Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Incidence and Predictors of Adverse Drug Events Among People Receiving Drug Resistant Tuberculosis Treatment in Uganda: 8-Year Retrospective Cohort Study
Authors Nasasira M , Kalyango JN, Mupere E , Baluku JB
Received 29 July 2022
Accepted for publication 12 December 2022
Published 15 December 2022 Volume 2022:18 Pages 1117—1127
DOI https://doi.org/10.2147/TCRM.S381800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Marble Nasasira,1 Joan N Kalyango,1,2 Ezekiel Mupere,3 Joseph Baruch Baluku4,5
1Clinical Epidemiology Unit, Makerere University College of Health Sciences, Kampala, Uganda; 2Department of Pharmacy, Makerere University College of Health Sciences, Kampala, Uganda; 3Department of Paediatrics and Child Health, Makerere University College of Health Sciences, Kampala, Uganda; 4Division of Pulmonology, Kiruddu National Referral Hospital, Kampala, Uganda; 5Makerere University Lung Institute, Kampala, Uganda
Correspondence: Marble Nasasira, Tel +256 778443346, Email [email protected]
Background: Adverse drug events (ADEs) are regarded as the most essential therapeutic issue during management of drug-resistant tuberculosis (DR-TB) due to the long duration of therapy and concurrent use of many second-line medications. This study aimed to determine the incidence and factors associated with ADEs among patients receiving DR-TB treatment at Mulago hospital in Uganda.
Methods: A retrospective cohort study was conducted among 417 DR-TB patient records at Mulago National Referral Hospital from January 2013 to December 2020. Using the data abstraction form, data were collected on socio-demographic and clinical factors, adverse drug events and treatment follow-up time. Data were double entered in Epi data version 3.2 and later exported to Stata version 14.0 for analysis. The incidence rate of adverse drug events was computed using number of cases of ADE divided by overall patient follow-up time. Poisson regression model was used to determine the factors associated with ADEs. The predictors were considered significant at if p< 0.05.
Results: The overall incidence was 5.56 ADEs per 100 person months (95% confidence interval (CI) 5.01, 6.15). Treatment regimens containing an aminoglycoside (incident rate ratio (IRR) 1.106, 95% CI 1.005– 1.216 p=0.0391), linezolid (IRR 1.145, 95% CI 1.008– 1.229 p = 0.037) or pyrazinamide (IRR 1.226, 95% CI 1.072– 1.401 p = 0.003) and the treatment duration (in months) (IRR 1.005, 95% CI 1.001– 1.010 p = 0.042) were associated with ADEs.
Conclusion: Regimens containing aminoglycosides, linezolid, or pyrazinamide and increase in treatment duration (months) were associated with an increased risk of ADEs. Clinicians should quickly adopt all oral shorter treatment regimens to obviate the need for aminoglycosides and reduce exposure duration.
Keywords: adverse drug events, drug-resistant tuberculosis, Uganda
Introduction
Drug-resistant Tuberculosis (DR-TB) continues to be a public health growing burden. Globally, about half a million people developed rifampicin-resistant TB (RR-TB), of which 78% had multidrug-resistant TB (MDR-TB) in 2019.1 In sub-Saharan Africa, the rate of decline in the burden of DR-TB is still low, at only 0.12% per year.2,3 About 12% and 25.3% of people who were previously treated for TB were found to have MDR-TB and DR-TB, respectively, in the Uganda National DR-TB survey.4 This high prevalence of DR-TB could have been attributed to poor adherence to TB treatment.5 Uganda is also among the highest TB/HIV burden countries in the world.1 DR-TB is a situation where TB organisms continue to grow in the presence of one or more anti-TB drugs confirmed using a drug susceptible test, RR is defined as Mycobacterium TB isolates that are resistant to rifampicin, with or without resistance to other anti-TB medications and MDR-TB is defined M. tuberculosis strains that are resistant to at least Rifampicin and Isoniazid.6
The World Health Organization (WHO) recently recommended shorter all-oral bedaquiline (Bdq)-containing regimen of 9–12 months in patients with confirmed MDR/RR-TB who have not been exposed to treatment with second-line TB medicines and in whom resistance to fluoroquinolones has been excluded. For those who are not eligible, the proposed total duration of longer MDR-TB regimens is about 18–20 months, modified according to the patient response.6 However, due to long duration of therapy and concurrent use of multiple second-line drugs, adverse drug events (ADEs) are regarded as a major challenge in patients.7 A study conducted in Rwanda reported that, ADEs were linked to a nearly two-fold increased probability of treatment failure.8 This association was consistent with a study done in Pakistan and Angola.9,10 Treatment failure resulting from non-adherence could lead to an increase in community transmission of DR-TB.
More than half of people with MDR-TB were reported to have experienced at least one ADE in a systematic review and meta-analysis. The three commonest ADEs were gastrointestinal disorders, ototoxicity and psychiatric disorders.7 However, this study was published 5 years ago and therefore does not reflect the several changes in DR-TB regimen. There has been progress in DR-TB drug development and new agents such as bedaquiline and delamanid (Dlm) have been developed.11 Moreover, clofazimine and linezolid have also been repurposed to be used as preferred drugs in DR-TB management. However, drug safety information from programmatic settings is still limited particularly for new and repurposed drugs. A more recent meta-analysis reported that that levofloxacin (Lfx), moxifloxacin (Mfx), bedaquiline (Bdq), and clofazimine (Cfz) were associated with a low risk of ADEs while amikacin (Am), kanamycin (Km), capreomycin (Cm), p-amino salicylic acid (PAS) and linezolid (Lzd) posted higher rates of ADEs leading to permanent discontinuation.12 However, the prevalence of HIV among the DR-TB patients in this meta-analysis was only 10% yet the prevalence of HIV among DR-TB patients in Uganda is 59.4%.13 Of concern is that ADEs are exacerbated in HIV co-infected individuals who have a weakened immune system, poor nutrition, and the propensity for anti-TB and antiretroviral drug-related adverse events and drug–drug interactions to overlap.14
In this study we determined the incidence and factors associated with adverse drug events among patients receiving DR-TB treatment at Mulago Hospital in Uganda.
Materials and Methods
Study Design and Setting
A retrospective cohort study was conducted among DR-TB patients who had started therapy from January 2013 to December 2020 at Mulago National Referral Hospital. The study utilized secondary data from the unit DR-TB registers and medical charts of DR-TB patients. Mulago Hospital TB unit is located in Kampala, an urban setting, which treats over 25% of the country’s DR-TB burden. Mulago serves as a specialist treatment and diagnostic center for DR-TB. Most patients are referrals from other units within and out of Kampala and services are free of charge including admissions.
DR – TB Treatment in Uganda
The programmatic management of DR-TB in Uganda began in 2012 although a local guideline was in place in 2011.15 The guidelines recommended a standardized regimen that consisted of a 6-months’ intensive phase with kanamycin (Km) or capreomycin (Cm), levofloxacin (Lfx), ethionamide (Eto), cycloserine (Cs), and pyrazinamide (Z) followed by a continuation phase of 18 months without the aminoglycoside. Individualized regimens were recommended when informed by drug susceptibility testing and tolerance. The alternative agents were ethambutol (E), amikacin (Am) and p-amino salicylic acid (PAS). In 2016, the revised guidelines recommended an intensive phase of 6 months of Km + Lfx + Eto + Cs + Z or 4 months after culture conversion, whichever was longer. A continuation phase without the aminoglycoside for a duration of 14 months or at least 20-months post-culture conversion (whichever was longer), was recommended. Bedaquiline (Bdq) and delamanid (Dlm), newly discovered drugs for DR-TB treatment, were initially available on a compassionate basis until they were increasingly available in the country by 2018. A modification to the standard regimen was recommended for the treatment of XDR-TB, pre-XDR-TB and other difficult-to-treat patients but the decision was to be made by the national DR-TB panel on a case-to-case basis. In 2017, an annex to the 2016 guidelines introduced the short-term regimen (STR) which consisted of 4–6 months of Km + moxifloxacin (Mfx) + clofazimine (Cfz) + Z + E + high-dose-Isoniazid (Hh) + Eto and 5 months of Mfx + Cfz + Z + E for patients with confirmed sensitivity to fluoroquinolones and an injectable aminoglycoside. Treatment was administered by health worker supervised directly observed therapy (DOT) using both facility and community-based models.13
Study Population and Sample Size Estimation
All confirmed DR-TB patients who received DR-TB treatment from January 2013 to December 2020 at Mulago Hospital were considered eligible for the study. Patients with missing charts and those who were transferred to other health facilities were excluded from the study.
The estimated sample size was 417 patient files. This was calculated using the formula for single proportions.16 We set the level of precision at 5% and confidence level at 95%. We assumed the proportion of ADEs among patients receiving DR TB treatment that was reported at 0.573 in a systematic review and meta-analysis.7 The sample size based on those assumptions was 376 patient records, which was inflated by 10% to cater for missing data. Systematic sampling method was used to sample 417 patient files from 820 files.
Study Definition and Measurements
The dependent variable was Adverse Drug Event (ADE). It was defined as any unwanted medical occurrence in a patient that received DR-TB treatment but did not necessarily have a causal relationship to the treatment17 The research team classified ADEs based on the Hartwig’s Severity Assessment Scale; Grade 1: Mild symptoms that necessitated monitoring and did not necessitate treatment. Grade 2: Moderate symptoms that necessitated medical intervention, such as the use of auxiliary medications. Grade 3: Severe symptoms that showed inability to carry social or functional activities requiring medical intervention or even hospitalization. Grade 4: Life-threatening symptoms accompanied by an incapacity of the health worker to provide basic health care, necessitating medical intervention or hospitalization to avoid permanent impairment, disability, or death.18 ADEs were documented in the patient files by the primary care physicians at the unit using the ADE monitoring form. Independent variables included socio-demographic factors (age, sex, area of residence, year of enrolment, marital status, any history of smoking and history of alcohol use) and clinical factors (weight, type of patients, resistance profile, drug regimen, duration of treatment, adherence, HIV status, known diabetes and known hypertension). Assessment of adherence within the entire treatment period was calculated by dividing number of DR-TB doses taken by number of DR-TB doses prescribed X 100. Greater than 95% was classified as very good, 94–85% as good and below 85% as poor. Specific ADEs are defined as shown in Table 1.
 |
Table 1 Description of ADE as Documented in the Patient File |
Data Collection Procedures
A data abstraction form in English language was used to retrieve data from patients’ register and patients’ charts by the principal investigator assisted by three trained research assistants. The research assistants were enrolled nurses working in the DR-TB department but employed as support staffs. The data abstraction form was pretested on 10 patient records to check for its feasibility before the study commenced. Data were checked for any inconsistencies, coding errors, out of range values, completeness, accuracy, clarity, missing values, and appropriate corrections were made on daily basis.
Data Processing and Analysis
Data was double entered into epi data version 4.6 and was later exported to Stata version 14 for analysis. Descriptive statistics were used to describe patient socio-demographic and clinical characteristics. The categorical variables were summarized using percentages and continuous variables were summarized using the mean and standard deviations (SD), or median and interquartile range (IQR) depending on the distribution.
Incidence rate was computed by dividing the new cases of ADEs by the overall patient follow-up time. Follow-up time was defined as a sum of total duration from the start of treatment up to the treatment outcome. The frequency of ADEs was presented by type and grade. The Kaplan Meier curve and Wilcoxon test were used to compare time to development of ADEs between HIV positive and HIV negative patients.
Factors associated with ADEs were evaluated using generalized linear model with family (Poisson) and link (log) and using robust standard error to adjust for over inflated variances. Variables with a p≤0.2 were included into the multivariate analysis. The measure of association was the rate ratio. The backward stepwise elimination method was used during model building considering a p-value of 0.05 as significant. We reported adjusted rate ratios (adjusted for confounding) and 95% level of confidence.
Ethical Approval and Consent to Participant
Makerere University’s Clinical Epidemiology Unit granted permission to perform the study. The School of Medicine Research and Ethics Committee (Mak-SOMREC-2021-186) and the Mulago National Referral Hospital ethics review board provided ethical approval. We sought waiver of consent since the study involved retrospective review of medical charts, participants were out of clinic care and were geographically dispersed from different districts in the country making informed consent not feasible. Waiver of consent was provided by the SOMREC and the Mulago National Referral Hospital ethics review board. Identifying information such patient names, contact details and patient file numbers were not extracted but we used unique study numbers during data collection to ensure privacy of participants. Data was backed up on an external hard drive with encrypted passwords known only by the researcher to ensure confidentiality of the patients’ information. Data is aggregated and anonymized in the reporting of findings. All study procedures were conducted according to the Declaration of Helsinki.
Results
Selection of Study Participants
A total of 417 patient records were sampled from DR-TB treatment register from 2013 to 2020. Of these, 28 were excluded; incomplete data were 20 (8 were missing the treatment chart for the first 2 months, 3 were missing adherence documentation log, 9 were missing detailed clinicians follow-up visit notes) and 8 were transferred out as summarized in Figure 1.
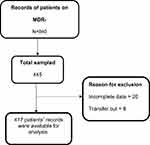 |
Figure 1 Study profile of study participants. |
Socio-Demographic Characteristics
Of the 417 patient files included in the study, the mean age was 33 years (SD=12.9), mean weight was 51kg (SD=12.0), 55.8% (n=232) were males, 42.7 (n=178) were initiated on treatment from 2018 to 2020 and only 15.6% (n=65) had a history of alcohol drinking. The socio-demographic characteristics are summarized in Table 2.
 |
Table 2 Socio Demographic Characteristics of 417 Patients That Received DR-TB Treatment from Mulago Hospital, 2013–2020 |
Clinical Characteristics
About half (52.5%, n=219) of the participants were HIV positive, 46% (n=192) were newly diagnosed DR-TB and 78.9% (n=329) had RR-TB. The most commonly prescribed class of drug was a fluoroquinolone at 98.3% (n=407) and 47.1% (n=196) of the participants had very good adherence as summarized in Table 3.
 |
Table 3 Clinical Characteristics of 417 Patients That Received DR-TB Treatment from Mulago Hospital, 2013–2020 |
Overall Incidence of Adverse Drug Events Among 417 Patients Receiving DR-TB Treatment from Mulago Hospital, 2013–2020
The overall incidence was 5.56 ADEs per 100 person months (95% CI 5.01, 6.15) among the 417 patients that received treatment at Mulago, between 2013 and 2020.
Prevalence and Grading of the ADEs
Musculoskeletal symptoms were the most prevalent ADEs at 64.3% (268/417) with 84.3% (226/268) of them recoded as moderate. Prevalence of ototoxicity was at 31.7% (132/417) and 55.5% (73/132) of them were recoded as moderate, neuropsychiatric disorders were reported at 23.5% (98/417) with 46.9% (46/98) of them documented as mild and cardiac toxicity was reported at 13.9% (58/417), however, 55.2% (32/58) of these were mild as summarized in Figure 2.
 |
Figure 2 Frequency and grading of ADEs among 417 patients that received DR-TB treatment from Mulago Hospital, 2013–2020. |
Factors Associated with ADEs Among the 417 Patients Receiving DR-TB Treatment from Mulago Hospital, 2013–2020
At multivariable analysis, a treatment regimen containing an aminoglycoside (incident rate ratio (IRR) 1.106, 95% CI 1.005–1.216), linezolid (IRR 1.145, 95% CI 1.008–1.229) or pyrazinamide (IRR 1.226, 95% CI 1.072–1.401); and the treatment duration (in months) (IRR 1.005, 95% CI 1.001–1.010) were significantly associated with ADEs as shown in Table 4. HIV co-infection was not significantly associated with ADEs (p=0.451).
 |
Table 4 Factors Associated with ADEs Among 417 Patients That Received DR-TB Treatment from Mulago Hospital, 2013–2020 |
Time to Development of ADEs by HIV Status
There was no significant difference in the cumulative probability of developing an ADE between HIV negative and HIV positive patients (Wilcoxon test =0.83, p=0.363) as summarized in Figure 3.
 |
Figure 3 Kaplan Meier failure curve by HIV status of DR-TB patients that received treatment from Mulago hospital, 2013–2020. |
Discussion
From this study, for every 100 patients with DR-TB each followed-up for 1-month, 6 of them developed ADEs. The most prevalent ADE was musculoskeletal symptoms and most of the ADEs were of moderate severity. Treatment regimen containing an aminoglycoside, linezolid (Lzd) or pyrazinamide (Z) and a longer treatment duration were significantly associated with ADEs.
The overall incidence observed in this study is consistent with the findings from a study conducted in Ethiopia that reported an incidence of 5.79 per 100 person months.19 Incidence from our study is however higher than that reported from a study conducted in Georgia where overall serious adverse event incidence rate was 1.16 per 100 person-months. The reason for this difference is because our population had more people with ADE risk factors, such as HIV, aminoglycosides-based regimen and longer treatment durations, while the other study focused on newer shorter treatment regimens in predominantly HIV negative population.20 The findings from our study could be an underestimate of the true value because the clinicians and patients could have under reported the ADEs during the review visits, particularly those that were mild or moderate.
From our study, a drug regimen containing an aminoglycoside (Am, Km, Cm) or linezolid or pyrazinamide were associated with the occurrence of an ADEs. This is consistent with findings from a systematic review that reported that ADEs leading to permanent discontinuation were associated with aminoglycosides and Lzd.12 In addition, other studies have also reported an association between ADEs and a Z based regimen.21,22 Two of the most problematic adverse effects associated with Z use include hepatotoxicity and polyarthralgia. We observed both mild and clinically important hepatocellular injury in 6.2% (n=26) of our patients. Z is extensively metabolized by the liver and the two Z metabolites, pyrazinoic acid and 5-hydroxypyrazinoic acid cause transient and cause asymptomatic elevations in the serum aminotransferase levels. Hepatotoxicity from Z is more frequent with higher doses, suggesting a direct toxic effect.23 We observed that 6 in every 10 patients reported musculoskeletal symptoms possibly because pyrazinamide inhibits the tubular secretion of uric acid resulting in hyperuricemia and attendant poly-arthralgias occur in up to 40% of patients.24 However, uric acid levels were not assessed during treatment to ascertain this causal relationship.
In this study, a quarter of the participants experienced peripheral neuropathy and more than a tenth experienced visual impairment. These results are similar to findings from a systematic review that reported that 30% of the participants experienced neurologic and ophthalmologic disorders.25 Linezolid inhibits mitochondrial protein synthesis and can lead to mitochondrial dysfunction in the neurons. Such effects are detrimental to sensory neurons, as described in antiretroviral-agent-induced small-fibre sensory neuropathy.26 Lzd has recently been reclassified as a group A drug that is strongly recommended for all longer regimens during management of DR TB.6 Patients ought to be monitored closely for the incidence of such ADEs.
In our study, 32% of people had ototoxicity. This is a higher prevalence compared to that reported by a study in Ethiopia (4.8%).27 This difference could be due to lack of audiometric follow-up in the Ethiopian study setting. However, our results are consistent with findings from a systematic review that reported prevalence of 28.3% of hearing loss, 14.5% of tinnitus and 8.1% of vertigo.28 Another study conducted in Cameroon reported a similar incidence of kanamycin-induced ototoxicity at 36.7%.29 Aminoglycosides generate free radicals within the inner ear, with subsequent permanent damage to sensory cells and neurons, resulting in permanent hearing loss.30 They have narrow therapeutic index and pharmacokinetic variability that require careful monitoring of serum levels, particularly during their prolonged use in MDR-TB patients, to prevent the occurrence of dose-dependent ototoxicity and nephrotoxicity. These findings support the current WHO guidelines that injectables should no longer be considered priority medicines when designing longer MDR-TB regimens.
Longer treatment duration was significantly associated with ADEs. This is consistent with results from other studies.10,31 Duration of treatment in DR-TB is guided by culture conversion. Traditionally, therapy would be continued for a minimum of 18 months after culture conversion. However, with the recent WHO guidelines the duration of treatment has been adjusted to a shorter 9–12 months duration all-oral, bedaquiline-containing regimen in eligible patients with confirmed MDR/RR-TB.6 Nonetheless, it is evident from our study that linezolid, a repurposed agent in the shorter regimen, is associated with ADEs. This implies that patients on shorter regimens containing Lzd would still need close monitoring.
There was no association between HIV status and ADE. This observation is similar to results from a systematic review by Schnippel et al.32 However, it is different from the findings obtained from a comprehensive review and meta-analysis which reported that HIV infection raised the probability of developing ADEs in DR-TB patients by 12%.33 The reason for the difference in findings could be because most of the patients studied had controlled HIV and were using less toxic ART regimens. The meta-analysis was also able to evaluate this association in different subsets of participants, combine results with a large sample size and improve the estimates of the effect size of an association.
Our study has some limitations. Because of the retrospective nature of the study, follow-up data regarding some risk factors such as pre-existing anaemia, liver or renal disease were lacking. Moreover, other medicines used by patients for co-morbid conditions were not documented yet these drugs may affect the occurrence of ADEs. Some variables such as history of alcohol consumption and smoking were based on self-reports which could have been underreported. Lastly, the study was carried out at one study site and the findings may not be generalized to other study settings in Uganda as different regions may have more or less frequent ADEs, depending on the host genetics and HIV prevalence.
Conclusion
From this study, for every 100 patients with DR-TB each followed-up for 1-month, 6 of them developed ADEs. A treatment regimen containing an aminoglycoside or linezolid or pyrazinamide and a longer treatment duration were significantly associated with ADEs. Clinicians should routinely monitor patients prescribed an aminoglycoside or linezolid or pyrazinamide-based regimen for incidence of ADEs such as ototoxicity, neuropathy, hepatotoxicity and musculoskeletal symptoms. Clinicians should quickly adopt all oral shorter treatment regimens to obviate the need for aminoglycosides and reduce exposure duration. The World Health Organization should develop validated tools to screen for DR-TB treatment related arthropathy, neuropathy since they are non-existent. Prospective studies should be designed to explore baseline complete blood count, liver and renal function that were not studied to further ascertain associations and occurrence.
Abbreviations
ADE, adverse drug event; AIDS, acquired immune deficiency syndrome; Am, amikacin; ART, antiretroviral therapy; Bdq, bedaquiline; Cfz, clofazimine; CI, confidence interval; Cm, capreomycin; Cs, cycloserine; Dlm, delaminad; DOTS, Directly Observed Treatment, short-course; DR-TB, drug resistant tuberculosis; DST, drug susceptibility test; E, ethambutol; Eto, Ethionamide; FQs, floroquinolones; Gfx, gatifloxacine; H, Isoniazid; HIV, human immunodeficiency virus; IQR, Interquartile range; Km, Kanamycin; Lfx, Levofloxacin; LTFU, Lzd, linezolid; Mfx, moxifloxacin; MOH, ministry of health; NDA, National Drug Authority; NTLP, National Tuberculosis and Leprosy Programme; PAS, P-amino salicylic acid; SD, Standard Deviation; S, Streptomycin; SOMREC, School of Medicine Research and Ethics Committee; Tb, tuberculosis; WHO, World Health Organization; XDR, Extensively Drug Resistant.
Acknowledgments
We would like to acknowledge the administration of Mulago National Referral hospital for granting us permission to utilize the patient records from the drug resistant tuberculosis clinic.
Disclosure
The authors declare no conflicts of interest.
References
1. World Health Organization. Global Tuberculosis Report. World Health Organization; 2020.
2. Musa BM, Adamu AL, Galadanci NA, Zubayr B, Odoh CN, Aliyu MH. Trends in prevalence of multi drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2017;12(9):e0185105. doi:10.1371/journal.pone.0185105
3. Kharsany AB, Karim QA. HIV infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34–48. doi:10.2174/1874613601610010034
4. Lukoye D, Adatu F, Musisi K, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS One. 2013;8(8):e70763. doi:10.1371/journal.pone.0070763
5. Rumende CM. Risk factors for multidrug-resistant tuberculosis. Acta Med Indones. 2018;50(1):1–2.
6. Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57(6). doi:10.1183/13993003.03300-2020
7. Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther. 2016;23(2):e521–e530. doi:10.1097/01.mjt.0000433951.09030.5a
8. Lorent N, Sebatunzi O, Mukeshimana G, Van den Ende J, Clerinx J. Incidence and risk factors of serious adverse events during antituberculous treatment in Rwanda: a prospective cohort study. PLoS One. 2011;6(5):e19566. doi:10.1371/journal.pone.0019566
9. Javaid A, Khan MA, Jan F, et al. Pakistan’da çok ilaca dirençli tüberküloz için toplum temelli tedavi alan hastalarda advers etki gelişimi [Occurrence of adverse events in patient receiving community-based therapy for multidrug-resistant tuberculosis in Pakistan]. Tuberk Toraks. 2018;66(1):16–25. doi:10.5578/tt.64054
10. Aznar ML, Rando Segura A, Moreno MM, et al. Treatment outcomes and adverse events from a standardized multidrug-resistant tuberculosis regimen in a rural setting in Angola. Am J Trop Med Hyg. 2019;101(3):502–509. doi:10.4269/ajtmh.19-0175
11. Mitnick CD, Rodriguez CA, Hatton ML, et al. Programmatic management of drug-resistant tuberculosis: an updated research Agenda. PLoS One. 2016;11(5):e0155968. doi:10.1371/journal.pone.0155968
12. Lan Z, Ahmad N, Baghaei P, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383–394. doi:10.1016/s2213-2600(20)30047-3
13. Baluku JB, Nakazibwe B, Naloka J, et al. Treatment outcomes of drug resistant tuberculosis patients with multiple poor prognostic indicators in Uganda: a countrywide 5-year retrospective study. J Clin Tuberc Other Mycobact Dis. 2021;23:100221. doi:10.1016/j.jctube.2021.100221
14. Resende LS, Santos-Neto ET. Risk factors associated with adverse reactions to antituberculosis drugs. J Bras Pneumol. 2015;41(1):77–89. doi:10.1590/s1806-37132015000100010
15. Ministry of Health. Uganda National Guidelines for the Programmatic Management of Drug Resistant Tuberculosis. Ministry of Healt; 2011.
16. Israel GD. Determining sample size; 1992.
17. Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49(3):1602308. doi:10.1183/13993003.02308-2016
18. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232.
19. Merid MW, Gezie LD, Kassa GM, Muluneh AG, Akalu TY, Yenit MK. Incidence and predictors of major adverse drug events among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara regional state public hospitals; Ethiopia: a retrospective cohort study. BMC Infect Dis. 2019;19(1):286. doi:10.1186/s12879-019-3919-1
20. Buziashvili M, Davtyan H, Sereda Y, et al. Incidence rate and time to serious adverse events among rifampicin resistant tuberculosis patients in Georgia treated with new and repurposed anti-tuberculosis drugs, 2016–2018. Monaldi Arch Chest Dis. 2021;91(1). doi:10.4081/monaldi.2021.1649
21. Papastavros T, Dolovich LR, Holbrook A, Whitehead L, Loeb M. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ. 2002;167(2):131–136.
22. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–1477. doi:10.1164/rccm.200206-626OC
23. Hussain Z, Zhu J, Ma X. Metabolism and hepatotoxicity of pyrazinamide, an antituberculosis drug. Drug Metab Dispos. 2021;49(8):679–682. doi:10.1124/dmd.121.000389
24. Steele MA, Des Prez RM. The role of pyrazinamide in tuberculosis chemotherapy. Chest. 1988;94(4):845–850. doi:10.1378/chest.94.4.845
25. Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2016;15(1):41. doi:10.1186/s12941-016-0156-y
26. Zhu Y, Antony JM, Martinez JA, et al. Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression. Brain. 2007;130(Pt 8):2011–2023. doi:10.1093/brain/awm148
27. Shibeshi W, Sheth AN, Admasu A, Berha AB, Negash Z, Yimer G. Nephrotoxicity and ototoxic symptoms of injectable second-line anti-tubercular drugs among patients treated for MDR-TB in Ethiopia: a retrospective cohort study. BMC Pharmacol Toxicol. 2019;20(1):31. doi:10.1186/s40360-019-0313-y
28. Wrohan I, Redwood L, Ho J, Velen K, Fox GJ. Ototoxicity among multidrug-resistant TB patients: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2021;25(1):23–30. doi:10.5588/ijtld.20.0217
29. Poka-Mayap V, Balkissou Adamou D, Pefura-Yone EW, Kuaban C. [Kanamycin-induced ototoxicity during treatment of multidrug-resistant tuberculosis] Ototoxicité liée à la kanamycine au cours du traitement de la tuberculose multirésistante. Rev Mal Respir. 2020;37(5):369–375. doi:10.1016/j.rmr.2019.12.005
30. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13(1):119–126. doi:10.2174/138161207779313731
31. Modongo C, Pasipanodya JG, Zetola NM, Williams SM, Sirugo G, Gumbo T. Amikacin concentrations predictive of ototoxicity in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother. 2015;59(10):6337–6343. doi:10.1128/AAC.01050-15
32. Schnippel K, Berhanu RH, Black A, et al. Severe adverse events during second-line tuberculosis treatment in the context of high HIV Co-infection in South Africa: a retrospective cohort study. BMC Infect Dis. 2016;16(1):593. doi:10.1186/s12879-016-1933-0
33. Lazarus G, Tjoa K, Iskandar AWB, et al. The effect of human immunodeficiency virus infection on adverse events during treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2021;16(3):e0248017. doi:10.1371/journal.pone.0248017
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
