Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Incidence and Predictors of Opportunistic Infections Among Adult HIV Infected Patients on Anti-Retroviral Therapy at Dessie Comprehensive Specialized Hospital, Ethiopia: A Retrospective Follow-Up Study
Authors Dagnaw Tegegne K, Cherie N , Tadesse F , Tilahun L , Kassaw MW , Biset G
Received 24 December 2021
Accepted for publication 27 March 2022
Published 19 April 2022 Volume 2022:14 Pages 195—206
DOI https://doi.org/10.2147/HIV.S346182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Kirubel Dagnaw Tegegne,1 Nigus Cherie,2 Fentaw Tadesse,2 Lehulu Tilahun,3 Mesfine Wudu Kassaw,4 Gebeyaw Biset5
1Department of Comprehensive nursing, College of medicine and Health Science, Wollo University, Dessie, Ethiopia; 2School of Public health, college of Medicine and Health science, Wollo University, Dessie, Ethiopia; 3Department of Emergency Nursing, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia; 4School of nursing, college of health science, Woldia University, Woldia, Ethiopia; 5Department of Pediatric Nursing, College of medicine and Health Science, Wollo University, Dessie, Ethiopia
Correspondence: Kirubel Dagnaw Tegegne, Tel +251938405728, Email [email protected]
Background: Opportunistic infections are the major causes for morbidity and mortality due to HIV infections. Despite advances in HIV diagnosis and management, the incidence of opportunistic infections remains high. This study aimed to assess the incidence and predictors of opportunistic infections among persons living with HIV/AIDS in Ethiopia.
Methods: A retrospective follow-up study was conducted on 354 samples of adults living with HIV on antiretroviral therapy at Dessie Comprehensive Specialized Hospital. Simple random sampling technique was used to select study participants. The data collection format was taken from national antiretroviral intake and follow-up forms. Epi-data Version 4.6.1 and STATA Version 16 software were used for data entry and data analysis respectively. The Cox-proportional hazards regression model was fitted. Kaplan–Meier survival curve was used to estimate opportunistic infections-free survival time. Both bi-variable and multivariable Cox-proportional hazard regression analysis were done to identify predictors of opportunistic infections.
Results: Of the total 354 peoples living with HIV, 114 (32.2%) developed OI, with an incidence rate of 13.5 per 100 person-year (95% CI: 10.8– 15.6). Advanced World Health Organization clinical disease stage (IV) (AHR: 2.1 (95% CI: 1.16, 3.8)), being bedridden (AHR: 1.66 (95% CI: 1.04, 2.65)), poor adherence (AHR: 1.7 (95% CI: 1.1– 2.63), and low CD4 count (AHR: 1.92 95% CI: 1.14– 3.22) were significant predictors of OIs.
Conclusion: Opportunistic infection among HIV/AIDS continues to be a significant public health concern in Ethiopian health care setting. Our results indicate that the incidence of OI is high. Besides, Stage IV HIV status, being bedridden, low CD4 count and poor adherence independently predicts an increased incidence/decreased survival time of OIs among PLWHIV. Early care-seeking and initiation of HAART and continuous follow-up of patients to take their drug timely are essential to curb the incidence of opportunistic infections and improve overall health. Further research on this area is highly recommended.
Keywords: incidence, predictors, opportunistic infections, HIV/AIDS, Dessie
Background
Opportunistic infections (OI) are diseases and cause infections in individuals with weakened immune systems, mainly in people living with Human Immunodeficiency Virus (PLWHIV).1–3 All people living with HIV are susceptible to develop many forms of OI,4 though the prevalence and incidence of HIV-associated OIs vary broadly.5,6
Despite advances in HIV diagnosis and treatment, opportunistic infections (OI) remained a major cause for significant morbidity and mortality among HIV/AIDS patients in low and middle income countries (LMIC).7,8 HIV weakens immune system and causes the risk of developing opportunistic infections that could accelerate HIV progression and transmission.9,10 The occurrence of the most frequent OI differs between low-income and industrialized countries; tuberculosis and recurrent bacterial infections are often observed in the former setting than in the latter.11,12
Studies from northern and northwestern part of Ethiopia indicate the most common OIs among PLWHIV are oropharyngeal candidiasis and tuberculosis (Tb), respectively.13,14 PLWHIV are more at risk of contracting OIs depending on exposure to potential pathogens, the virulence of the pathogens, the degree of host immunity, and the use of different antimicrobial prophylaxis to prevent OI.15
Although OIs are major causes of morbidity and mortality among PLWHIV are OI,16 results related to these infections; particularly in the study area, are scarce. When OIs are present in people living with HIV, It indicates that they are in the severe phase of AIDS, and most of these infections often contribute to the death of infected individuals.17–19 More than 90% of HIV/AIDS deaths are due to opportunistic infections and malignancies.10 The type of opportunistic infections which affect people living with HIV/AIDS vary from region to region.20
The incidence of OI in Ethiopia is also rising at a high rate, shown by a study conducted in Mekelle, Ayder Hospital, which reported 7.5 cases per 100 person-year of observations.13 In recent years, efforts have been made to integrate OI diagnosis and treatment with HIV care to prevent, diagnose and treat OI among PLWHIV. However, this also needs a good understanding of the situation through research especially in the study area where OI incidence rate and related factors are not fully studied yet. Hence, this study aimed to assess the incidence and predictors of OI among PLWHIV who attended ART care at Dessie Comprehensive Specialized Hospital.
Methods
Study Design, Area, and Period
A retrospective follow-up study was conducted at Dessie Comprehensive Specialized Hospital (DCSH) from January 1, 2015, to December 31, 2020. Dessie located about 400 km from Addis Ababa, the capital city of Ethiopia. The hospital provides service for more than five million people of South Wollo and neighboring zones and regions. The hospital has been providing ART follow-up care services since 2005. Currently, more than 10,000 patients are on active follow-up, of who over 7832 started highly active antiretroviral treatment (HAART).
Study Participants
In this study, all patients living with HIV who had follow-up at the hospital and whose age is 15 years and above were our source population. Besides, all patients living with HIV who were enrolled to treatment between January, 2015 and December, 2016 were our study population.
All PLHIV, who has been receiving treatment in HIV clinic at the DCSH from January 2015 to December 2016, were included and followed until December 2020. We excluded patients who already developed OI at the start of the study. Similarly, records which had incomplete baseline information such as CD4 count, hemoglobin level, WHO clinical stage, weight, unknown date of ART initiation time and OI diagnosis were excluded from the study.
Sampling Size Determination and Procedure
The required sample size (n = 362) was determined by survival analysis sample size estimation using STATA 16 in which functional status, WHO clinical stage, and male considered as exposure variable. Statistical assumptions of 95% confidence level, 80% power, and a Hazard ratio of 1.35 that would give maximum sample size taken from a previous study21 was considered. The sampling frame was prepared based on the Participants medical registration number. Simple random sampling was used to select samples from the prepared sampling frame. 362 samples were selected randomly by lottery method among 832 ART users who started ART between January 1, 2015 and December 31, 2016. This period was chosen to get a complete five-year follow-up study period and to have the advantage of standardized ART documentation and reporting formats used during this period.
Variables of the Study
In this study, the dependent variable was time to occurrence of opportunistic infections during the follow-up period. The independent variables were Socio-demographic characteristics (ie, age, sex, residence, marital status, occupation);
Clinical and laboratory predictors (ie, WHO clinical stage, CD4 count, hemoglobin (hgb) level, underweight, functional status,) and medication-related predictors (ie, past OI prophylaxis, type of baseline ART regimen, ART eligibility criteria, change in drug regimen, level of ART adherence, ever taking Isoniazid Preventive Therapy (IPT), ART side effects, and ART treatment failure), Functional status of the patient, ie, working, ambulatory or bedridden.
Operational Definition of Variables
Event
Was labeled when HIV-infected adults developed any OI after ART initiation during the follow-up period.
The Incidence Rate of OI
Was calculated by dividing all newly OI occurrences by the total follow-up time of patients in a year.
Opportunistic Infections (OIs)
Were recorded when any infections occur after initiation of HAART.
Survival
The person without occurrence of any OI during follow-up Time.
Censored
Was recorded when HIV-infected Adults quit or transferred out (whether dead or alive) to other health institutions or are still on active ART follow-up but no evidence for any OI occurrence by the end of the study.
Level of ART Adherence
Good adherence: If PLHIV are adherent >85% (ie, the percentage of missed doses is <3 doses of 30 doses or <4 doses of 60 doses), as documented by ART health personnel. Poor adherence: If PLHIV are adherent <85% (ie, the percentage of missed doses is >5 doses of 30 doses or >9 doses of 60 doses), as documented by ART health personnel. Time to develop opportunistic infection: The time from adult ART initiation to the event (OI) of occurrence during the follow-up period.
Data Collection Procedures and Quality Assurance
The data extraction form was based on The Federal Ministry of Health’s HIV-care/ART follow-up and intake records which are currently used in Ethiopian ART clinics. The data extraction form included the following variables: socio-demographic characteristics, ART and other medication, clinical and laboratory-related information. Data was collected over three weeks in April, 2021 by three BSc well prepared nurses in the ART clinic of DCSH. To ensure data quality, a well-adapted data extraction form from a standardized ART intake and follow-up form was used, and data collectors took one day training. All the filled variables were double-checked for completeness by taking some randomly selected cards and the supervisor carefully monitored the entire data collection process. The predictor variables were recorded at the baseline and occurrences of OI were registered during follow up period based on the health professionals’ reporting on the patient chart. Before data entry, data were checked for completeness and consistency.
Data Analysis
Epi-data Version 4.6.1 and STATA Version 16 were used for data entry and data analysis, respectively. Descriptive statistics presented as percentage, mean, median and visualized using tables and charts. Kaplan–Meier method was used to estimate the OI free survival time.
The total number of OI cases per 100 person-year observations calculated and labeled as incidence rate. In addition, a generalized Log rank test was used to compare the OI free survival time between different categorical predictor variables. Multi-collinearity test was used to check if there was associations among predictor variables (all values of VIF<3). The cox proportional hazard regression model assumptions were checked using the Schoenfeld residuals test (global test = 0.2). Cox-Snell residual test was used to observe the goodness of fit. A bi-variable Cox-proportional hazard regression model was used to select variables that would be considered for the final model. In the bi-variable analysis, Variables having a p-value of ≤0.25 were fitted into the multivariable Cox-proportional hazard regression model.
Finally, the presence of a significant association between the predictor and outcome variables was reported by adjusted hazard ratio with its corresponding 95% confidence interval.
Ethical Considerations
Ethical clearance was obtained from Wollo University, College of Medicine and Health Science ethical review board. Additionally, Letter of permission was obtained from the medical director of Dessie Comprehensive Specialized Hospital. Appropriate measures were taken to ensure confidentiality of the data. Furthermore, this study was conducted according to the principles of Declaration of Helsinki.
Results
Socio-Demographic Characteristics
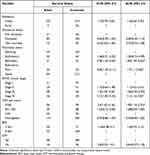
A total of 362 medical records of Adults on ART from January 2015 to December 2020 were reviewed, and from this, 354 (97.7%) complete records of adults analyzed in the study (Figure 1). The mean age was 32.83 (± 10.8 SD) years, and 71.5% of the study participants were female. More than half (n = 236, 66.7%) were unemployed. The majority of participants (90.7%) were urban residents. A total of 267 (75.4%) patients disclosed their HIV status. (Table 1)
 |
Table 1 Socio-Demographic Characteristics of PLHIV on HIV Care at Dessie Comprehensive Specialized Hospital, January 2015 to December 2020 |
 |
Figure 1 Flowchart of PLWHIV on treatment at Dessie comprehensive specialized hospital, Northeast Ethiopia, 2015 to 2020. |
Clinical, Laboratory, and Medication-Related Characteristics
Describing on functional status, half (50.3%) of study participants were classified as working functional status. The mean baseline CD4 cell count was 342.04 cell/mm3 (SD: ± 192.7 cell /mm3). Clinically, one-third (34%) of the study participants were classified as WHO clinical stage II. Besides, 87.3% of study participants had 10 g/dl and above hemoglobin level. At baseline, one-third (32.5%) of the participants were undernourished (BMI < 18.5). Patients were taking a standardized three-drug ART regimen. These three drug regimens used in different combinations were tenofovir (TDF), lamivudine (3TC), nevirapine (NVP), zidovudine (AZT), and efavirenz (EFV). We found that the commonly taken regimen combinations by patients living with HIV were: TDF+3TC + EFV (68.4%), TDF+3TC + NVP (15%), AZT+3TC + NVP (11.6%), and Others (5.1%). Majority (84.5%) of the participants had a history of good adherence. Moreover, 79.7% of participants used CPT, but, below half (45.8%), received IPT. (Table 2)
 |
Table 2 Clinical, Laboratory, ART, and Other Medication-Related Information of HIV Patients on HIV Care at Dessie Comprehensive Specialized Hospital, Northeast, Ethiopia |
Incidence of Opportunistic Infections During Follow-Up
In this study, 354 study participants were followed for a different period, resulting a total of 10,130 months or 844.16 years of observation with a median individual follow-up of 32 months (range: 1–60). During the follow-up period, 114 (32.2%) of the study participants experienced OI with an overall incidence rate of OIs 13.5 (95% CI: 10.8–15.6) per 100 person-year of observation. Among all types of OI occurring during the follow-up time, TB (37.7%) was the most common, followed by bacterial pneumonia (14.9%) and oral candidiasis (14%). (Figure 2) The highest Incidence of OI was observed during the first year of enrolment (8.3 cases per 100 person-years) and then dropped into 1.3 cases per 100 person-years in the second year of follow-up.
 |
Figure 2 Incidence of common opportunistic infection among PLWHIV at Dessie Comprehensive Specialized Hospital, 2021 (n=354). |
Opportunistic Infections Free Survival Time of Predictor Variable
The result of this study showed the OI-free survival time of patients classified as bedridden functional status was less when compared to patients classified as working active status. (Figure 3) Additionally, adult patients with poor ART drug adherence had less OI free survival time than patients with good ART drug adherence. (Figure 4)
 |
Figure 4 Kaplan–Meier curves of OI-free survival proportion based on adherence to ART drugs at Dessie Comprehensive Specialized Hospital from January 2015 to December 2020. |
Predictors of OI Among Patients Living with HIV on ART
Residency, functional status, disclosure status, WHO clinical stage, drug adherence, taking CPT, BMI, hemoglobin level, and CD4 count were variables for multivariable analysis. Of these: functional status, WHO clinical staging, CD4 counts, and ART drug adherence were found to be significant predictors of OI. In this study, the hazard of developing OI among PLWHIV classified as bedridden at baseline was 1.6 times (AHR: 1.6, 95% CI: 1.04, 2.65) higher than those classified as working functional status. Similarly, the hazard of developing OI among PLWHIV classified as WHO clinical stage IV at baseline was 2.1 times (AHR: 2.1, 95% CI: 1.16, 3.8) higher than those classified as WHO Stages I.
On top of that, the hazard of developing OI among PLWHIV who had poor adherence was 1.7 times (AHR: 1.7, 95% CI: 1.1, 2.63) higher than those classified as having good adherence. Kaplan–Meier analysis for specific OI in different categories of adherence level showed no crossing of lines between the poor and good adherence level.
Moreover, the hazard of developing OI among HIV- positive patients who had low CD4 count at baseline was 1.92 times (AHR: 1.92, 95% CI: 1.14, 3.22) times higher than those categorized as CD4 count of ≥351 cells. The goodness of fit for the model is illustrated using a Cox-Snell residual test. (Figure 5)
 |
Figure 5 The goodness of fit test for the Cox-proportional hazard regression model. |
Discussion
This facility-based retrospective follow-up study was aimed to determine the incidence of OI among patients living with HIV on ART at Dessie Comprehensive Specialized Hospital. In this study, almost one third (32.2%) of the study participants experienced OI, which results an overall incidence rate of OI 13.5 per 100 person-year of observation.
This finding is higher than the previous Ethiopian study conducted in Ayder Referral Hospital (7.5 cases/100 person-years).13 This higher figure might be attributed to significant number of patients followed in this study had poor ART drug adherence and were at advanced stages of HIV/AIDS which could probably elevate the incidence. In addition, sample size variations between the two studies could contribute for this difference.
In this study, the incidence of OI in patients with HIV is quite lower than a study conducted in India, Pune, which is 35 per 100 people-year of follow up.22 This difference might be the retrospective nature of the data in this study, but in the Indian study, a prospective cohort study. The incidence of OI in this study was comparable to the study conducted in Senegal.23 However, the incidence of OI found by this study is higher than the study conducted in Brazil (2.63 per 100 person-years)24 in the United States and Canada (2.3 cases per 100 person-years).25 This could be due to variations in follow-up periods, used outcome variable criteria, and study population, including adults and children. Moreover, this difference might be due to developed countries and middle-income countries provide better healthcare service to prevent the occurrence of OIs than countries with resource-limited settings like Ethiopia.
According to this study, the most common type of OIs occurred after initiation of HAART is tuberculosis, 37.7%. This result is in line with a study conducted in Mekelle Ayder referral hospital, which reported tuberculosis (32.3%) as the highest proportion among the other OIs.13 The reason for higher number of tuberculosis in our study could be due to only 45.8% of PLWHIV took IPT. Lower IPT intake is associated with increased incidence of tuberculosis.26 After analysis have been done, predictors significantly associated with OI-free survival time were bed redden functional status, advanced WHO clinical stage IV, low CD4 count at baseline, and poor drug adherence (Table 3).
In this study, patients with poor adherence had a significantly higher risk of experiencing OI than their counterparts (AHR was 1.8). This finding is in agreement with results from other studies conducted in Arbaminch hospital.27 The study methods used in our study were similar to those of the study from Arbaminch Hospital, except that the survey from Arbaminch Hospital considered patients taking Cotrimoxazole preventive therapy. Poor adherence results in decline of CD4 count which is directly related to development of OIs.28
In this study, patients categorized into to baseline CD4 count (CD4 count < 200 cell/mm3) had a significant association with the development of OI, which is consistent with studies conducted in India,22 England.29 A 10-year retrospective study in Uganda26 also supported this finding. Opportunistic infections did occur more frequently in PLWHIV with lower CD4 count (< 200 cell/mm3). The result of association between CD4 count and incident OIs confirms the known biological relationship between low CD4 count and OIs.30,31
In our study, the advanced WHO clinical disease stage (IV) is a crucial predictor of OI among HIV-positive adults on ART. This finding is consistent with studies previously conducted in different regions of Ethiopia27,32 and South Africa.33 As WHO clinical disease staging becomes more advanced, the risk of developing OI and re occurrence significantly increase.34 Patients at stage (IV) HIV/AIDS are worse in terms of immune system35,36 and at higher risk of mortality and morbidity due to various types of OIs, especially tuberculosis, bacterial and fungal infections.37 The most severe and life-threatening OI become more apparent at stage IV HIV/AIDS in peoples living with HIV/AIDS. Therefore, early screening and ART initiation immediately after clients know their status will significantly improve ART drug adherence and reduce HIV related sufferings from advanced stages of diseases and opportunistic infections in peoples living with HIV. Being bedridden functional status are more likely to develop OIs because patients become bedridden due to advanced disease stage and severe immune-compromised stage of HIV, this finding, bedridden patients had 17% more chance of being affected by OIs (AHR: 1.707) which is in line with a study conducted in Debre Markos referral hospital.38 But the Debre Markos hospital study was only operated on the incidence of TB in HIV patients.
Limitation of the Study
Since the nature of the data collection was retrospective, the study relied on the available information for the number and types of diseases reported during follow-up. Therefore some data might be missed. Some of the diagnoses were not clinical as there was limited capacity to make definitive diagnoses.
Conclusion
The incidence rate of OI among HIV-positive people treated at Dessie Comprehensive Specialized Hospital ART clinic is high. More importantly, baseline bedridden functional status, poor patient adherence to ART drugs, advanced stages of diseases (WHO stage IV), and CD4 count less than < 200 cell/mm3 independently predicts an increased incidence/decreased survival time of OIs among PLWHIV. Early care-seeking and initiation of HAART and continuous follow-up of patients to take their drug timely are essential to curb the incidence of opportunistic infections and improve overall health. Further research should be done using a prospective design to explore additional factor contributing to the high incidence rate of OIs.
Abbreviations
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CPT, cotrimoxazole preventive therapy; DCSH, Dessie Comprehensive Specialized Hospital; HAART, highly active antiretroviral therapy HIV, human immuno virus; IPT, isoniazid preventive therapy; OIs, opportunistic infections; PLWHIV, persons living with human immuno virus; PCP, pneumocystis pneumonia; TB, tuberculosis; WHO, World Health Organization.
Data Sharing Statement
Data will be available upon request of the corresponding author.
Acknowledgments
The author would like to thank advisors; Nigus Cherie and Fentaw Tadesse, for their unreserved support and health care professionals working at the ART clinic of Dessie Comprehensive Specialized Hospital for their kindness and valuable support during data collection and chart retrieval. The authors also extend their special thanks to both data collectors and supervisors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300. doi:10.1001/jama.296.3.292
2. Iroezindu M, Ofondu E, Hausler H, Van Wyk B. Prevalence and risk factors for opportunistic infections in HIV patients receiving antiretroviral therapy in a resource-limited setting in Nigeria. J AIDs Clin Res. 2013;3:2.
3. Podlekareva D, Mocroft A, Dragsted UB, et al. Factors associated with the development of opportunistic infections in HIV-1–infected adults with high CD4+ cell counts: a EuroSIDA study. J Infect Dis. 2006;194(5):633–641.
4. WHO. Global health sector strategy on HIV. 2016–2021; 2019. Available from: https://wwwwhoint/news-room/fact-sheets/detail/hiv-aids.
5. Kharsany AB, Karim QA. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34.
6. Low A, Gavriilidis G, Larke N, et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low-and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(12):1595–1603.
7. Ethiopian Public Health Institute and ICAP at Columbia University. Ethiopia Population-Based HIV Impact Assessment (EPHIA). Summary Sheet: Preliminary Findings; 2017–2018. 2018:4–7.
8. IeDEA T. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-and high-income countries. J Acquired Immune Deficiency Syndromes. 2014;65(1):e8.
9. Control CfD, Prevention. Guidelines for the Prevention and Treatment of opportunistic Infections in HIV-infected Adults and Adolescents. MMWR. 2009;58(4):1–206.
10. Edwards LA. Establishing a Pastoral Care Ministry for Those Infected and Affected by HIV/AIDS at the Robert Pinn Memorial Baptist Church. Eastern University; 2015.
11. Seyler C, Messou E, Gabillard D, Inwoley A, Alioum A, Anglaret X. Morbidity before and after HAART initiation in Sub-Saharan African HIV-infected adults: a recurrent event analysis. AIDS Res Hum Retroviruses. 2007;23(11):1338–1347.
12. Zhou J, Paton N, Ditangco R. AIDS-defining illness diagnosed within 90 days after starting highly active antiretroviral therapy among patients from the TREAT Asia HIV Observational Database. Int J STD AIDS. 2007;18(7):446–452.
13. Arefaine ZG, Abebe S, Bekele E, Adem A, Adama Y. Incidence and predictors of HIV related opportunistic infections after initiation of highly active antiretroviral therapy at Ayder Referral Hospital, Mekelle, Ethiopia: a retrospective single centered cohort study. PLoS One. 2020;15(4):e0229757.
14. Moges N, Kassa G. Prevalence of opportunistic infections and associated factors among HIV positive patients taking anti-retroviral therapy in DebreMarkos Referral Hospital, Northwest Ethiopia. J AIDs Clin Res. 2014;5(5):1–300.
15. World Health Organization. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. World Health Organization; 2007.
16. Mariam ZT, Abebe G, Mulu A. Opportunistic and other intestinal parasitic infections in AIDS patients, HIV seropositive healthy carriers and HIV seronegative individuals in southwest Ethiopia. East Afr J Public Health. 2008;5(3):169–173.
17. Nsagha DS, Njunda AL, Assob NJC, Ayima CW, Tanue EA, Kwenti TE. Intestinal parasitic infections in relation to CD4+ T cell counts and diarrhea in HIV/AIDS patients with or without antiretroviral therapy in Cameroon. BMC Infect Dis. 2015;16(1):1–10.
18. Patel SD, Javadekar TB, Kinariwala D. Enteric opportunistic parasitic infections in HIV seropositive patients at tertiary care teaching hospital. Natl J Med Res. 2015;5(3):190–194.
19. Shenoy N, Ramapuram JT, Shenoy A, Ahmed J, Srikant N. Incidence of opportunistic infections among HIV-positive adults on highly active antiretroviral therapy in a teaching hospital, India: prospective study. J Int Assoc Providers AIDS Care. 2017;16(3):309–311.
20. Sun H, Chen M, Hsieh S, et al. Changes in the clinical spectrum of opportunistic illnesses in persons with HIV infection in Taiwan in the era of highly active antiretroviral therapy. Jpn J Infect Dis. 2006;59(5):311.
21. Shearer K, Maskew M, Ajayi T, et al. Incidence and predictors of herpes zoster among antiretroviral therapy-naive patients initiating HIV treatment in Johannesburg, South Africa. Int J Infect Dis. 2014;23:56–62.
22. Ghate M, Deshpande S, Tripathy S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. Int j Infect Dis. 2009;13(1):e1–e8.
23. De Beaudrap P, Etard J-F, Diouf A, et al. Incidence and determinants of new AIDS-defining illnesses after HAART initiation in a Senegalese cohort. BMC Infect Dis. 2010;10(1):1–9.
24. Candiani T, Pinto J, Cardoso CAA, et al. Impact of highly active antiretroviral therapy (HAART) on the incidence of opportunistic infections, hospitalizations and mortality among children and adolescents living with HIV/AIDS in Belo Horizonte, Minas Gerais State, Brazil. Cadernos de Saúde Pública. 2007;23:S414–S23.
25. Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000–2010. J Infect Dis. 2016;214(6):862–872.
26. Sabasaba A, Mwambi H, Somi G, Ramadhani A, Mahande MJ. Effect of isoniazid preventive therapy on tuberculosis incidence and associated risk factors among HIV infected adults in Tanzania: a retrospective cohort study. BMC Infect Dis. 2019;19(1):1–8.
27. Dalbo M, Tamiso A. Incidence and predictors of tuberculosis among HIV/AIDS infected patients: a five-year retrospective follow-up study. Adv Infect Dis. 2016;6(02):70.
28. Wachamo D, Bonja F. Magnitude of Opportunistic Infections and Associated Factors Among HIV-Positive Adults on ART at Selected Public Hospitals in Sidama National Regional State, Southern Ethiopia. HIV/AIDS. 2020;12:479.
29. Mocroft A, Youle M, Phillips AN, et al. The incidence of AIDS-defining illnesses in 4883 patients with human immunodeficiency virus infection. Arch Intern Med. 1998;158(5):491–497.
30. Damtie D, Yismaw G, Woldeyohannes D, Anagaw B. Common opportunistic infections and their CD4 cell correlates among HIV-infected patients attending at antiretroviral therapy clinic of Gondar University Hospital, Northwest Ethiopia. BMC Res Notes. 2013;6(1):1–7.
31. Tewachew AS, Mekonnen WN, Mekuria AD, Amare YE. Determinants of Opportunistic Infections Among HIV-Positive Patients on HAART in Debre Berhan Referral Hospital, North Shoa Zone, Ethiopia, 2020: a Case–Control Study. HIV/AIDS. 2021;13:337.
32. Alene KA, Nega A, Taye BW. Incidence and predictors of tuberculosis among adult people living with human immunodeficiency virus at the University of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect Dis. 2013;13(1):1–9.
33. Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. Aids. 2005;19(18):2109–2116.
34. Melkamu H, Seyoum B, Dessie Y. Determinants of tuberculosis infection among adult HIV positives attending clinical care in western Ethiopia: a case-control study. AIDS Res Treat. 2013;2013:64.
35. Diaz A, Del Romero J, Rodriguez C, et al. Effects of region of birth, educational level and age on late presentation among men who have sex with men newly diagnosed with HIV in a network of STI/HIV counselling and testing clinics in Spain. Eurosurveillance. 2015;20(14):21088.
36. Sobrino-Vegas P, Miguel LG-S, Caro-Murillo AM, et al. Delayed diagnosis of HIV infection in a multicenter cohort: prevalence, risk factors, response to HAART and impact on mortality. Curr HIV Res. 2009;7(2):224–230.
37. Ford N, Vitoria M, Hirnschall G, Doherty M. Getting to zero HIV deaths: progress, challenges and ways forward. J Int AIDS Soc. 2013;16(1):54.
38. Temesgen B, Kibret GD, Alamirew NM, et al. Incidence and predictors of tuberculosis among HIV-positive adults on antiretroviral therapy at Debre Markos referral hospital, Northwest Ethiopia: a retrospective record review. BMC Public Health. 2019;19(1):1–9.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.