Back to Journals » Clinical Ophthalmology » Volume 8
In vivo laser confocal microscopy findings of a cornea with osteogenesis imperfecta
Authors Kobayashi A , Higashide T, Yokogawa H , Yamazaki N, Masaki T, Sugiyama K
Received 22 November 2013
Accepted for publication 24 December 2013
Published 21 February 2014 Volume 2014:8 Pages 429—433
DOI https://doi.org/10.2147/OPTH.S58087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Akira Kobayashi, Tomomi Higashide, Hideaki Yokogawa, Natsuko Yamazaki, Toshinori Masaki, Kazuhisa Sugiyama
Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
Objective: To report the in vivo laser confocal microscopy findings of a cornea with osteogenesis imperfecta (OI) with special attention to the abnormality of Bowman's layer and sub-Bowman's fibrous structures (K-structures).
Patients and methods: Two patients (67-year-old male and his 26-year-old son) with OI type I were included in this study. Slit lamp biomicroscopic and in vivo laser confocal microscopic examinations were performed for both patients. Central corneal thickness and central endothelial cell density were also measured.
Results: Although the corneas looked clear with normal endothelial density for both eyes in both patients, they were quite thin (386 µm oculus dexter (OD) (the right eye) and 384 µm oculus sinister (OS) (the left eye) in the father and 430 µm OD and 425 µm OS in the son). In both patients, slit lamp biomicroscopic and in vivo laser confocal microscopic examination showed similar results. Anterior corneal mosaics produced by rubbing the eyelid under fluorescein were completely absent in both eyes. In vivo laser confocal microscopy revealed an absent or atrophic Bowman's layer; a trace of a presumed Bowman's layer and/or basement membrane was barely visible with high intensity. Additionally, K-structures were completely absent in both eyes.
Conclusion: The absence of K-structures and fluorescein anterior corneal mosaics strongly suggested an abnormality of Bowman's layer in these OI patients.
Keywords: osteogenesis imperfecta, K-structure, confocal microscopy, Bowman's layer
Introduction
Osteogenesis imperfecta (OI) is a connective tissue disorder of the skeleton, ears, eyes, teeth, skin, and joints that is caused by an abnormality of type I collagen.1,2 Due to considerable phenotypic variability, Sillence et al developed a classification of OI subtypes: OI type I with blue sclera, perinatal lethal OI type II, also known as congenital OI, OI type III with a progressive deformation with a normal sclera, and OI type IV with a normal sclera.3 Aside from the blue sclera, the absence of Bowman’s layer is also reported in OI.4
Previously, we demonstrated for the first time by using in vivo laser confocal microscopy that there are sub-Bowman’s fibrous structures located just beneath subbasal nerves.5 These sub-Bowman’s fibrous structures, also designated as K-structures, were 5 to 15 μm in diameter and appeared to consist of many filaments.5 We have subsequently confirmed a strong association between K-structures and the presence/health of Bowman’s layer.6,7
In this case report, we report for the first time the in vivo laser confocal microscopic findings of OI type I, and confirmed the associated abnormality of Bowman’s layer and K-structures.
Patients and methods
This study was approved by the Ethical Committee of Kanazawa University Graduate School of Medical Science and followed the tenets of the Helsinki Declaration. Two patients (a 67-year-old male and his 26-year-old son) with OI type I were included in this study. Slit lamp biomicroscopic, in vivo laser confocal microscopic examinations, and central endothelial cell density measurements were performed. Also, central corneal thickness was measured by OPTOVUE optical coherence tomography (Optovue, Inc., Fremont, CA, USA).
In vivo laser confocal microscopy
Informed consent was obtained after explaining the nature and possible consequences of microscopic study (such as superficial punctate keratopathy). A cornea specific, in vivo, laser confocal microscope (Heidelberg Retina Tomograph 2 Rostock Cornea Module, Heidelberg Engineering GmbH, Heidelberg, Germany) was used to investigate all cell layers of the central cornea.
Results
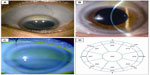
A 67-year-old Japanese man with OI Type I, case 1, was referred to our clinic for ophthalmic evaluation. At the age of 62, the patient had been treated with vitreous surgery, phacoemulsification, and intraocular lens implantation for a left rhegmatogenous retinal detachment. He had been deaf from the age of 24, and had no history of bone fractures. The patient used complete dentures, his height was 168 cm and his weight was 63 kg. The past history of case 2, the son of case 1, is shown in Table 1. At the initial visit, bilateral blue sclera and iris atrophy was observed using slit lamp biomicroscopy in case 1 (Figure 1A and B). Although bilateral corneas were clear, the anterior corneal mosaic (ACM) usually apparent after eyelid rubbing with fluorescein staining was absent in both eyes (Figure 1C). The corneas were quite thin (386 μm oculus dexter (OD) (the right eye) and 384 μm oculus sinister (OS) (the left eye) in Case 1 [Figure 1D] and 430 μm OD and 425 μm OS in Case 2). The diameters of the corneas in both patients were approximately 11 mm. The endothelial cell density was 2,557 cells/mm2 OD and 2,207 cells/mm2 OS in Case 1, and 2,563 cells/mm2 OD in case 2 (the data in OS was missing in case 2). No abnormal findings were noted by fundus examination except for chorioretinal scars after retinal detachment surgery in the left eye. The patient’s corrected visual acuity in case 1 was 20/20 and his intraocular pressure was 10 mm Hg in both eyes. The ocular findings of case 2 are shown in Table 1. In both patients, keratoconus was ruled out by corneal topography.
In vivo laser confocal microscopy
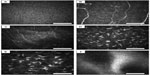
Normal epithelial cell layer and subbasal nerves were observed in case 1 using in vivo laser confocal microscopy (Figure 2A and B). A trace of what was presumed to be Bowman’s layer and/or basement membrane was barely visible, indicating that the Bowman’s layer was missing or nearly atrophic (Figure 2C). The subepithelial stroma seemed normal (Figure 2D). However, K-structures usually seen between Bowman’s layer and subepithelial stroma were completely absent. The keratocyte nuclei in the stroma (Figure 2E) and endothelial cells (Figure 2F) had a normal appearance. Similar findings of in vivo laser confocal microscopy were observed in case 2.
Discussion
Four clinical types of OI are recognized and are caused by abnormalities of the α1 or α2 chains of type I collagen.1,2 In OI, there is a failure of type I collagen fibers to mature to their normal diameter. Mutations in the COL1A (17q21.31-q22) and COL1A2 genes (7q22.1) account for most cases in all four types.8,9 Patients with OI type I have a blue sclera; the blue coloration results from visualization of the underlying choroid through a thin sclera. Ocular rigidity is reduced due to a thin sclera, which may confer a high risk of globe rupture.10 Interestingly, Bowman’s layer may also be absent in OI.4 Therefore, we hypothesized that there might be some abnormality in Bowman’s layer and/or adjacent superficial stroma in these OI patients.
Previously, we have speculated that K-structures (Figure 3) may correspond with anterior collagen fiber bundles running at the posterior surface of Bowman’s layer based on light/electron microscopic observations11–13 and two photon generated second harmonic signal observations.14 We have subsequently reported that the overall distribution of K-structures in normal human corneas by in vivo laser confocal microscopy formed a net like pattern and corresponded quite well with the fluorescein ACM pattern observed using slit lamp biomicroscopy.6 These results support the hypothesis that the K-structures are the anterior collagen fiber bundles running at the posterior surface of Bowman’s layer, and thus are the structural basis for ACM formation. Therefore, the presence of K-structures by in vivo laser confocal microscopy and fluorescein ACM by slit lamp biomicroscopy can be an indicator of the health of Bowman’s layer.6,7 Furthermore, we have reported that both K-structures and ACM disappeared only after epipolis laser assisted in situ keratomileusis, in which Bowman’s layer is destroyed, but not after laser assisted in situ keratomileusis in which Bowman’s layer is preserved.7
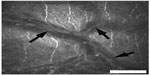  | Figure 3 Normal K-structures (arrows) by in vivo laser confocal microscopy seen in a healthy volunteer (23-year-old female). |
In this study, we report a family with clinically diagnosed OI type I with blue sclera. Most notably, in vivo laser confocal microscopy showed Bowman’s layer to be atrophic or nearly absent, with complete loss of K-structures in both patients. By slit lamp biomicroscopy, fluorescein ACM was completely missing in both patients. These observations suggest an association of Bowman’s layer abnormality and OI in these patients. Therefore, we surmise that Bowman’s layer abnormality in OI may be associated with corneal stromal thinning. Also, loss of K-structure and absence of fluorescein ACM in suspected OI patients may suggest corneal complications and have supportive diagnostic value of OI. Further studies using larger groups of patients with different subtypes of OI are required to fully elucidate the abnormality of Bowman’s layer in OI.
Disclosure
None of the authors has any proprietary interest in any product mentioned in this article.
The corresponding investigator (AK) had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Sillence DO, Barlow KK, Garber AP, Hall JG, Rimoin DL. Osteogenesis imperfecta type II delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet. 1984;17(2):407–423. | |
Byers PH, Cole WG. Osteogenesis imperfect. In: Royce BM, Steinmann B, editors. Connective Tissue and its Heritable Disorders: Molecular, Genetic and Medical Aspects. New York: Wiley-Liss; 1993:385–430. | |
Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. | |
Mietz H, Kasner L, Green WR. Histopathologic and electron-microscopic features of corneal and scleral collagen fibers in osteogenesis imperfecta type III. Graefes Arch Clin Exp Ophthalmol. 1997;235:405–410. | |
Kobayashi A, Yokogawa H, Sugiyama K. In vivo laser confocal microscopy of Bowman’s layer of the cornea. Ophthalmology. 2006;113:2203–2208. | |
Yokogawa H, Kobayashi A, Sugiyama K. Mapping of normal corneal K-structures by in vivo laser confocal microscopy. Cornea. 2008;27:879–883. | |
Yokogawa H, Kobayashi A, Tagawa K, Sugiyama K. In vivo laser confocal microscopic analysis of corneal K-structures after keratorefractive surgery (LASIK and epi-LASIK). Ophthalmic Surg Lasers Imaging. 2010;41:494–498. | |
Marini JC, Grange DK, Gottesman GS, Lewis MB, Koeplin DA. Osteogenesis imperfecta type IV: detection of a point mutation in one alpha-1(I) collagen allele (COL1A1) by RNA/RNA hybrid analysis. J Biol Chem. 1989;264:11893–11900. | |
Wenstrup RJ, Cohn DH, Cohen T, Byers PH. Arginine for glycine substitution in the triple-helical domain of the products of one alpha-2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988;263:7734–7740. | |
Kaiser-Kupfer MI, McCain L, Shapiro JR, Podgor MJ, Kupfer C, Rowe D. Low ocular rigidity in patients with osteogenesis imperfecta. Invest Ophthalmol Vis Sci. 1981;20:807–809. | |
Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye: An Atlas and Textbook. Philadelphia: WB Saunders; 1971:55–111. | |
Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–2258. | |
Smolek MK, Klyce SD. Cornea. In: Tasman W, Jaeger EA, editors. Duane’s Foundation of Clinical Ophthalmology. Volume 1. Ocular Anatomy, Embryology, and Teratology. Philadelphia: Lippincott Williams & Wilkins; 2006. | |
Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg. 2006;32:1784–1791. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.