Back to Journals » Infection and Drug Resistance » Volume 15
Incidence and Predictors of Viral Load Suppression After Enhanced Adherence Counseling Among HIV-Positive Adults in West Gojjam Zone, Amhara Region, Ethiopia
Authors Atnafu GT, Moges NA , Wubie M , Gedif G
Received 30 September 2021
Accepted for publication 31 December 2021
Published 25 January 2022 Volume 2022:15 Pages 261—274
DOI https://doi.org/10.2147/IDR.S341392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Gezahegn Terefe Atnafu,1 Nurilign Abebe Moges,2 Moges Wubie,2 Getnet Gedif2
1Anti-Retroviral Treatment Clinic, Dembecha Health Center, Dembecha, Amhara Region, Ethiopia; 2Department of Public Health, Debre Markos University, Debre Markos, Amhara Region, Ethiopia
Correspondence: Getnet Gedif
Department of Public Health, Debre Markos University, P.O.Box: 251-269, Debre Markos, Amhara Region, Ethiopia
Tel +251-58-771-4281
Fax +251-58-771-1764
Email [email protected]
Background: Viral load suppression among people living with HIV is the main goal of antiretroviral therapy (ART). The most cause for high viral load is poor adherence to ART. World Health Organization (WHO) recommends intensive enhanced adherence counseling for people with a high viral load, which is greater or equal to 1000 RNA copies per mL and at least on treatment for six months. However, little is known about the outcome of enhanced adherence counseling. The study aimed to assess the incidence of viral load suppression after enhanced adherence counseling and its predictors among HIV-positive adults in high caseload health facilities in the Amhara region, Ethiopia.
Methods: An institution-based retrospective follow-up study was employed among 346 HIV-positive adults enrolled in enhanced adherence counseling in a high caseload health facility in the West Gojjam zone from June 2016 to June 2020. The data on relevant variables were collected from the patient’s medical cards by trained data collectors. The collected data were entered into EpiData version 3.1 and then exported to Stata version 14 for analysis. Descriptive analysis was performed to describe the variables. Cox proportional regression model was used to identify independent predictors of viral load suppression after enhanced adherence counseling.
Results: Overall, 51.73% of the study participants achieved viral load suppression after enhanced adherence counseling. The incidence of viral load suppression rate was 11.17 per 100-person month. During the multivariate analysis, it was observed that being female (AHR = 1.50, 95% CI: 1.05– 2.15), CD4 count of greater than or equal to 350 cells/mm3 (AHR = 1.98, 95% CI: 1.12– 3.51) and no recurrent OI (AHR = 1.85, 95% CI: 1.06– 3.24) were an independent predictor of viral load suppression after enhanced adherence counseling.
Conclusion: Incidence of viral load suppression rate was still far from the WHO target (70%). Therefore, higher priority should be given to patients with low CD4 counts with improved enhanced management of opportunistic infections.
Keywords: HIV/AIDS, antiretroviral therapy, viral load suppression, predictors, enhanced adherence counseling, Ethiopia
Background
Human Immunodeficiency Virus (HIV) created an enormous challenge to the survival of human beings. About 36.3 million people have died due to HIV since the beginning of the epidemic according to the UNAIDS report.1 In 2020, 37.7 million people were living with HIV/AIDS (PLWHA) worldwide. East and southern Africa contribute about 54.6% of the global burden of HIV. According to the UNAIDS factsheet 2020 estimates, 620,000 people are infected with HIV in Ethiopia.2 Among this, 35.5% reside in Amhara Regional State.3 By the end of March 2020, 10,202 people received HIV care and treatment in the West Gojjam Zone.4,5
To end Acquired Immune Deficiency Syndrome (AIDS) epidemic and lay the foundation for a healthier, more just, and equitable world for future generations, the Joint United Nations Program on HIV/AIDS (UNAIDS) set new, final, ambitious, but achievable targets the “so” called 90-90-90.6 That was by the end of 2020, 90% of all people living with HIV will know their HIV status, 90% of all people with diagnosed HIV infection will receive sustained ART, and 90% of all people receiving ART will have viral suppression.6 By the end of 2020, 84% of all people living with HIV knew their HIV status, 73% of them had access to treatment, and 66% of them achieved viral suppression worldwide.7,8
In Ethiopia, through implementing a 90-90-90 end HIV/AIDS epidemic target, by the end of 2020, 83% of people living with HIV knew their status, 78% of people living were on treatment and 72% achieved viral suppression. Achieving the third 90’s target has been troublesome for a while.2
Routine viral load testing, which is the golden standard for the monitoring of HIV treatment, was recommended by WHO in 2013.9 Based on WHO recommendations, viral load monitoring will be done at 6 and 12 months after initiating ART and annually thereafter for stable people.10,11 Cascading of viral load testing has been implemented across all regions of the country.12 A low viral load indicates the treatment is effective.10,12,13 A high viral load in a person on treatment indicates either that the medication is not being taken properly or that the virus is becoming resistant to the medication.9,11,12 WHO and the Ministry of Health (MOH) Ethiopia, recommend enhanced adherence counseling, followed by an additional viral load test to establish re-suppression or to confirm treatment failure and switch to an alternative regimen.10,12
The ART regimens used in Ethiopia are based on the use of three ART drugs with different mechanisms of action for the treatment of HIV infection to have effective viral load suppression. These include Nevirapine (NVP)-based, Efavirenz (EFV), and Dolutegravir (DTG)-based regimens. CD4 testing used at the baseline of ART initiation, to determine discontinuation of OI prophylaxis every six months and to diagnose immunological failure is outlined in Ethiopian ART guideline.14
Enhanced adherence counseling (EAC) for those patients with high viral load ≥1000 RNA per mL addresses all adherence barriers like cognitive, social, behavioral, and emotional using a multidisciplinary team approach for 3 to 6-months results in 70% of viral re-suppression.10,12 Viral load re-suppression is a repeat viral load measurement of HIV RNA to less than or equal to 1000 copies/mL while continuing to receive first-line ART regimen after an initial viral load measurement of greater than 1000 copies/mL. Before the repeat, a viral load is taken; the patient should complete the three-month enhanced adherence counseling session, be perfectly adherent for 3 months, and have fever ruled out.14
Timely completion of the EAC session and taking of the first viral load within three to six months is crucial for achieving viral re-suppression and reducing virological failure by 22% and 27%, respectively.15 For each additional month of delay in taking the first viral load (between 2.5 and 9 months), the risk of virological failure increased by 9% and the risk of treatment switching second-line antiretroviral therapy (ART) increased by 13%.15
Having an unsuppressed viral load while taking ARV medication has been the area of concern in the ART treatment program. In 2019, only 65% of people living with HIV in Ethiopia had achieved viral suppression.16 The National ART treatment guideline recommends that all patients with initial high viral undergo EAC.12 Implementation of EAC for patients with high viral load with routine viral load monitoring showed some promising results but failed to achieve the WHO recommended target of 70% viral re-suppression in a patient taking antiretroviral (ARV) medication.17
The cause of the high viral load while on EAC has been the area of controversy. Some studies have found that it is linked to earlier ARV medication exposure, while others showed that patients have already developed drug resistance.18 However, the most common reason associated with high viral load was poor medication adherences.19
Unless implementing EAC for the patient with high viral load, HIV will continue to be easily transmitted and result in a rapid decline in CD4 count and re-currying occurrence of opportunistic infections (OIs) eventually leading to death.20
However, resolving all adherence barriers to ARV medication is often comes up with difficulty. Since there is an individual-level variation of behavioral, cognitive, and psychosocial barriers associated with ARV medication. Therefore, this study aimed to assess the incidence of viral load suppression after EAC and its predictor among HIV-positive adults in high caseload health facilities in the West Gojjam Zone.
It could also provide important information concerning the aspect of a predictor of re-suppression and forward recommendations to health care providers, health facilities, non-governmental organizations (NGOs), and policymakers in enhancing the implementation of ART programs and the development of evidence-based interventions to improve the survival of adults on ART.
Methods and Materials
Study Design and Setting
An institution-based retrospective follow-up study was carried out from September 20/2020 to November 20/2020. The study was conducted in the west Gojjam zone, with high caseload health facilities, which are located in the Amhara region, Ethiopia. Finote-Selam is the capital city of West Gojjam Zone, 178km far from Bahir Dar (the capital city of Amhara Regional state) and 387 km from Addis Ababa. West Gojjam Zone is one of the largest zones in Amhara region with an estimated over 2 million population and has 15 rural districts and 2 city administrations. There are 7 hospitals, 26 health centers, and 1 private hospital providing ART services.5 By the end of March 2020, 10,202 people were living with HIV in West Gojjam. The study was carried out in health facilities with a high caseload that had a patient greater than or equal to 500 people living with HIV on ART. Six health centers, namely, Adet Health center, Burie health center, Durebete health center, Dembecha health center, Merawi health center, and one hospital, Fenote-Selam hospital, were included. The number of patients enrolled at those ART centers was 1003, 1075, 783, 539, 538, and 1880 adults, respectively. From June 2016 to June 2020, 466 adult patients were registered in these health facilities at EAC.
Sample Size and Study Participants
The study populations were all HIV-positive adult patients on ART for the last six months, had viral load results of ≥1000RNA per mL, and enrolled to EAC in high caseload health facilities in West Gojjam Zone. The sample size was determined using the previous study in North Wollo Zonal public Hospital, the overall viral load suppression after EAC was 64.4%.17 By considering the factor analysis: gender, period on ART, and baseline viral load count greater than or equal to 1000 copies/mL by using epi info TM 7 stat-Calc, and by assuming a margin of error 5%, power 80%, 95% confidence level, and 10% non-response rate. As a result, the final sample size was 347.
Sampling Procedure
All high caseload ART center health facilities were included in the study and the total sample size was allocated proportionally among each ART center based on the number of patients with a viral load count of greater than 1000 copies/mL. The study participants were selected through a simple random sampling technique from a viral load registration book.
Data Collection Tools and Procedures
The data extraction tool was prepared based on the Ethiopian National HIV care/ART intake and ART follow-up form on routinely collected patient chronic care. Data were extracted from patient ART follow-up clients with high viral load follow-up charts, and EAC register.
As a result, all charts containing detailed information about ART patients were examined. When there were incomplete data, the data collectors have sought to acquire from several data sources (patient’s chart and follow-up form). If clinical parameters and laboratory results (CD4 count and WHO clinical stage) were not available at the start of enhanced adherence counseling sessions, the most recent data were used as baseline data. Eight BSc degree nurses as data collectors and three ART data managers as supervisors participated in the data collection process.
Variables
The main outcome of interest or dependent variable was viral load suppression (suppressed/unsuppressed).
Independent variables included were sex, age, educational status, residency, baseline viral load count, time to start EAC, weight, height, functional status, WHO clinical staging, presence of recurrent OI, baseline CD4 count and baseline hemoglobin level, current ART regimens, regimen change, duration on ART, prior level of adherence, CPT treatment status, INH treatment status, presence of drug interactions, presence of severe drug side effects, history of ART/PMTCT prophylaxis, nutritional status, lack of food, disclosure status, family support, presence of treatment supporter, use of reminder, confidence to take ARVs, presence of depression, presence of substance abuse, presence of diagnosed mental health illness, unsafe sex practice and discontinue ARVs for remedies.
Measurement
Viral load suppression is the second viral load measurement of less than 1000 RNA copies per mL after 3–6 months of EAC while continuing to receive first-line ART.12
Enhanced Adherence Counseling (EAC)
It is a continual and repeated process that involves a structured assessment of the current level of adherence, and explores the specific barriers the patient must overcome, motivates and assists patients to identify solutions and address barriers and develop an individualized adherence intervention plan. It is recommended for patients with viral load results >1000 copies/mL and has been on treatment within six months, which lasts for 3−6 months.10,12
Level of ART adherence was classified based on patient self-report on the percentage of ARV drugs taken as prescribed. Good ART adherence is defined as ≥95% of doses taken as prescribed and poor adherence, defined as <95% of doses taken as prescribed.21
Censored
Lost, drop out, transfer out, died of other causes, or completed study period before viral load suppression.
Data Quality Assurance
To ensure quality, data was collected by ART-trained health professionals after one day of training on the techniques of data collection. The completeness of the data was checked by two trained ART data clerk supervisors to provide feedback in the data collection process and to correct when necessary. Moreover, pretest was performed on 5% of the sample size to assess the reliability and consistency of the data collection tool.
Data Management and Analysis
The data were checked for completeness, then entered in using EpiData version 3.1, and exported to Stata version 14. Descriptive statistics, including mean and frequency, were used to describe the characteristics of the study participants. Incidence of viral load suppression rate concerning person-time at risk was calculated and reported as many suppressed per 100 Person-months of follow-up by assessing the date of enrolment for EAC. A life table was used to estimate the cumulative viral load suppression probability of adults and the Kaplan–Meier survival curve together with Log rank test was used to compare survival between different categories of independent variables. Bivariate and multivariate Cox proportional hazards models were used to identify predictors. Multivariate Cox analysis was done and hazard ratio with 95% CI and P-value was used to assess the strength of association and statistical significance. Variables significant at a p-value less than 0.25 were included in final multivariate Cox-regression analysis to identify independent predictors of viral load suppression after EAC. The model was built by a backward stepwise procedure and compared by the likelihood ratio test and Harrell’s concordance statistics test. Interactions and confounders were tested. A cut-off point with a beta change greater than 20% was used. Before fitting the covariate into model, all the proportionality assumption was tested by a global test based on Schoenfeld residuals and by examining log minus log plots. The overall goodness of model fitness was checked by Nelson Aalen’s cumulative hazard function against Cox Snell residual. Multi-collinearity was checked using Pearson correlation, tolerance, or variance inflation factor. To decide whether a variable is significant, associations with viral re-suppression were estimated with a p-value less than 0.05 in the final model. The crude and adjusted hazard ratios together with their corresponding 95% confidence intervals were computed and interpreted accordingly.
Results
Sociodemographic Characteristics
The study contained 347 medical records of adult study participants and 346 (99.7%) records were included in the review. Of the study participants, 126 (36.42%) were located in the 30–39 age group. The mean age of the study participants was 35.6 (±9.42SD). More than half 187 (54%) of the study participants were married and 22% completed primary level of education. Concerning religious status, most of the study participants 329 (95.09%) were Ethiopian Orthodox Christian followers (Table 1).
Clinical and Laboratory Related Characteristics
The median baseline CD4 count was 306.00 cells/mm3 (IQR 183.00–503.00 cells/mm3) and the median HIV viral load was 8793.00 copies/mL (IQR 2296.00–40,456.50 RNA copies/mL). Moreover, of the study participants, 142 (42.9%), had CD4 count of greater than 350 cells/mm3. About 40.17% of the study participants had a baseline viral load count between 5000 and 49,999 RNA copies/mL.
On the other hand, 305 (88.15%) study participants were WHO had clinical stage one. Most of the participants, 279 (80.64%), had no history recurrent of opportunistic infection. About 169 (51.52%) of the study participants started their EAC session within two weeks after receiving their viral load results. Just 32% of participants in the study completed the EAC session within three months (Table 2).
ART and Treatment Related Characteristics
More than half 260 (75.14%) of the study participants were on ART for more than 59 months and 169 (48.84%) of them had a good adherence level to the ARV medications (see Table 3). Cotrimoxazole prophylaxis was used by around 166 (47.98%) study participants and isoniazid prophylaxis was used by 287 (82.95%) at the time of EAC registration (Figure 1).
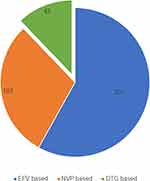 |
Figure 1 ART regimen of the HIV positive adults enrolled on enhanced adherence counseling at high caseload health facilities in Amhara region, Ethiopia from June 2016 to June 2020. |
Nutrition and Related Characteristics
The majority of the study participants had a normal BMI of 241 (70.88%) and lack of food was seen only in 66 (19.08%) of them (Table 4).
 |
Table 4 Actuarial Life Table Cumulative Survival of Adult on Enhanced Adherence Counseling After the Start of at the Interval, High Caseload Health Facilities, Amhara Region, Ethiopia, January 2021 |
Psychosocial, Mental and Behavioral Related Characteristics
More than half (67.63%) of the study participants who disclosed their HIV status were men and 201 (58.09%) had treatment supporters. A 77.46% of the study participants were confident to take their ARV medication. Around 7.8% of the participants in the study were diagnosed with depression. About 36.13% of the study participants had reported persistent use of a condom (Table 5).
Incidence of Viral Re-Suppression
Out of the 346 cohort adults on EAC, 321 (92.7%) were alive, 7 (2.02%) were lost to follow-up, 8 (2.31%) were transferred out to other facilities, and 10 (2.89%) were reported dead. Out of the 179 viral suppressed study participants on EAC 78 (43.57%) were virally suppressed after completion of 6 month EAC session, 18 (10%) in 5 months, 24 (13.4%) in 4 months, and 59 (32.96%) in 3 months.
After starting the EAC session, participants were followed for different periods, which is a minimum of 3 months and a maximum of 6 months with a median follow-up of 5 months, and the cohort contributed to 1602 person-months of follow-up. The overall viral suppression rate of the cohort was found to be 11.17 (95% CI=0.09–0.12) per 100 person-months of observation.
Out of the 179 viral suppressed, the highest incidence of viral suppression was observed after completion of 3 months of EAC session 59 (17.71 per 100 person month), while other 11.53 per 100 person month, 9.72 per 100 person-months and 8.90 per 100 person-months at four, five and six months. The cumulative probability of survival at the end of the 3rd, 4th, 5th, and 6th months were 81.56%, 72.71%, 65.16%, and 19.78%, respectively (Table 4).
Comparison of Viral Suppression Probability Among Categories of Covariates
Kaplan–Meier survival curve together with Log rank test was used to check for the existence of any significant differences in viral suppression probability between the various categories of variables considered in this study. Accordingly, the study participants who had a baseline CD4 count greater than or equal to 350 cells/mm3 had a significantly higher probability of viral load suppression than those who had a CD4 count of fewer than 200 cells/mm3 (log-rank, P-value less 0.05). Study participants that had a baseline viral load count of greater than 50,000 RNA copies per mL had a higher viral load suppression probability compared to those who had a viral load count of less than or equal to 5000 RNA copies per mL (log-rank, P-value less than 0.05).
Study participants who were on DTG-based regimens had a higher viral load suppression probability than those who were on EFV-based regimens (log-rank P-value less than 0.05). However, the p-values of the Log rank test do not show that the mean survival experience of patients among those categories of WHO clinical staging, prior history of adherence, disclosure status, duration on ART, and regimen change (Figure 2).
Predictors of Viral Load Suppression
In bivariate Cox Proportional hazard, factors such as sex, WHO clinical staging, baseline viral load, CD4 count, presence of recurrent OI, current ART regimen, duration on ART, INH prophylaxis and last adherence level are statistically significant at a 25% level of significance.
When variables that had an association with viral load suppression in the bivariate analysis (P-value < 0.25) were all included in the multivariate Cox proportional hazard model using backward stepwise regression. It was found that sex, CD4 count, and presence of recurrent OI had a statistically significant association with viral load suppression (p-value < 0.05).
In this study, females were 1.50 times more likely to have viral load suppression compared to male participants (AHR=1.50, 95% CI: 1.05–2.15).
The study participants with a CD4 count of greater than or equal to 350 cells/mm3 were estimated to have 1.98 (AHR = 1.98, 95% CI: 1.12–3.51) times higher hazard of viral suppression compared to those who had a CD4 count of less than 200 mm3, all other variables held constant.
The hazard of viral load suppression after EAC was 1.85 times more among participants who had no recurrent OI than those who had (AHR = 1.85, 95% CI: 1.06–3.24) (Table 5).
Model Adequacy
After fitting the Cox proportional hazard model, the adequacy was checked by Nelson Aalen cumulative hazard with Cox Snell residual (Figure 3).
Discussion
This study assessed the incidence rate of viral load suppression after EAC and its predictors among HIV-positive adults in high caseload health facilities. A total of 346 adults were followed for 1602 person-month of observation. The study showed that the overall incidence density rate of viral suppression of 11.17 (95% CI=0.09–0.12) per 100 person-months of observation. Viral load suppression probability at the end of the 3rd, 4th, 5th, and 6th month was 81.56%, 72.71%, 65.16%, and 19.78%, respectively, and the overall median viral load suppression time was 5 months (95% CI: 4–6). Being Female, CD4 count of greater than or equal to 350 cells/mm3 and no history of recurrent OIs associated with a higher rate of viral load suppression.
The study results showed that the average incidence of viral load suppression was 11.17 per 100 months of observation. The study also showed that the highest incidence of viral suppression after 3 months of EAC session was observed. The average incidence of viral load suppression was higher than the study conducted in Kenya, which was 3.9 per 100 months of observation.22 The disparities in viral suppression rate could be explained by the fact that the two countries may differ in systems for the management of HIV patients. The median time to viral load suppression in this study is comparable to the studies conducted in Oromia, Ethiopia, and South Africa.23,24 The study findings showed that the median time to suppression of viral load was longer than the study conducted in Southern Ethiopia25 but less than the study conducted in Canada and Botswana.26,27 This difference might be due to the difference in the follow-up duration of the study participants. This study followed the participants for three to six months, while the participants in other studies followed for a minimum of 12 months. Secondly, it might be associated with the availability of an optimized, potent, and well-tolerable ART regimen that has a significant impact on viremia replication.
In this study, the hazard of viral load suppression after EAC was 1.50 times more among females than males. This finding is supported by studies conducted in North Wollo, North East Ethiopia, and Uganda.17,28 The finding was contrary to the study conducted in South Carolina.29 This might be explained in two forms and be due to the first timely start of the EAC session, which might have contributed to better adherence and response to ART therapy in the female participants. Secondly, in the current study, females had higher level of baseline CD4 count than men, which might contribute to better viral load suppression. Nevertheless, the gender difference in viral load suppression rate should be studied further.10,12
The hazard of viral load suppression after EAC was 1.98 times greater among those who had a CD4 count of greater than or equal to 350 cells/mm3 compared to those who had a CD4 count of less than 200 cells/mm3. This finding is supported by a study conducted in Southern Ethiopia, Zimbabwe, Mumbai, and Swaziland.30–32 It is well known that CD4 T-cell count has an inverse relationship with viral replication. As patients’ immune status declines, the rate of viral replication increases as compared to their immune-competent counterparts. In addition, clients with compromised immunity are more vulnerable to different opportunistic infections that sustain a vicious cycle of immunity and viral replication.
The hazard of viral load suppression after EAC was 1.85 times greater among participants who had no recurrent OI than those who had. The findings of this study were supported by the studies conducted in South Africa and Uganda.33,34 This could be explained in two forms, first a large number of the study participant was put on cotrimoxazole and isoniazid prophylaxis that might prevent the occurrence opportunistic infections like TB. Secondly, it could be due to the fact that those patients who had developed recurrent OI were treated while on EAC.
The findings of this study using multivariate Cox modeling showed that baseline viral load count was not a significant predictor of viral load suppression as in most studies.30–32 This might be because poor adherence was associated with high viral load and modified by intensive adherence support.
Factors associated with psychosocial, mental, and behavioral of the study participants were not a significant predictor of viral load suppression.35–37 This contrast might be associated with the diagnosis procedure as well as the skill of profession.
Conclusion
The study showed that the overall incidence density rate of viral suppression was 11.17 per 100 person-months of observation. The highest incidence of viral load suppression was observed after completion of the first 3 months of enhanced adherence counseling. Being female, a CD4 count of greater than or equal to 350 cells/mm3 and no history of recurrent OI were associated with a higher rate of viral load suppression upon initiation of treatment. As a result, ART case managers, adherence counselors, and the multidisciplinary team in both hospitals and health centers should focus on patients with a low current CD4 count in order to improve management of recurrent OI.
Abbreviation
AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; ARV, antiretroviral; CI, confidence interval; HIV, human immunodeficiency virus; PLWHA, people living with HIV/AIDS; WHO, World Health Organization; USAID, United States Aid for International Development; AHR, adjusted hazard ratio; EAC, enhanced adherence counseling; OI, opportunistic infections; MRN, medical record number; EFV, efavirenz; NVP, nevirapine; DTG, dolutegravir.
Data Sharing Statement
All the data supporting the study findings are within the manuscript. Additional detailed information and raw data are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the ethical review committee of college of Health science, Debre Markos University, with the approval number of HSC/R/C/Scr/Co/105/11/13 dated on 13 October 2020. Study participants’ informed consent was waived by the review board as the data source of the patient’s medical record numbers (MRN) that were anonymously registered using codes without personal identifiers such as names of patients. Supporting letter was obtained from the West Gojjam zonal health department office and permission letters were obtained from each hospital administration and head of the health center. The data were collected by keeping confidentiality while reviewing the card of the patient. All methods were performed in accordance with the relevant regulations and guidelines. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
The authors wish to thank the academic staff of Debre Markos University, College of Health Science, for the suggestion and selection of the topic. The authors would also like to thank health institutions providing ART services under the west Gojjam zonal health department. North-west Ethiopia and health personals in ART center, data collectors, and supervisors for giving the chance to conduct this study.
Author Contributions
All authors made a substantial contribution to the conception and design, acquisition of data, or analysis and interpretation of data. They took part in drafting the article and revising it critically for important intellectual content; agreed to submit to the current journal, gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
Gezahegn Terefe Atnafu is principal investigator. The authors report no conflicts of interest in this work.
References
1. UNAIDS. Fact sheet - global HIV statistics; 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
2. UNAIDS. Country factsheets Ethiopia AIDSinfo; 2020. Available from: https://aidsinfo.unaids.org/.
3. ARHB. EFY 2013 Eight Month HIV Prevention & Control Program Performance Review. Debre Tabor: ARHB; 2021.
4. Instiute eph. HIV Related Estimates and Projections in Ethiopia for the Year-2019. Addis Ababa; February, 2020:6.
5. Department WGZH. Annual Report on HIV/AIDS. unit WGZHdoHcat, ed. Fenotselam: West Gojjam Zone Health Department; 2019/2020.
6. UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDSEpidemic. Geneva, Switzerland; 2017.
7. (1)PEPFAR. The global HIV/AIDS epidemic; 2019. Available from: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics.
8. Acollaboration of UNAIDS UaW. Global factsheets AIDSinfo; 2021. Available from: https://aidsinfo.unaids.org/.
9. Organisation WH. Adapting and Implementing New Recommendation on HIV Patient Monitoring. Geneva, Switzerland; 2017.
10. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection, Recommendations for a Public Health Approach. Geneva, Switzerland; 2016.
11. Reference U. The need for routine viral load testing; 2016. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2845_en.pdf.
12. Ethiopia MoHo. National Guidelines for Comprehensive HIV Prevention Care and Treatment, 2019. Addis Ababa, Ethiopia; 2019.
13. Etoori D, Ciglenecki I, Ndlangamandla M, et al. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second-line switches in Swaziland. J Int AIDS Soc. 2018;21(10):e25194. doi:10.1002/jia2.25194
14. Minstery of Health E. National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. Addis Ababa: MoH; 2018.
15. Kerschberger B, Boulle AM, Kranzer K, et al. Superior virologic and treatment outcomes when viral load is measured at 3 months compared to 6 months on antiretroviral therapy. J Int AIDS Soc. 2015;18(1):20092. doi:10.7448/IAS.18.1.20092
16. (1)USAIDS. Global and regional statistics on the status of the AIDS epidemic. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
17. Diress G, Dagne S, Alemnew B, Adane S, Addisu A. Viral load suppression after enhanced adherence counseling and its predictors among high viral load HIV seropositive people in North Wollo Zone Public Hospitals, Northeast Ethiopia, 2019: Retrospective Cohort Study. AIDS Res Treat. 2020;2020:1–9. doi:10.1155/2020/8909232
18. Fox Z, Phillips A, Cohen C, et al. Viral re-suppression and detection of drug resistance following interruption of a suppressive NNRTI-based regimen. AIDS. 2008;22(17):2279. doi:10.1097/QAD.0b013e328311d16f
19. Leisegang R, Stewart A, Sunpath H, et al. Short term adherence tool predicts failure on second line protease inhibitor-based antiretroviral therapy: an observational cohort study. BMC Infect Dis. 2014;14(1):664. doi:10.1186/s12879-014-0664-3
20. Aidsmap. viral load; 2017. Review date May, 2017. Available from: https://www.aidsmap.com/about-hiv/viral-load.
21. Ahmed M, Merga H, Jarso H. Predictors of virological treatment failure among adult HIV patients on first-line antiretroviral therapy in Woldia and Dessie hospitals, Northeast Ethiopia: a case-control study. BMC Infect Dis. 2019;19(1):1–7. doi:10.1186/s12879-019-3924-4
22. Maina E, Mureithi H, Adan A, Muriuki J, Lwembe R, Bukusi E. Incidences and factors associated with viral suppression or rebound among HIV patients on combination antiretroviral therapy from three counties in Kenya. Int J Infect Dis. 2020;97:151–158. doi:10.1016/j.ijid.2020.05.097
23. Ali JH, Yirtaw TG. Time to viral load suppression and its associated factors in cohort of patients taking antiretroviral treatment in East Shewa zone, Oromiya, Ethiopia, 2018. BMC Infect Dis. 2019;19(1):1084. doi:10.1186/s12879-019-4702-z
24. Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12(1):83.
25. Hussen S, Mama M, Mekonnen B, et al. Predictors of time to viral load suppression of adult PLWHIV on ART in Arba Minch General Hospital: a Follow up Study. Ethiop J Health Sci. 2019;29(6):751–758. doi:10.4314/ejhs.v29i6.12
26. d’Arminio Monforte A, Testa L, Adorni F, et al. Clinical outcome and predictive factors of failure of highly active antiretroviral therapy in antiretroviral-experienced patients in advanced stages of HIV-1 infection. AIDS. 1998;12(13):1631–1637. doi:10.1097/00002030-199813000-00010
27. Thiébaut R, Morlat P, Jacqmin-Gadda H, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d’Epidémiologie du SIDA en Aquitaine (GECSA). AIDS. 2000;14(8):971–978. doi:10.1097/00002030-200005260-00008
28. Kipp W, Alibhai A, Saunders LD, et al. Gender differences in antiretroviral treatment outcomes of HIV patients in rural Uganda. AIDS Care. 2010;22(3):271–278. doi:10.1080/09540120903193625
29. Haider MR, Brown MJ, Harrison S. Sociodemographic factors affecting viral load suppression among people living with HIV in South Carolina. AIDS Care. 2021;33(3):290–298. doi:10.1080/09540121.2019.1703892
30. Bvochora T, Satyanarayana S, Takarinda KC, et al. Enhanced adherence counselling and viral load suppression in HIV seropositive patients with an initial high viral load in Harare, Zimbabwe: operational issues. PLoS One. 2019;14(2):e0211326. doi:10.1371/journal.pone.0211326
31. Hicham T, Ilyas E, Tarik H, et al. Risk factors associated with unsuppressed viral load in HIV-1 infected patients at the first antiretroviral therapy in Morocco. Int J Mycobacteriol. 2019;8(2):113. doi:10.4103/ijmy.ijmy_41_19
32. Jobanputra K, Parker LA, Azih C, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):e0116144. doi:10.1371/journal.pone.0116144
33. Joseph Davey D, Abrahams Z, Feinberg M, et al. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29(6):11–14. doi:10.1177/0956462417748859
34. Ekwaru JP, Campbell J, Malamba S, Moore D, Were W, Mermin J. The effect of opportunistic illness on HIV RNA viral load and CD4+ T cell count among HIV-positive adults taking antiretroviral therapy. J Int AIDS Soc. 2013;16:17355. doi:10.7448/IAS.16.1.17355
35. Yehia BR, Stephens-Shield AJ, Momplaisir F, et al. Health outcomes of HIV-infected people with mental illness. AIDS Behav. 2015;19(8):4–5. doi:10.1007/s10461-015-1080-4
36. Hartzell JD, Janke IE, Weintrob AC. Impact of depression on HIV outcomes in the HAART era. J Antimicrob Chemother. 2008;62(2):246–255. doi:10.1093/jac/dkn193
37. Modi RA. HIV Disclosure, Retention in HIV Care, and Viral Load Suppression: A Study Among New to HIV Care Patients. The University of Alabama at Birmingham; 2018.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






