, the recommendation combines between mechanical and pharmacological prophylaxis with an extended duration of pharmacological prophylaxis for 4 weeks in patients with malignancy.5
In Asian populations, only two large studies from Korea (N=993,459) and Taiwan (N=5347) reported on patients undergoing major surgery. The incidence of VTE in general surgery was lower compare with Western populations in previous studies.6–8 In a systematic review, which included 14 publications with 11,218 patients, the incidence of above-knee DVT was 0.08%, whereas and the incidence of PE was 0.18%. This systematic review suggested that the risk of VTE in Asian populations after general surgery is low.9 Other studies have demonstrated a higher rate of VTE after abdominal surgery.10–13 In Thailand, Laohapensang et al (N=167) reported a low incidence of VTE (3.6%) compared with Western populations, an increased risk of VTE in patient with a high body mass index and postoperative bed rest.14
This aim of this study was to clarify the risk factors for postoperative VTE in larger Thai populations. Thus, we will explore the need of thromboprophylaxis in real-world practice in Thailand.
Patients and Methods
Patient Characteristics and Study Design
All patients signed an informed consent form for the personal data process. This study was approved by Ethic Committee for Human Research, Chulabhorn Research Institute (Ethic number 004/2562) and has been performed according to the Good Clinical practice guidelines and the Declaration of Helsinki. All data were retrospectively collected from records in patients who underwent elective open or laparoscopic major abdominal surgery between January 2008 to December 2018 at Chulabhorn Hospital, Bangkok, Thailand.
The inclusion criteria included were operated abdominal surgery and close follow-up at Chulabhorn Hospital. The exclusion criteria were incomplete medical records. The primary outcome was the incidence of postoperative VTE in our population. The secondary outcomes were significant risk factors for VTE, overall survival, and reduction in VTE events.
The patients were divided into two groups: the non-VTE and the VTE group. Non-VTE group referred to patients who had no postoperative VTE, both clinical presentation and imaging finding. The VTE group referred to patients who were diagnosed as VTE either by duplex ultrasound or computer tomography (CT) scan. Patients were followed up to December 2019.
Definition
Abdominal surgery included gastric surgery, small bowel surgery, colorectal surgery, appendices surgery, urological surgery, gynecological surgery, abdominal reconstruction surgery, and retroperitoneal or intraabdominal tumor surgery (Table 1). Major abdominal surgery was abdominal surgery that took longer than 45 minutes.15 General medical information and specific data for the Caprini risk scoring system were collected (Table 2).
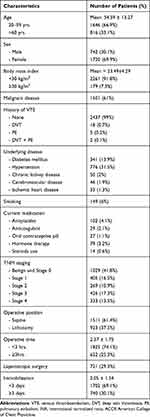 |
Table 1 Demographic Characteristics of Patients |
 |
Table 2 Factors Influencing the Increase and Reduction in VTE Events |
VTE was diagnosed by duplex ultrasound and CT. Duplex ultrasonography was performed by an experienced radiologist and diagnosed in 6-months after surgery. The criteria for diagnosis of VTE by duplex ultrasound were echogenic material within the lumen, uncompressible veins or non-visualized flow on color doppler imaging. Respiratory variation was also assessed by evaluating of iliac vein or inferior vena cava patency. CT revealed presence of PE, DVT and thrombosis of visceral veins.
Institution Protocol
Mechanical prophylaxis was performed using an intermittent pneumatic device composed of three segmental compressors (ankle, calf, and thigh). Mechanical prophylaxis was started on the day of surgery and continued until patients were able to ambulate.
Pharmacological prophylaxis was performed using low-molecular-weight heparin (1mg/kg) once daily at 12 hours before surgery and then continue once daily for 7 days.
Statistical Analysis
The incidence and associated 95% confidence interval (CI; two-sided) of VTE were calculated. Continuous variables were compared using the Student’s t-test or the non-parametric Mann–Whitney U-test, as appropriate. Continuous data are presented as mean ± standard deviation and median (interquartile range) for non-normally distributed data. Categorical variable was compared using the Chi-square test or Fisher’s exact test as appropriate and were presented as number (percentage), risk ratio and 95% confidence interval (CI). Overall survival was calculated from the date of surgery to the date of death, and the timing of postoperative VTE was calculated from the date of surgery to the date of VTE. Survival rates were calculated using the Kaplan-Meier method and compared using Log rank tests. The Cox proportional-hazards model was used to calculate hazard ratios with 95% confidential intervals (CI) for overall survival. Overall survival was analyzed and compared between the non-VTE and VTE groups. A P-value of 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 11.0 (STATA Corp., College Station, TX, USA).
Results
We enrolled 2462 patients in this study. The incidence of VTE in Thai patients who underwent major abdominal surgery was 0.48%. The majority of patients were female (69.9%) and the mean age of patients was 54.59±13.27 years. Seven percent of patients were classified as obese if their body mass index was more than 30 kg/m2. The most common co-morbidity was hypertension (31.5%) and only 6% of patients were smokers. Sixty-one percent of cases were diagnosed as malignant, and the most common malignant diseases were colorectal cancer (43%), gynecological cancer (27.3%) and hepatobiliary cancer (7.7%). Twenty-five patients (0.9%) had a previous history of VTE (18 cases of DVT, 5 cases of PE, and 2 cases of both DVT and PE), as shown in Table 1. Our population had a high Caprini score, the mean Caprini score was 4.72 ± 1.87 (range 0–16), and the VTE group had significantly higher Caprini score (P=0.001). Thus, in this study, 58.9% of patients were at a high risk of VTE, 29.2% were at a moderate risk, 11% were at a low risk, and 0.6% were at a very low risk.
Figure 1 shows that the most common type of surgery was gynecological surgery (45.5%), followed by colorectal surgery (29.5%) and hepatobiliary surgery (15.4%).
 |
Figure 1 Percentage of patients undergoing each prophylaxis method according to adherence to ACCP guidelines. Abbreviation: ACCP, American College of Chest Physicians. |
Compliance to ACCP guidelines was 24% for appendix surgery, 22% for gynecological surgery, 4.3% for urological surgery, 2.8% for colorectal surgery and 2.1% for hepatobiliary surgery. No compliance to ACCP guidelines in gastric surgery, small bowel surgery, abdominal reconstruction surgery and retroperitoneal tumor removal, as shown in Figure 2. Approximately 60% of patients were operated on in a supine position, while the remaining 40% were operated on in a lithotomy position. Approximately 30% of patients underwent laparoscopic surgery, as shown in Table 1.
 |
Figure 2 Comparison of type of surgery adherence to with ACCP guidelines. Abbreviation: ACCP, American College of Chest Physicians. |
In total,17% of patients received preoperative chemotherapy, and 12% of patients underwent preoperative radiotherapy. According to TNM staging, 61% of patients were malignancy. The most common cell type was adenocarcinoma. Most patients had an operation time <3 hours. The duration of immobilization was 2.05 ±1.54 days. According to the ACCP classification, 58.9% of patients were allocated to high-risk group. Sixty-six percent of patients in this groups received of this group received mechanical prophylaxis. Most patients (87.9%) did not follow ACCP guidelines, as shown in Table 1.
There were 1501 cases of malignancy. The most common malignant condition was colon cancer (43.6%), followed by gynecological cancer (27.3%) and hepatobiliary cancer (12.6%). VTE was found in most frequently observed in patients with appendiceal cancer (20%), followed by lung cancer (12.5%) and gastric cancer (11.1%).
The most relevant factor related to VTE was a previous history of VTE. The risk ratio of history of PE was 98.28 and the risk ratio of a history of DVT was 12.34. The risk ratio of other factors is shown in Table 2. There were no VTE events in patients with early ambulation (within first 24 hours).
According to the Caprini score, patients were allocated a score of 1 to 16. According to ACCP guidelines, 2.97% of patients were classified as high-risk and 0.97% as moderate risk in term of VTE events, as shown in Figure 3.
 |
Figure 3 ACCP classification categories in our population and incidence of VTE in each of classification. Abbreviation: VTE, venous thromboembolism. |
Mechanical and combined prophylaxis did not reduce the rate of VTE in this population. Sixty-six percent of patients received mechanical prophylaxis, and all of them underwent intermittent pneumatic compression. Combined mechanical and pharmacological prophylaxis was used in 0.5% of patients and isolated pharmacological prophylaxis was used in 0.3% of patients, as shown in Table 2. Only 12% of cases followed ACCP guidelines.
In total, 12 patients (0.48%) were diagnosed with VTE. There were 5 cases of unilateral DVT and 1 case of bilateral DVT. Five cases of patients were proximal DVT and only case was distal DVT. Most cases (66%) of DVT were symptomatic and were diagnosed by duplex ultrasound whereas 33% of cases of DVT were incidentally identified by CT. Most cases of PE (75%) were also asymptomatic. There were 2 cases of symptomatic PE and no cases of fatal PE. There were two cases of combined DVT and PE. Postoperative VTE occurred most frequently at 1 month (25%), and 58% of cases were identified within the first 3 months postoperatively. There were two cases of bleeding complications after pharmacological prophylaxis. They have upper gastrointestinal bleeding and were indicated for inferior vena cava filter placement.
ACCP guidelines were unable to reduce the risk of VTE in our population. The survival rate of patients with VTE event was significantly lower compared with patients without VTE, as shown in Figure 4. Following ACCP guidelines was a preventative factor for mortality as shown in Table 3.
 |
Table 3 Multivariate Analysis of Mortality from VTE and with Adherence to ACCP Guidelines |
 |
Figure 4 Overall survival between the VTE and non-VTE groups. Abbreviation: VTE, venous thromboembolism. |
Discussion
In this study, the incidence of VTE in Thai patients who underwent major abdominal surgery was 0.48%, which is much lower compared with Western patients who underwent general surgery (15–30%) in a previous study.16 The overall rate of VTE in patients undergoing major abdominal surgery was lower compared with a previous study in Thailand (3.6%). Previous studies have also shown that an Asian population had a swing incidence of postoperative VTE ranging between 0.49% and 7.5% similar to our populations.17,18
In term of laboratory findings, white blood cell (WBC) counts, hemoglobin, hematocrit, platelet counts, and cholesterol were not significantly difference between the VTE and non-VTE groups. High hematocrit is a risk factor for VTE in the general population.19 Hematocrit correlates with thrombotic risk and is also associated with increased plasma viscosity and platelet reactivity.20,21 A cohort study of cancer-free subjects reported no association between WBC count and VTE.22 In contrast, leukocytosis was identified as a risk factor for VTE and mortality in ambulatory patients with cancer.23 In previous study, reactive thrombocytosis was associated with increased risk of VTE.24 A meta-analysis found a significant association between low high-density-lipoprotein/cholesterol levels, high triglyceride levels and the risk of VTE.25
We found that endometrial cancer, chemotherapy and advanced TNM stage significantly increased VTE. Current evidence supported that four prothrombotic pathway consist of activation of extrinsic and intrinsic coagulation pathway, platelet activation and impair fibrinolysis.26 Even though cancer may be considered in neoadjuvant setting and therefore may be amenable to surgical excision, certain tumor types with high thrombogenic potential such as gastric or pancreatic cancers could activate blood coagulation, leading to thrombosis.27,28 Furthermore, neoadjuvant chemotherapy increases the risk of VTE by directly damaging the endothelium, reducing blood anticoagulant levels, increasing procoagulant factor, stimulating platelet activation and increasing tissue factor activity.28 Chemotherapy increases the risk of VTE by two-to-six fold, and specific chemotherapeutic agents such as platinum-based regimens have been associated with higher rates of VTE.28 In some studies, the highest rates of VTE were observed in patients with cancer of the pancreas, stomach, uterus, kidney, lung, and primary brain.16,20 These cancer types could be associated with a pro-coagulant state even when diagnosed at an earlier stage. However, the most widely used model for identification VTE risk in cancer patients with chemotherapy is the Khorana score and the COMPASS-CAT model. The stratification model scoring identify risk of VTE and focus on thromboprophylaxis.26
In addition to the variable incidence of VTE in different types of malignancy. The incidence of postoperative VTE with endometrial cancer varies between 0.8% and 8.1%.29–31 A previous study showed that the most common cancer associated with VTE in Thailand is gynecologic cancers, followed by gastrointestinal and hepatobiliary cancers, lung cancer, and lymphoma.32,33 Cancer-associated VTE was more severe and associated with higher morbidity and mortality.33
However, perioperative interruption of anticoagulant is associated with high postoperative rates of VTE and major bleeding.34 The present study found that anticoagulant use increased risk of VTE by 16.78-fold, which may have causing perioperative interruption of anticoagulation.
In term of VTE history, 25 patients (1%) of our study had a history of VTE before undergoing abdominal surgery, including 18 cases of DVT, 5 cases of PE and 2 cases of combined PE + DVT. Nearly all patients with a history of VTE had malignant disease, and one patient had antithrombin III deficiency. All patients in this group were administered anticoagulants before surgery. Previous studies showed that a history of prior VTE predicted VTE occurrence, both in the general population and in patients with cancer.35–37 As part of the Ottawa scoring system, which is a recently developed risk assessment model for predicting VTE recurrence in patients with cancer, a history of VTE is included as one of only four clinical patient characteristics.38
In term of operation, more than half of our population (53.2%) underwent gastrointestinal surgery (especially colorectal surgery) and gynecological surgery (45.5%). We found that VTE was not significantly different between type of surgery and operation time.
We found that VTE event 0.76% in high risk, 0.13% in moderate risk, 0% in low risk and very low risk. In this study was lower incidence than ACCP guideline in every classification.39 Asian venous thromboembolism guidelines for the prevention of VTE suggest risk assessment following ACCP guidelines. These guidelines also recommend that low-molecular-weight heparin should be considered in patients with a surgery for cancers with a high risk of VTE. Mechanical prevention using IPC should be considered in patients with a high bleeding risk.17
In term of prophylaxis methods, following ACCP guidelines did not reduce VTE including with prophylaxis methods (ie, mechanical, pharmacological, and combined prophylaxis) were not reduced VTE event. In this study, pharmacological prophylaxis was provided only in gynecological surgery was provided only for the first 7 days postoperatively, despite ACCP guidelines recommending continuous anticoagulant use for 28 days postoperatively in high-risk patients with malignancy. Thus, ACCP guidelines related to pharmacological prophylaxis in gynecological cancer surgery were incompletely followed. In general surgery, we found that most general surgeons worried about bleeding complications; thus, pharmacological prophylaxis was performed in case of a history of VTE and incidental identification of venous thrombosis. However, every case of colorectal surgery and gynecological surgery used routine mechanical prophylaxis by IPC. However, in this study, we found that mechanical prophylaxis could not significantly reduce VTE. Thus, ACCP guidelines were unable to reduce the risk of VTE in our population.
Bleeding complications after pharmacological prophylaxis occurred in 0.07% of patients compared with a previous study using low-molecular-weight heparin according to meta-analysis. The risk of major bleeding was 1.2% (95% CI, 0.9–1.7%).1 Another meta-analysis reported that the mean risk of wound hematoma and bleeding requiring reoperation in the control groups of randomized trials on thromboprophylaxis with low-dose-unfractionated heparin or low-molecular-weight heparin were 0.8% and 0.7%, respectively.40 From a literature review of study performed in Asian patients, major bleeding rates were less than 1% following pharmacological prophylaxis.9
In term of mortality, VTE significantly increased mortality rate (P=0.042), while following ACCP can reduced mortality (P=0.014).
The present study had some limitations. First, selection bias may exist due to clinician preference. Second, the study adopted a retrospective design. Data were extracted from medical records; thus, patients were not randomized, and unrecognized confounding factors could have introduced bias in our results and weakened the conclusions.
Conclusion
Post-operative VTE incidence in Thai patients undergoing major abdomino-pelvic surgery was lower compared with Western patients. Factors influencing for VTE were history of VTE, anticoagulant use, postoperative chemotherapy, preoperative chemotherapy, endometrium cancer, TNM stage 4 and ACCP class 4. Following ACCP guideline reduced the incidence of mortality.
Acknowledgments
This study was supported by Chulabhorn Hospital Research Grant. The authors would like to extend our special thanks to all patients and staff of Chulabhorn Hospital for their kind assistance and support towards the success and accomplishment of this study.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S–47S. doi:10.1378/chest.1412S3
2. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi:10.1016/S0140-6736(16)30514-1
3. Angchaisuksiri P. Venous thromboembolism in Asian unrecognised and under-treated problem? J Thromb Haemost. 2011;106:585–590. doi:10.1160/TH11-03-0184
4. Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251:344–350. doi:10.1097/SLA.0b013e3181b7fca6
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in non-orthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:277.
6. Yhim H, Jang MJ, Bang SM, et al. Incidence of venous thromboembolism following major surgery in Korea: from the health insurance review and assessment service database. J Thromb Haemost. 2014;12:1035–1043. doi:10.1111/jth.12611
7. Wu PK, Chen CF, Chung L, Liu C, Chen WM. Population-based epidemiology of postoperative venous thromboembolism in Taiwanese patients receiving hip or knee arthroplasty without pharmacological thromboprophylaxis. Thromb Res. 2014;133:719–724. doi:10.1016/j.thromres.2014.01.039
8. Lee CH, Lin L, Cheng C, Kao Yang YH, Chen JY, Tsai L. Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost. 2010;8:1515–1523. doi:10.1111/j.1538-7836.2010.03873.x
9. Yeo DX, Junnarkar S, Balasubramaniam S, et al. Incidence of venous thromboembolism and its pharmacological prophylaxis in Asian general surgery patients: a systemic review. World J Surg. 2015;39:150–157. doi:10.1007/s00268-014-2763-0
10. Kim JW, Chun EJ, Choi SI, et al. A prospective study on the incidence of postoperative venous thromboembolism in Korean gastric cancer patients: an inquiry into the application of Western guidelines to Asian cancer patients. PLoS One. 2013;8:e61968. doi:10.1371/journal.pone.0061968
11. Sugimachi K, Tajiri H, Kinjo N, et al. Incidence and predictors of deep venous thrombosis after abdominal oncologic surgery: prospective Doppler ultrasound screening. J Surg Res. 2012;178:657–661. doi:10.1016/j.jss.2012.06.002
12. Yang SS, Choi DW, Kim SJ, Choi SH, Sohn TS, Noh JH. Incidence and risk factors for deep vein thrombosis after abdominal surgery. Korean J Vasc Endovasc Surg. 2012;28:37–42.
13. Kim IG, Kim KH, Seo HJ, et al. Deep vein thrombosis after surgery for gastrointestinal cancer: incidence and correlation with risk factors. J Korean Soc Vasc Surg. 2004;20:237–241.
14. Vachirasrisirikul S, Laohapensang K. Incidence and risk factors of venous thromboembolism following major abdominal surgery. J Med Assoc Thai. 2016;99(6):665–674.
15. Nicolaides AN, Breddin HK, Fareed J, et al. Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence. Int Angiol. 2001;20:1–37. doi:10.1177/0003319701052001S01
16. Kakkar AK, Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ. Hemostasis and Thrombosis: Basic Principles and Clinical Practice.
17. Ngoh CI, Lai HL, Angchaisuksiri P, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017;36(1):1–20. doi:10.23736/S0392-9590.16.03765-2
18. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2):e227S–e277S. doi:10.1378/chest.11-2297
19. Braekkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population (The Tromso study). Haematologica. 2010;95:270–275. doi:10.3324/haematol.2009.008417
20. Santos MT, Valles J, Marcus AJ, et al. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J Clin Invest. 1991;87:571–580. doi:10.1172/JCI115032
21. Valles J, Santos MT, Aznar J, et al. Erythrocytes metabolically enhance collagen-induced platelet responsiveness via increased thromboxane production, adenosine diphosphate release, and recruitment. Blood. 1991;78:154–162. doi:10.1182/blood.V78.1.154.154
22. Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am J Med. 2002;113:636–642. doi:10.1016/S0002-9343(02)01345-1
23. Connolly GC, Khorana AA, Kuderer NM, et al. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126:113–118. doi:10.1016/j.thromres.2010.05.012
24. Ho KM, Yip CB, Duff O. Reactive thrombocytosis and risk of subsequent venous thromboembolism: a cohort study. J Thromb Haemost. 2012;10:1768–1774. doi:10.1111/j.1538-7836.2012.04846.x
25. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi:10.1161/CIRCULATIONAHA.107.709204
26. Prandoni P, Campello E. Venous thromboembolism in cancer patients undergoing chemotherapy: risk factor and prevention. Semin Thromb Hemost. 2021. doi:10.1055/s-0040-1718927
27. Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. Thromb Haemost. 2016;117:57–65. doi:10.1160/TH15-08-0686
28. Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi:10.1200/JCO.2011.35.5669
29. Matsuo K, Yessaian AA, Lin YG, et al. Predictive model of venous thromboembolism in endometrial cancer. Gynecol Oncol. 2013;128:544–551. doi:10.1016/j.ygyno.2012.12.014
30. Rauh-Hain JA, Hariton E, Clemmer J, et al. Incidence and effects on mortality of venous thromboembolism in elderly women with endometrial cancer. Obstet Gynecol. 2015;125:1362–1370. doi:10.1097/AOG.0000000000000866
31. Graul A, Latif N, Zhang X, et al. Incidence of venous thromboembolism by type of gynecologic malignancy and surgical modality in National Surgical Quality Improvement Program. Int J Gynecol Cancer. 2017;27:581–587. doi:10.1097/IGC.0000000000000912
32. Angchaisuksiri P, Atichartakarn V, Aryurachai K, et al. Risk factors of venous thromboembolism in Thai patients. Int J Hematol. 2007;86:397–402. doi:10.1007/BF02983995
33. Mutirangura P, Ruengsethakit C, Wongwanit C. Epidemiologic analysis of proximal deep vein thrombosis in Thai patients: malignancy, the predominant etiologic factor. Int J Angiol. 2004;13:81–83. doi:10.1007/s00547-004-1027-5
34. Shaw JR, Douketis J, Le Gal G, Carrier M. Periprocedural interruption of anticoagulation in patients with cancer‐associated venous thromboembolism: an analysis of thrombotic and bleeding outcomes. J Thromb Haemost. 2019;17:1171–1178. doi:10.1111/jth.14468
35. Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160:761–768. doi:10.1001/archinte.160.6.761
36. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi:10.1182/blood-2002-01-0108
37. Kroger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol. 2006;17:297–303. doi:10.1093/annonc/mdj068
38. Louzada ML, Carrier M, Lazo-Langner A, et al. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation. 2012;126:448–454. doi:10.1161/CIRCULATIONAHA.111.051920
39. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54(12):1496–1502. doi:10.1097/DCR.0b013e31823302a1
40. Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: a systematic review of 33 randomized controlled trials. Arch Surg. 2006;141(8):790–797. doi:10.1001/archsurg.141.8.790
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
