Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
In vivo Guinea Pig Model Study for Evaluating Antifungal Effect of a Dual-Diode Laser with Wavelengths of 405 Nm and 635 Nm on Dermatophytosis
Authors Ahn JC, Mo SJ , Choi M , Kim B, Cho SB
Received 4 April 2023
Accepted for publication 13 June 2023
Published 17 June 2023 Volume 2023:16 Pages 1559—1567
DOI https://doi.org/10.2147/CCID.S415679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Jin-Chul Ahn,1,* Sang Joon Mo,1,* Min Choi,2 Bora Kim,2 Sung Bin Cho3
1Medical Laser Research Center, College of Medicine, Dankook University, Cheonan, Korea; 2R&D Center, Shenb Co., Ltd, Seoul, Korea; 3Yonsei Seran Dermatology and Laser Clinic, Seoul, Korea
*These authors contributed equally to this work
Correspondence: Sung Bin Cho, Yonsei Seran Dermatology and Laser Clinic, Geumcheon REMAIN CITY 6F, 224 Siheung-Daero, Geumcheon-Gu, Seoul, 08628, Korea, Tel +82 2-2135-1375, Fax +82 70-8250-1375, Email [email protected]
Background: Various laser- and light-based devices have been introduced as complementary or alternative treatment modalities for dermatophytosis, particularly for finger or toenail onychomycosis.
Objective: This study aimed to comparatively evaluate the antifungal effects of 405-nm and 635-nm dual-band diode lasers using an in vivo guinea pig model of dermatophytosis.
Materials and Methods: A guinea pig model was developed by the repetitive application of fungal spore preparations to the back skin of guinea pigs. Dual-diode laser treatment was delivered to the guinea pig skin at a power of 24 mW at a wavelength of 405 nm and 18 mW at 635 nm for 12 min. The treatments were administered three times weekly for 2 weeks, and a mycological study was performed.
Results: Mycological studies using scraped samples obtained from treatment groups A (N = 8) and B (N = 8) after dual-diode laser treatment revealed that seven of eight (87.5%) samples in each group had negative results for direct potassium hydroxide microscopy and fungal culture studies. Skin specimens from each infected laser-untreated guinea pig exhibited spongiotic psoriasiform epidermis with parakeratosis. Meanwhile, skin specimens from infected laser-treated guinea pigs in groups A and B demonstrated thinner epidermal thickness than those from infected untreated controls but thicker than those from uninfected treated controls without noticeable inflammatory cell infiltration in the dermis.
Conclusion: The guinea pig dermatophytosis model can be used to comparatively evaluate the efficacy and safety of various treatment modalities, including dual-diode lasers, for superficial fungal skin infection.
Keywords: dermatophytosis, Trichophyton rubrum, Trichophyton mentagrophytes, skin, toenail, dual-diode laser, wavelengths of 405 nm and 635 nm
Introduction
Superficial mycosis is a common fungal infection of the skin, hair, and nails caused by dermatophytes, including the genus Trichophyton, Epidermophyton, and Microsporum; non-dermatophytic molds; and yeasts.1,2 Most cases of dermatophyte-induced superficial mycosis, termed dermatophytosis, can be effectively treated with topical and/or systemic antifungal agents.3 The choice of treatment for dermatophytosis is usually determined based on the infection location, infective genus, and species.3 Thus, dermatophytosis treatment involving hair-bearing scalp skin and nails requires systemic agents.2,3 Oral antifungal agents for dermatophytosis treatment are limited in patients presenting hepatotoxicity and drug–drug interactions. Moreover, failure of dermatophytosis treatment with systemic and topical antifungal agents has been considered an emerging clinical and mycological resistance issue.3
Various laser/light-based devices have been introduced as complementary or alternative treatment modalities for dermatophytosis, particularly for finger or toenail onychomycosis, since carbon dioxide (CO2) lasers were used for treating infectious toenail disorders.4–10 These devices include long-pulsed 1064-nm neodymium (Nd):yttrium-aluminum-garnet (YAG) laser, Q-switched 532-nm and 1064-nm Nd:YAG laser, fractional CO2 laser, 870-nm and 930-nm near-infrared dual-band diode laser, 1444-nm Nd:YAG laser, 1319-nm diode laser, infrared broadband system, and 405-/635-nm dual-band diode laser.4–12 Studies have demonstrated the action mechanisms of energy-based antifungal treatments: fungal chromophores in the cell membranes absorb irradiated energy sources to generate thermomechanical reaction that destroys cell walls and induces cellular apoptosis or necrosis of pathogens.13,14 Moreover, they have shown that laser/light-based treatments generated increased production of mitochondrial reactive oxygen species that induced apoptotic pathways of fungi.14,15
Mycological tests for evaluating the effects of antifungal treatments include direct microscopic examination of a smear preparation with potassium hydroxide (KOH) pretreatment, fungal culture study, histopathological test with hematoxylin and eosin (H&E), and Periodic acid-Schiff (PAS) staining, and KOH-treated nail clipping stained with PAS.16,17 In this study, we comparatively evaluated the antifungal effects of 405-nm and 635-nm dual-band diode lasers using an in vivo guinea pig model of dermatophytosis. To do so, the skin of guinea pigs was infected with T. rubrum and T. mentagrophytes and treated with two different 405-/635-nm dual-band diode lasers. Then, pre-treatment and post-treatment mycologic tests, including KOH preparation, fungus culture, and histopathologic tests, were performed to evaluate the antifungal effects of each 405-/635-nm dual-band diode laser on dermatophytosis.
Materials and Methods
Preparation of in vivo Guinea Pig Model of Dermatophytosis
Twenty-four Hartley-specific pathogen-free female guinea pigs weighing 300–345 g were purchased from Orient Bio Corp. (Seongnam, Korea). Eight guinea pigs were divided into two control groups: an uninfected, mycologically negative control group (N = 4) and an infected, mycologically positive control group (N = 4). The remaining 16 guinea pigs with mycologically confirmed dermatophytosis were randomly divided into two treatment groups (eight guinea pigs per group). Ethical approval was obtained from the Ethics Committee of the Dankook University Institutional Animal Care and Use Committee in Cheonan, Korea (DKU-22-010). All animal experiments were performed in accordance with the guidelines of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee.
The standard 0.1-mL suspensions of Trichophyton rubrum (KCCM 60443) and T. mentagrophytes (KCCM 60446) were seeded onto Sabouraud dextrose agar (Difco, Detroit, MI, USA) and incubated at 25°C for 14 days. Mixed fungal colonies of T. rubrum and T. mentagrophytes were suspended in 0.9% sterile saline to prepare a fungal spore suspension at 1 × 108 CFU/mL concentration. Back hair of guinea pigs (N = 24) was gently shaved and cleansed with a mild soap and 70% alcohol. After a gentle abrasion of the upper part of the stratum corneum to a size of 2 cm × 2 cm using sterile sandpaper, 1 mL of the fungal spore suspension was applied to the back skin of guinea pigs (N=20) five times a week for 4 weeks.
Treatment of Guinea Pig Skin with a 405-/635-Nm Dual-Diode Laser
General anesthesia was induced and maintained by increasing from 0.5% to 3.0% isoflurane. Then, two uninfected control guinea pigs and eight mycologically positive guinea pigs (group A) were treated with the AF® laser (Shenb Co., Ltd., Seoul, Korea), and other two uninfected control guinea pigs and eight mycologically positive guinea pigs (group B) were treated with the Lunula® laser (Erchonia Lasers, Ltd., Wallingford, UK). Dual-diode laser treatment was delivered to the back skin of experimental animals at a power of 24 mW at a wavelength of 405 nm (17.28 J/session) and 18 mW at 635 nm (12.96 J/session) for 12 min. The treatments were performed three times/week for 2 weeks.
Mycological Study
Development of a dermatophytosis animal model was confirmed by direct KOH microscopy and fungal culture studies. After repetitive application of the fungal spore suspension for 4 weeks, the back skin of guinea pigs was cleaned with 70% alcohol, and stratum corneum samples were obtained by gently scraping the skin with a sterile scalpel. Then, direct preparations were made in 20% KOH and microscopically evaluated. The scraped samples were also inoculated onto Sabouraud’s dextrose agar and incubated to evaluate the colony formation of T. rubrum and T. mentagrophytes in each sample.
The therapeutic effect of the dual-diode laser treatment on dermatophytosis was evaluated after completing the treatment protocol. As mentioned, scraped stratum corneum samples from guinea pigs in the control and dual-diode laser-treated groups were obtained for direct KOH microscopy. The scraped samples were inoculated onto Sabouraud’s dextrose agar, and the colony-forming abilities of T. rubrum and T. mentagrophytes in each sample were evaluated over 14 days of incubation. The experimental guinea pigs were euthanized for a humane sampling of the treated tissues according to the standard protocol. Full-thickness skin specimens from guinea pigs in the control and treatment groups were obtained immediately for histological evaluation after the completion of the treatment protocol. Samples were fixed in 10% buffered formalin and embedded in paraffin. Next, 4-μm-thick skin tissue sections were prepared and stained with H&E and PAS.
Results
Mycological Test Results of the Guinea Pig Model Before Dual-Diode Laser Treatment
All four uninfected control guinea pigs showed negative results on direct KOH microscopy. Furthermore, a fungal culture study performed by inoculating skin-scraped samples from uninfected control guinea pigs onto Sabouraud’s dextrose agar found that none of the samples presented fungal colonies of T. rubrum or T. mentagrophytes over 14 days. Meanwhile, the infected areas presented marked macroscopic changes in skin redness with scales compared with the mycologically negative controls (Figure 1). All 20 guinea pigs infected with the fungal spore suspension tested positive for fungal hyphae and spores by direct KOH microscopy. Moreover, all 20 guinea pigs that were infected with the fungal spore suspension tested positive for fungal colonies of T. rubrum or T. mentagrophytes in a fungal culture study (Figure 2A).
Mycological Test Results of the Guinea Pig Model After Dual-Diode Laser Treatment
All 4 uninfected control guinea pigs and 16 infected guinea pigs underwent complete experimental protocol for dual-diode laser treatment three times/week for 2 weeks (Figure 2B). All four uninfected dual-diode laser-treated guinea pigs showed complete hair regrowth without skin redness or scales (Figure 3). Direct KOH microscopy and fungal culture studies with scraped samples from uninfected dual-diode laser-treated guinea pigs revealed that none of the samples presented fungal hyphae and spores or fungal colony formation of T. rubrum and T. mentagrophytes over 14 days. Additional mycological studies using eight scraped samples obtained from group A after dual-diode laser treatment revealed that seven of the eight (87.5%) samples had negative results from direct KOH microscopy and fungal culture studies. Furthermore, seven of the eight (87.5%) guinea pigs in group B presented no fungal hyphae or spores in direct KOH microscopy and no fungal colony formation in the fungal culture study. Two guinea pigs in groups A and B presented with incomplete hair regrowth, skin redness, and scales. The remaining four infected untreated guinea pigs showed positive results in direct KOH microscopy and fungal culture studies. The infected areas of untreated guinea pigs exhibited skin redness with scales and incomplete restoration of hair growth.
Histologic Features of the Guinea Pig Model After Dual-Diode Laser Treatment
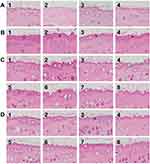
Skin specimens from the uninfected dual-diode laser-treated guinea pigs showed no remarkable histological changes in the epidermis or dermis without noticeable inflammatory cell infiltration (Figure 4A). Meanwhile, the skin specimens obtained from each infected guinea pig without dual-diode laser treatment exhibited spongiotic psoriasiform epidermis with parakeratotic stratum corneum compared with the skin specimens from each uninfected dual-diode laser-treated guinea pig (Figure 4B). Moreover, neutrophilic infiltration was noted in the epidermis, and mixed inflammatory cell infiltration was observed in the upper dermis. Fungal hyphae were apparent in the hyperkeratotic stratum corneum of the PAS-stained sections (Figure 5).
Histological changes in group A correlated with direct KOH microscopy and fungal culture study results. In group A, H&E-stained sections showed that the epidermis was notably thinner than infected, untreated controls but thicker than uninfected, treated controls in seven of eight guinea pigs (87.5%) without noticeable inflammatory cell infiltration in the dermis (Figure 4C). The specimen of the remaining guinea pig in group A presented spongiotic psoriasiform epidermis with parakeratotic stratum corneum and inflammatory cell infiltration in the dermis. In group B, H&E-stained sections presented a milder spongiotic and thickened epidermis than untreated controls in seven of eight guinea pigs (87.5%) without noticeable inflammatory cell infiltration in the dermis as shown in group A (Figure 4D). The specimen of the remaining one guinea pig in group B also exhibited spongiotic psoriasiform epidermis with parakeratosis and dermal inflammation. The histological changes in group B correlated with the results of direct KOH microscopy and fungal culture studies.
Discussion
This pilot study compared the antifungal effects of 405-/635-nm dual-band diode lasers. This study used guinea pigs to develop an in vivo animal model of dermatophytosis because their skin shares many structural similarities with human skin.18 Then, the skin of guinea pigs was infected with mixed preparation of T. rubrum and T. mentagrophytes suspensions to reduce the number of enrolled experimental animals. Mycological studies using scraped samples after completing six sessions of dual-diode laser treatment in the infected guinea pig groups revealed that seven of eight (87.5%) samples in each group had negative results in direct KOH microscopy and fungal culture studies.
A previous study investigated fungal flora in 103 asymptomatic guinea pigs.19 They isolated fungi of the genera Penicillium, Mucor, Rhizopus, Cladosporium, Aspergillus, Trichophyton, Alternaria, Fusarium, and Humicola guinea pigs in the order of frequency.19 Although 3.5–10.7% of the asymptomatic guinea pigs can carry T. mentagrophytes, symptomatic fungal infection, none of our uninfected control animals presented T. mentagrophytes-positive results.19,20 All guinea pigs carrying T. rubrum or T. mentagrophytes showed clinical signs of fungal infection, including skin redness with scales and alopecia. However, because our study design lacked controlled evaluation of the treatment responses to fungal pathogens, further in vitro studies are needed to support our findings.
In the present study, skin specimens from an animal model of dermatophytosis demonstrated the typical histopathological features of fungal skin infection, including hyperkeratosis, parakeratosis, spongiosis, psoriasiform changes in the epidermis, and inflammatory cell infiltration in the dermis with exocytosis into the epidermis. Six sessions of 405-/635-nm dual-band diode laser treatment improved the overall fungal infection-related histopathological changes in the epidermis and dermis. However, although epidermal exocytosis and dermal inflammatory cell infiltration notably improved along with the negative results from direct KOH microscopy and fungal culture studies, the histopathological changes in the epidermis were incompletely recovered during the 2 weeks of treatment.
The effects of diode lasers at the wavelength of 635 nm have been shown to accelerate wound repair by reducing inflammation and inducing neovascularization and collagen synthesis, in addition to the fungicidal effects for treating onychomycosis.21 A previous investigation revealed that 635-nm light-emitting diode treatment decreased cyclooxygenase, prostaglandin E2, and reactive oxygen species (ROS).22 Low-level laser therapy-induced photobiomodulation has been suggested to enhance the wound healing process by upregulating cytokine and chemokine release.23 Meanwhile, light sources at 400- and 500-nm wavelengths have been proven to have antibacterial effects by inducing ROS production, such as superoxide anion, hydroxyl radical, and singlet oxygen.24,25 Blue light treatments at 400–470-nm wavelengths have antimicrobial effects on Cutibacterium acnes, Helicobacter pylori, Porphyromonas gingivalis, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA).25 Moreover, a previous comparative study between the 405-nm light-emitting diodes and lasers during the treatment of MRSA infection suggested that antimicrobial outcomes seemed to depend on wavelength, treatment time interval, and fluence but not the coherence of light sources.25
Non-thermal laser therapy, particularly using 405-/635-nm dual-diode lasers, has been used to treat fungal infections of the toenails.4 However, the precise mechanisms of action of antifungal effects during non-thermal laser therapy are under investigation.4,18 Nonetheless, the antimicrobial or antifungal effects of non-thermal laser therapy have been suggested to result from photobiomodulation but not thermal destruction of pathogens.4,18 Therefore, 405-/635-nm dual-diode laser treatments can be clinically delivered to patients with infectious diseases without pain or burning sensations.4 The treatment parameters of the 405-/635-nm dual-diode laser in our experimental study were determined according to the previous clinical reports.4,18 The simultaneously activated wavelengths of 405 nm at a power of 24 mW and 635 nm at 18 mW for 12 min have been known to be unaffecting temperature changes in treated areas.4
In our study, the 405-/635-nm dual-diode laser treatment in guinea pigs with dermatophytosis achieved 87.5% negative conversion of mycologic studies. A previous retrospective review demonstrated that the 405-/635-nm dual-diode laser treatment in patients with mild-to-severe onychomycosis achieved 67% clear nail growth.4 Another study reported that 870-/930-nm near-infrared light treatment in patients with mild-to-severe onychomycosis achieved 65% clear nail growth.26 The higher clearance rate in our study could be associated with superficial fungal infection of the skin compared with fungal infection of the toenail, different treatment protocols, and higher skin permeability in animal models than in human skin.4,18
Conclusion
Mycological studies, including direct KOH microscopy and fungal culture studies, using scraped samples from fungus-infected, dual-diode laser-treated guinea pigs revealed that 87.5% of the samples had negative results. The results correlated with the histopathological features of the guinea pig skin after dual-laser treatment. We suggest that a guinea pig model of dermatophytosis can be used for a comparative evaluation of the efficacy and safety of various treatment modalities for superficial fungal skin infections. However, further experimental and clinical studies are needed to evaluate treatment responses to fungal pathogens and establish cost-effective treatment protocols.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Statement of Ethics
Ethical approval was obtained from the Ethics Committee of the Dankook University Institutional Animal Care and Use Committee in Cheonan, Korea (DKU-22-010). All animal experiments were performed in accordance with the guidelines of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee.
Author Contributions
All authors contributed significantly to the work reported, including the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. They participated in drafting, revising, or critically reviewing the article, and provided final approval of the version to be published. All authors agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2020R1A6A1A03043283).
Disclosure
No competing financial interest exists. The authors have no conflicts of interest to declare.
References
1. Verma S, Vasani R, Reszke R, Matusiak Ł, Szepietowski JC. Prevalence and clinical characteristics of itch in epidemic-like scenario of dermatophytoses in India: a cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34:180–183. doi:10.1111/jdv.15877
2. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151–159. doi:10.1016/j.clindermatol.2009.12.006
3. Saunte DML, Pereiro-Ferreirós M, Rodríguez-Cerdeira C, et al. Emerging antifungal treatment failure of dermatophytosis in Europe: take care or it may become endemic. J Eur Acad Dermatol Venereol. 2021;35:1582–1586. doi:10.1111/jdv.17241
4. Zang K, Sullivan R, Shanks S. A retrospective study of non-thermal laser therapy for the treatment of toenail onychomycosis. J Clin Aesthet Dermatol. 2017;10:24–30.
5. Apfelberg DB, Rothermel E, Widtfeldt A, Maser MR, Lash H. Preliminary report on use of carbon dioxide laser in podiatry. J Am Podiatry Assoc. 1984;74:509–513.
6. Borovoy M, Fuller TA, Holtz P, Kaczander BI. Laser surgery in podiatric medicine-present and future. J Foot Surg. 1983;22:353–357.
7. Rothermel E, Apfelberg DB. Carbon dioxide laser use for certain diseases of the toenails. Clin Podiatr Med Surg. 1987;4:809–821.
8. Bristow IR. The effectiveness of lasers in the treatment of onychomycosis: a systematic review. J Foot Ankle Res. 2014;7:34. doi:10.1186/1757-1146-7-34
9. Gupta AK, Venkataraman M, Quinlan EM. Efficacy of lasers for the management of dermatophyte toenail onychomycosis. J Am Podiatr Med Assoc. 2022;112:20–236. doi:10.7547/20-236
10. Zhang J, Lin P, Li J, Guo C, Zhai J, Zhang Y. Efficacy of laser therapy combined with topical antifungal agents for onychomycosis: a systematic review and meta-analysis of randomised controlled trials. Lasers Med Sci. 2022;37:2557–2569. doi:10.1007/s10103-022-03561-9
11. Choi MJ, Zheng Z, Goo B, Cho SB. Antifungal effects of a 1444-nm neodymium:yttrium-aluminum-garnet laser on onychomycosis: a pilot study. J Dermatolog Treat. 2014;25:294–297. doi:10.3109/09546634.2012.714455
12. Waibel J, Wulkan AJ, Rudnick A. Prospective efficacy and safety evaluation of laser treatments with real-time temperature feedback for fungal onychomycosis. J Drugs Dermatol. 2013;12:1237–1242.
13. Cao Y, Xu S, Kong W, Xu Y, Fang H. Clinical retrospective analysis of long-pulsed 1064-nm Nd:YAG laser in the treatment of onychomycosis and its effect on the ultrastructure of fungus pathogen. Lasers Med Sci. 2020;35:429–437. doi:10.1007/s10103-019-02840-2
14. Bhatta AK, Keyal U, Wang X, Gellén E. A review of the mechanism of action of lasers and photodynamic therapy for onychomycosis. Lasers Med Sci. 2017;32:469–474. doi:10.1007/s10103-016-2110-9
15. Francuzik W, Fritz K, Salavastru C. Laser therapies for onychomycosis-critical evaluation of methods and effectiveness. J Eur Acad Dermatol Venereol. 2016;30:936–942. doi:10.1111/jdv.13593
16. Liu HN, Lee DD, Wong CK. KONCPA: a new method for diagnosing tinea unguium. Dermatology. 1993;187:166–168. doi:10.1159/000247235
17. Haghani I, Shokohi T, Hajheidari Z, Khalilian A, Aghili SR. Comparison of diagnostic methods in the evaluation of onychomycosis. Mycopathologia. 2013;175:315–321. doi:10.1007/s11046-013-9620-9
18. Marquet F, Grandclaude MC, Ferrari E, Champmartin C. Capacity of an in vitro rat skin model to predict human dermal absorption: influences of aging and anatomical site. Toxicol In Vitro. 2019;61:104623. doi:10.1016/j.tiv.2019.104623
19. Kottferová L, Molnár L, Čonková E, et al. Fungal flora in asymptomatic pet Guinea pigs and rabbits. Animals. 2022;12:2387. doi:10.3390/ani12182387
20. Vangeel I, Pasmans F, Vanrobaeys M, De Herdt P, Haesebrouck F. Prevalence of dermatophytes in asymptomatic Guinea pigs and rabbits. Vet Rec. 2000;146:440–441. doi:10.1136/vr.146.15.440
21. Lee HS, Lee Y, Jeong U, Oh S, Hwang CW, Kang HW. Transoral low-level laser therapy via a cylindrical device to treat oral ulcers in a rodent model. Lasers Surg Med. 2020;52:647–652. doi:10.1002/lsm.23203
22. Lim W, Lee S, Kim I, et al. The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med. 2007;39:614–621. doi:10.1002/lsm.20533
23. Wagner VP, Curra M, Webber LP, et al. Photobiomodulation regulates cytokine release and new blood vessel formation during oral wound healing in rats. Lasers Med Sci. 2016;31:665–671. doi:10.1007/s10103-016-1904-0
24. Ghate VS, Ng KS, Zhou W, et al. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. Int J Food Microbiol. 2013;166:399–406. doi:10.1016/j.ijfoodmicro.2013.07.018
25. Masson-Meyers DS, Bumah VV, Biener G, Raicu V, Enwemeka CS. The relative antimicrobial effect of blue 405 nm LED and blue 405-nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers Med Sci. 2015;30:2265–2271. doi:10.1007/s10103-015-1799-1
26. Landsman AS, Robbins AH, Angelini PF, et al. Treatment of mild, moderate, and severe onychomycosis using 870- and 930-nm light exposure. J Am Podiatr Med Assoc. 2010;100:166–177.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.