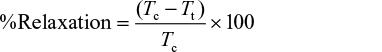Back to Journals » Journal of Experimental Pharmacology » Volume 8
In vitro vasodilatory activity and possible mechanisms of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in isolated thoracic aorta of guinea pigs
Authors Geleta B , Makonnen E , Debella A , Abebe A, Fekadu N
Received 19 July 2016
Accepted for publication 26 August 2016
Published 12 October 2016 Volume 2016:8 Pages 35—42
DOI https://doi.org/10.2147/JEP.S117545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Bekesho Geleta,1,2 Eyasu Makonnen,2 Asfaw Debella,1 Abiy Abebe,1 Netsanet Fekadu,1
1Directorate of Traditional and Modern Medicine Research, Ethiopian Public Health Institute, 2Department of Pharmacology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Abstract: Moringa stenopetala, a plant belonging to the family of Moringaceae, is traditionally used for the treatment of hypertension and diabetes in Ethiopia. This study evaluates the in vitro vasodilatory effect of the extract of M. stenopetala leaves and the possible mechanisms in precontracted isolated thoracic aorta of guinea pigs. A guinea pig was sacrificed by gentle cervical dislocation, and the thoracic aortic ring was removed, cut spirally, and mounted in an organ bath containing Krebs–Henseleit physiological solution maintained at 37°C, and then the solution was aerated with carbogen (95% O2 and 5% CO2). The vasodilatory activity of cumulative doses of M. stenopetala extracts and fractions was evaluated on intact and denuded endothelium of isolated whole, spirally cut thoracic aortic strips of guinea pigs precontracted with potassium chloride (80 mM), epinephrine (1 µM), methylene blue (10 µM), and glibenclamide (10 µM) using polygraph. All extracts showed a relaxant effect in precontracted isolated whole, spirally cut thoracic aortic strips of guinea pigs in a dose-dependent manner, whereas the greater percentage of relaxant effect was shown with the addition of crude extracts in 80 mM of potassium chloride (99.10% and 95.56% for ethanol and aqueous crude extracts, respectively), and 1 µM of epinephrine (82.85% and 90.16% for ethanol and aqueous crude extracts, respectively) in precontracted isolated whole, spirally cut thoracic aortic strips of guinea pigs. Hence, the possible mechanism of relaxation might be mediated through the blockade of receptor-operated calcium influx and L-type voltage-dependent calcium channels. The aqueous extract showed more significant in vitro vasodilatory effect than its fractions and 70% ethanol extract.
Keywords: in vitro vasodilatory, Moringa stenopetala, aortic strips, organ bath, mechanism of action, guinea pigs
Introduction
Blood pressure (BP) is created by the force of blood pushing against the walls of blood vessels (arteries) as it is pumped by the heart.1,2
It is primarily the result of interactions of the functions of the nervous, endocrine, and renal systems. Endothelial dysfunction, increased vascular reactivity, and vascular remodeling may be the causes, rather than consequences, of BP elevation; increased vascular stiffness contributes to isolated systolic hypertension in the elderly.3
Agents such as potassium chloride (KCl), epinephrine (EPI), methylene blue (MB), and glibenclamide (GLIB) induce vascular contraction through different mechanisms. KCl bypasses G-protein-coupled receptors and activates smooth muscle by a mechanism involving stimulation of voltage-operated Ca2+ channels, which results in increases in cytosolic-free Ca2+ ([Ca2+]i), Ca2+-calmodulin-dependent myosin light chain (MLC) kinase activation, and MLC phosphorylation and contraction.4 EPI also activates smooth muscle by a highly reproducible and relatively “simple” mechanism involving activation of receptor-operated Ca2+ channels that leads to increases in cytosolic-free Ca2+ ([Ca2+]i), MLC kinase activation, and MLC phosphorylation and contraction.5 MB inhibits guanylate cyclase enzyme and decreases the synthesis of cyclic guanine monophosphate, thereby preventing MLC dephosphorylation and contraction. In addition, it might interact directly with endothelium-derived relaxing factor and cause inhibition of the endothelium-independent relaxation.6 On the other hand, GLIB is an inhibitor for opening the adenosine triphosphate (ATP)-sensitive potassium channel that causes contraction.7–9
Hypertension leads to complications leading to considerable morbidity and mortality by causing damage to the arteries, brain (cerebrovascular accident or stroke), heart (heart failure, coronary artery disease, myocardial infarction, and heart attack), retina (retinopathy or visual impairment), and kidneys (renal failure).1,2,10
Treatment is usually lifelong. Therefore, drugs must be effective and safe over a long period of use. Many antihypertensive drugs act directly on blood vessel smooth muscles as vasodilators (α1-adrenegic block, release of NO in situ, calcium channel blockers, and α2-adrenegic agonist).11
Despite the availability of a wide range of antihypertensive drugs, hypertension and its complications are still important causes of adult morbidity and mortality in Africa. More than 50% of treated hypertensive patients have uncontrolled hypertension.12 Moreover, the efficacy of these drugs is only 40%–60%, and usually two or more antihypertensive drugs from different categories need be combined to achieve optimal results; however, side effects from these medications are important concerns.13 On the other hand, various herbal preparations have been claimed to have benefit for hypertension. Moringa stenopetala (Baker f.) Cufod. is one of such plants used in Ethiopia. It grows abundantly in Southwestern Ethiopia at an altitude range of 1,000–1,800 m, where the leaves are not only used for medicinal purposes, but are also eaten as vegetables.14 The species is known by different vernacular names such as “Shiferaw” in Amharic, “Aleko” in Wollaytegna and Gamugna, and “Cabbage tree” in English.15 It is a multipurpose plant16 and has been reported to possess antihypertensive,17 hypotensive,18 antihyperglycemic, hypoglycemic,16,18–21 antileishmanial and antifertility properites,15 in addition to use for treatment of stomach pain, as an antispasmodic, to expel retained placenta following birth,22 and as an antimicrobial23 and also has a nutritional value.16 The aim of this study was, therefore, to investigate the vasodilatory activity of M. stenopetala (Baker f.) Cufod. in precontracted isolated thoracic aorta of guinea pigs together with its possible mechanism of action.
Materials and methods
Chemicals and reagents
EPI (lot no: 111K1610; Sigma-Aldrich Co., St Louis, MO, USA), MB (lot no: 073K3413; Sigma-Aldrich Co.), acetylcholine chloride (lot no: 12134/1; Sigma-Aldrich Co.), GLIB (lot no: 53917; Remedica, Limassol, Cyprus), d-glucose anhydrous (lot no: GL2863; Eurostar Scientific Limited, Liverpool, UK), potassium chloride (lot no: 8114/86; Park Scientific Limited, Northampton, UK), sodium hydrogen carbonate (lot no: 205-633-8; Eurostar Scientific Limited), calcium chloride (lot no: 1501; Allied Chemical, Camden, NJ, USA), magnesium sulfate (lot no: 400290; The British Drug Houses Limited, UK), sodium chloride (lot no: 108278; Riedel-de Haen, Seelze, Germany), and ethyl acetate (Park Scientific Limited) were used in the study. All the drugs and reagents used complied with the required standard and were of analytical grade.
Instruments and apparatus
Balance (Mettler Toledo, Seoul, South Korea), Whatman filter paper number 1 (Whatman International Ltd, Maidstone, UK), orbital shaker (VWR DS-500; The LabWorld Group, Boston, MA, USA), rota vapor (Rotavapor R-bb210/215 B-490; Buchi, Flawil, Switzerland), water bath (DVE-Kottermann, D-3162; Uetze-Hanigsen/W, Berlin, Germany), lyophilizer/freeze dry system (Labconco, 12L Console Freeze Dry 230v-60 (7754040; Freeze Dry System, Labconco, Kansas City, MO, USA), and grass polygraph (Model 7E; Diversified Equipment Company Inc., Alexandria, VA, USA) were obtained.
Plant material
The fresh M. stenopetala leaves were collected from Southern Ethiopia around Arbaminch, 500 km far south of Addis Ababa, in September 2014. The plant material was authenticated by a taxonomist, Doctor Dawit Abebe, in the Ethiopian Public Health Institute, and a voucher number AL-001 was deposited for future reference.
Experimental animals
Ethics approval for this study was obtained from the Scientific and Ethical Review Committee of Ethiopian Public Health Institute. The experiments were performed on adult male guinea pigs (Cavia porcellus) weighing 350–400 g. The guinea pigs were bred and obtained from the Ethiopian Public Health Institute. All the animals used for this study were kept in standard animal cages and maintained under laboratory conditions of temperature (22°C±3°C), relative humidity (40%–70%), and 12-hour day/night and had free access to food (standard cabbage and pellet diet) and water. The animals were treated with care throughout the study period and were kept in a well-controlled area according to the National Guide for the Care and Use of Laboratory Animals.24
Plant material preparation and extraction
Fresh M. stenopetala (Baker f.) Cufod. leaves were garbled, chopped, dried under shade (at room temperature), grinded to powder using mortar and pestle, and stored in a cool and dry place. Weighed amounts of 1.208 kg and 2.130 kg powdered leaves were kept in Erlenmeyer flasks and macerated with water (distilled and deionized) and 70% ethanol at room temperature under a rotator shaker until exhaustion for 4 hours and 72 hours, respectively. The 70% EtOH extract was filtered using cotton gauze and then with Whatman filter paper number 1. The filtrate was concentrated under reduced pressure using rota vapor. The semidried residue was kept on a water bath at 400°C overnight and then with a lyophilizer to completely remove the solvent residue. Aqueous (AQ) crude extract was filtered using a Whatman filter paper number 1, kept in refrigerator overnight to freeze, and lyophilized to remove the water. The total yields of the AQ and 70% EtOH extract were calculated as 17.1% and 4.9% (w/w), respectively. Approximately 178.95 g of dried AQ crude extract was defatted by petroleum ether and partitioned with ethylacetate (EtAc); the solvent was removed using rota vapor and lyophilizer to obtain EtAc (18.6% w/w yield) and AQ residue (35% w/w yield), respectively. The dried extracts were kept in a refrigerator until used for the experiment (Figure 1).
  | Figure 1 Flowchart showing plant material preparation and extraction process of Moringa stenopetala leaves. Abbreviation: M. stenopetala, Moringa stenopetala |
Phytochemical screening
All extracts used for the in vitro study were subjected to phytochemical screening following methods described by Tiwari et al25 and Trease and Evans.26 The extracts along with negative controls were tested for the presence of alkaloids, saponins, polyphenols, flavonoids, coumarins, terpenoids, anthraquinones, tannins, phytosterols, and glycosides, which are described in the following sections.
Alkaloids
Approximately 1.5 mL of 10% HCl was added to 0.5 mg of the extracts in a test tube. The mixture was heated for 20 minutes. It was then cooled and filtered. To 1 mL of the filtrate, five drops of Mayer’s and Dragendorff’s reagents were added separately, and the formation of cream- and orange-colored precipitates, respectively, indicated the presence of alkaloids in the extracts.
Saponins
Froth test: An aqueous solution of 0.5 mg of the extract in a test tube was vigorously shaken for 2 minutes. Foam that persisted for 30 minutes and does not disappear upon warming was taken as an indication of the presence of saponin in the extract.
Polyphenols (phenolic compounds)
Three drops of a mixture of 1 mL of 1% FeCl3 and 1% K3Fe(CN)6 each were added to 2 mL of extracts. The formation of green or blue color was taken as an indication of the presence of polyphenols.
Flavonoids
To 2 mL of aqueous solution of the extract, four drops of 2% lead acetate solution were added. The development of yellow or orange color confirms the presence of flavonoids.
Coumarins
Two milliliters of 10% ammonia solution was added to 5 mL of concentrated alcoholic solution of the extracts. The occurrence of an intensive fluorescence under ultraviolet light indicates the presence of coumarin derivatives.
Terpenoids (ketonic)
One milliliter of 2,4-dinitrophenylhydrazine solutions (0.5 g dissolved in 100 mL of 2 M HCl) was added to 2 mL of aqueous solution of the extract. The formation of yellow–orange coloration indicates the presence of a ketonic terpenoids.
Anthraquinones
Borntrager’s test: Five milliliters of the extract was dried and shaken with 3 mL of petroleum ether. The filtrate was added to 2 mL of a 25% ammonia solution. The mixture was shaken, and the formation of a red coloration was taken as an indication of the presence of free anthraquinones.
Tannins
Three drops of 5% ferric chloride solution was added to 1 mL of the extract solution in water. A greenish or blue coloration or precipitation was taken as an indication of the presence of tannins.
Phytosterols with anoids
Five drops of 3% vanillin in concentrated H2SO4 were added to a concentrated chloroform solution of extracts. The formation of a rose or reddish brown color indicates the presence of anoids or phytosterols.
Test for glycosides (Keller–Kiliani test)
To 0.5 g of each extract suspended in 5 mL of water, 2 mL of glacial acetic acid containing one drop of ferric chloride hexahydrate (FeCl3×6H2O) solution was added. This was mixed with 1 mL of concentrated sulfuric acid and observed for a brown ring at the interface or a violet ring below the brown ring; alternatively, acetic acid was added and observed for a greenish ring above the brown ring that gradually spread throughout this layer.
Evaluation of in vitro vasodilatory activity
The in vitro vasodilatory study was conducted on isolated whole, spirally cut thoracic aortic strips of guinea pigs according to the methods described by Vogel et al.27 The guinea pig was sacrificed by gentle cervical dislocation, the thoracic cavity was opened, and the aorta was identified. The descending thoracic aorta was then immediately removed and placed in Krebs–Henseleit physiological solution maintained at 37°C. Excess adherent fat and connective tissue were trimmed off and cleaned; each aorta was cut spirally using heparinized capillary tube with plastic sealing to a strip of ~2 mm wide and 4 mm long. The whole, spirally cut strip was immediately mounted in an organ bath containing 20 mL Krebs–Henseleit physiological solution (118.4 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4×7H2O, 2.2 mM KH2PO4, 1.3 mM CaCl2, 24.9 mM NaHCO3, 11.1 mM glucose, pH 7.4). The whole, spirally cut aortic strip was attached to isometric transducers connected to a polygraph, and a resting tension of 1 g was applied to strip. Whole, spirally cut strip was mounted under this resting tension onto two 0.2 mm L-shaped stainless steel wire hooks gently inserted into the lumen to avoid damage to the endothelium in 20 mL organ baths containing Krebs–Henseleit physiological solution and allowed to stabilize for ~1 hour before commencing an experiment during which period it was washed by overflowing every 15 minutes.
The physiological solution was allowed to pass through a warm water jacket to maintain its temperature and was continuously aerated with carbogen (95% O2 + 5% CO2 gas mixture) at a pH of 7.4. The pH of buffer was checked every 60 minutes of aeration with carbogen.
Experiments were performed on aortic strip with intact (E+) and denuded (E-) endothelium. For the experiment on whole, spirally cut aortic strip with intact endothelium, the presence and functional integrity of the endothelium was tested by administration of 1 μM acetylcholine, which should result in a transient relaxation. On the other hand, the experiment was performed on denuded endothelium by removing endothelial cells from strip and by gently rubbing the intimal surface with a moist wooden stick for 30–60 seconds. The effectiveness of this procedure was subsequently investigated using acetylcholine (1 mM), which normally relaxed aorta strips, but had no such effect on rubbed strip precontracted with 80 mM of KCl.28
After aortic strip equilibration period of 1 hour under a resting tension of 1 g, tissue viability was confirmed by adding 80 mM of KCl. Contraction of whole, spirally cut aortic strip was then induced by administering one of the following contracting agents to organ bath: potassium chloride (80 mM), EPI (1 µM), MB (10 µM), and GLIB (10 µM).
Once a contraction plateau was achieved, extracts of M. stenopetala (Baker f.) Cufod. were administered cumulatively every 15 minutes, and tension responses of the tissue were detected with transducers and recorded isometrically and were displayed on a Grass model 7E polygraph.27,29
At the end of the experiment, relaxation, which is a measure of inhibition of contraction in whole, spirally cut aortic strip precontracted with contracting agent, was determined using a measurement before and after extract administration and calculated using the following formula:30
|
|
where Tc stands for tension due to contracting agents, while Tt stands for tension after adding extract.
For each experiment, aortas from four animals were tested to ensure sufficient biological variability.
Statistical analysis
All experimental data were expressed as mean values (%relaxation) ± standard error of the mean and were subjected to biostatistical interpretation by SPSS windows Version 20 statistical packages (IBM Corporation, Armonk, NY, USA) all the way through a one-way analysis of variance followed by post hoc test (Tukey’s test) for multiple comparisons of the mean differences and responses of different drugs and extracts. Statistical significance of P-value <0.05 was considered as the level of significance.
Results and discussion
This study investigated the in vitro vasodilatory effects of different extracts of M. stenopetala leaves in isolated whole, spirally cut strips of guinea pigs. In addition, the phytochemical screening of different extracts of M. stenopetala leaves was performed. This is the first in-depth study to investigate the effect of different extracts of M. stenopetala leaves on precontracted isolated whole, spirally cut aortic strips of guinea pigs, in particular, blood vessel in an attempt to explore potential vasodilatory mechanism(s).
Phytochemical screening
Basic investigations of the extracts for their major phytocompounds are vital as the active principles of many drugs are secondary metabolites found in plants. Several phytochemical screening tests performed on the crude extracts and solvent fractions of M. stenopetala leaves revealed the presence of different secondary metabolites (Table 1).
  | Table 1 Phytochemical screening of crude extracts and solvent partitions of Moringa stenopetala leaves Abbreviations: AQ, aqueous; EtAc, ethylacetate. |
Phytochemicals are non-nutritive plant chemicals that may have some disease prevention or treatment properties.31 The four solvent extracts (AQ crude, 70% EtOH crude, EtAc partition, and AQ partition residue of AQ crude) of the fresh M. stenopetala (Baker f.) Cufod. leaves were screened for the presence of different phytochemicals.
The qualitative phytochemical screening of AQ extract showed the presence of all tested secondary metabolites. This finding is in agreement with the study performed on AQ extract of Moringa oleifera.32 In addition, 70% EtOH extract showed the presence of all tested phytochemicals except saponins. This finding is in line with the study performed on EtOH extract of M. oleifera.33 Tannin and phytosterol were present in all tested extracts. Saponin was present in all tested extracts but not in 70% EtOH crude extract. Only crude extracts showed a positive test result for alkaloids and glycosides. One of the earlier studies showed the presence of alkaloids, tannins, and glycosides but no saponins and anthraquinones in EtOH extract of M. oleifera.34 Another study showed the presence of all tested metabolites (tannin, alkaloid, saponin, and phenol) in both EtOH and EtAc crude extract of M. oleifera.35 On the other hand, another study showed the presence of all tested metabolites (flavonoid, anthraquinone, alkaloid, saponin, terpenoid, glycoside, and tannin) in both EtOH and AQ crude extract of M. oleifera.36 The current study indicated that the extracts of fresh leaves of M. stenopetala (Baker f.) Cufod. contain different classes of secondary metabolites. The yield obtained for secondary metabolites of M. stenopetala (Baker f.) Cufod. leaves in the current study was recorded to be highest in the case of AQ crude extract followed by 70% EtOH crude, AQ residue, and EtAc fraction of AQ crude extract in succession. The presence of these phytochemicals gave a great potential for the extracts of M. stenopetala (Baker f.) Cufod. leaves in producing vasodilatory effect that signifies the potential of the plant as a source of therapeutic agent.
In vitro vasodilatory activity
BP is the product of cardiac output and peripheral vascular resistance, and it is affected by a change in either or both of these parameters. The goal of any antihypertensive therapy is to bring a reduction in either or both of these parameters, preferably peripheral vascular resistance.37
From the current study, it is observed that all extracts of M. stenopetala (Baker f.) Cufod. produced a relaxation of KCl (on intact and denuded endothelium), MB, EPI, and GLIB induced contraction in isolated whole, spirally cut aortic strips of guinea pigs in a dose-dependent manner. The greatest vasodilatory activity was observed at maximum tested doses of 10 mg/mL (Table 2).
AQ and 70% EtOH crude extracts (Table 2) elicited a higher relaxation than the fraction and residue of AQ crude extract (Table 2) on precontracted isolated whole, spirally cut aortic strips. AQ and 70% EtOH crude extracts have shown 95.56% and 99.10% relaxation against KCl and 90.16% and 82.85% relaxation against EPI, respectively (P<0.001), whereas fraction and residue of AQ crude extract have shown 48.07% and 66.88% relaxation against KCl and 36.19% and 61.31% relaxation against EPI, respectively (P<0.001). This might be due to the synergistic action of different metabolites in crude extracts. This finding is in line with those of the studies performed on AQ extract of M. stenopetala leaves in isolated thoracic aorta of guinea pigs18 and on isolated compounds from crude extracts of M. oleifera leaves in isolated thoracic aorta of rabbit.38
This activity might be attributed to the presence of alkaloids and glycosides in crude extracts, which is in agreement with the study performed on isolated ileum of guinea pigs with alkaloids of leaves of M. oleifera,39 and thiocarbamate glycosides isolated from M. oleifera pods and seeds.40
The percent relaxation of KCl-induced contraction was found to be greater in intact than denuded endothelium of isolated whole, spirally cut aortic strips of guinea pigs for all extracts. This showed that the presence of endothelium has a contribution for increment in the percent relaxation of precontracted isolated whole, spirally cut aortic strips, which may be attributed to the stimulation of endothelium-derived relaxing factor and release of nitric oxide (NO) by some phytoconstituents present in the extracts. As the extracts also produced significant relaxation in denuded endothelium in KCl precontracted isolated whole, spirally cut aortic strips, they have a potential to induce endothelium-independent vasodilation.
The largest dose-dependent percent relaxation was achieved by KCl (intact endothelium) followed by KCl (denuded endothelium) and then by EPI (intact endothelium) precontracted isolated whole, spirally cut aortic strips. The mechanism of relaxation by M. stenopetala might, therefore, be through Ca2+ channel blockade followed by a significant α1-blocking effect, which is indicative of the blockade of Ca2+ influx through both L-type voltage-dependent and receptor-operated Ca2+ channels, respectively. This finding is in-line with those of studies performed on AQ extract of M. stenopetala leaves.18 The glycosides present in the crude extracts might have contributed to the vasodilatory effect by α1-blocking, which is in agreement with the study performed on thiocarbamate glycosides isolated from M. oleifera pods and seeds.40
In addition, the crude extracts and fractions also revealed a relatively dose-dependent vasorelaxant action against aorta precontracted with MB and GLIB , which has shown guanylate cyclase activation and ATP-sensitive potassium channel activation, respectively.
Conclusion
In general, this study demonstrated the in vitro vasodilatory activity of the AQ and 70% EtOH crude extracts of M. stenopetala leaves in precontracted isolated whole, spirally cut aortic strips of guinea pigs perhaps due to different active secondary metabolites found in the extracts. The possible mechanisms of action of the extracts are Ca2+ channel blockade and α1-blockade activity. Further studies, however, have to pursue to confirm the mechanism of action.
Acknowledgments
The current article includes the work based on the Master’s thesis submitted by the main author Bekesho Geleta. The authors are grateful for the financial support provided by the School of Graduate Studies of Addis Ababa University and Ministry of Finance and Economic Development (grant number: OBN6.34/2007) through Ethiopian Public Health Institute. In addition, they would like to appreciate technical assistance of Mr Ashenafi Assefa during the laboratory work. The staffs of the Traditional and Modern Medicine Research Directorate and Finance, for planning and monitoring, are hereby sincerely appreciated for their relentless assistance and much noted contribution during the study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors report no other conflicts of interest in this work.
References
WHO. A Global Brief on Hypertension: Silent Killer Global Public Health Crisis; 2013. Available from: http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf. Accessed July 9, 2014. | ||
Samardeep. Experimental models of hypertension review: a tailored approach. Int J Pharm Res Biosci. 2013;2(6):348–362. | ||
Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Int Med. 2003;139(9):761–776. | ||
Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288(4):C769–C783. | ||
Waugh WH. Role of calcium in contractile excitation of vascular smooth muscle by epinephrine and potassium. Circ Res. 1962;11(6):927–940. | ||
Martin W, Villani GM, Jothianandan DE, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232(3):708–716. | ||
Nakanishi T, Gu H, Hagiwara N, Momma K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res. 1993;72(6):1218–1228. | ||
Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99(11):2602. | ||
Hernández M, Prieto D, Orensanz LM, et al. Involvement of a glibenclamide-sensitive mechanism in the nitrergic neurotransmission of the pig intravesical ureter. Br J Pharmacol. 1997;120(4):609–616. | ||
AU Conference of Ministers of Health (CAMH6). Status Report on Hypertension in Africa. Addis Ababa: Sixth Ordinary Session, CAMH/Exp/6(VI) iii; 2013. Available from: http://www.carmma.org/sites/default/files/PDF-uploads/Background%20Report%20on%20Hypertension%20-%20English.pdf. Accessed July 7, 2014. | ||
Williams EM, Okpako DT, Evans FJ. Pharmacological methods in phytotherapy research. Selection, Preparation and Pharmacological Evaluation of Plant Material I. Chichester: John Wiley; 1996:69–71. | ||
Salako BL, Ajose FA, Lawani E. Blood pressure control in a population where antihypertensives are given free. East Afr Med J. 2003;80(10):529–531. | ||
Du YL, Chen SX. Combinative application of antihypertension drugs. World Clin Drugs. 2005;26:592–602. | ||
Arora DS, Onsare JG, Kaur H. Bioprospecting of Moringa (Moringaceae): microbiological perspective. J Pharmacogn Phytochem. 2013;1(6):193–215. | ||
Mekonnen Y, Gessesse A. Documentation on the uses of Moringa stenopetala and its possible antileishmanial and antifertility effects. Ethiop J Sci. 1998;21(2):287–295. | ||
Abuye C, Urga K, Knapp H, et al. A compositional study of Moringa Stenopetala leaves. East Afr Med J. 2003;80(5):247–252. | ||
Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. Leaves in rats. Front Pharmacol. 2016;7:97. | ||
Mengistu M, Abebe Y, Mekonnen Y, Tolessa T. In vivo and in vitro hypotensive effect of aqueous extract of Moringa stenopetala. Afr Health Sci. 2012;12(4):545–551. | ||
Toma A, Makonnen E, Debella A, Tesfaye B. Antihyperglycemic effect on chronic administration of butanol fraction of ethanol extract of Moringa stenopetala leaves in alloxan induced diabetic mice. Asian Pac J Trop Biomed. 2012;2(3):S1606–S1610. | ||
Sileshi T, Makonnen E, Debella A, Tesfaye B. Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. J Coast Life Med. 2014;2(3):214–221. | ||
Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15:242–249. | ||
Mekonnen Y. Effects of ethanol extracts of Moringa stenopetala leaves on guinea-pig and mouse smooth muscle. Phytother Res. 1999;13(5):442–444. | ||
Biffa D. In vitro antimicrobial activities of bark and leaf extracts of Moringa stenopetala against mastitis causing bacterial pathogens. Ethiop Pharm J. 2005;23(1):15–22. | ||
National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academy Press; 2011. | ||
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. | ||
Trease GE, Evans WC. Pharmacognosy. 13th ed. London: Bailliere Tindall; 1989:135–250. | ||
Vogel GH, Vogel WH, Scholkens BA, Sandow J, Muller G, Vogel WF. Drug Discovery and Evaluation. Pharmacological Assays. 3rd ed. 2008:85. Berlin: Springer. | ||
Nwokocha CR, Ajayi IO, Ebeigbe AB. Altered vascular reactivity induced by malaria parasites. West Indian Med J. 2011;60(1):13–18. | ||
Madingou KNO, Souza A, Lamidi M, et al. Study of medicinal plants used in the management of cardiovascular diseases at Libreville (Gabon): an ethnopharmacological approach. Int J Pharm Sci Res. 2012;3(1):111–119. | ||
Bamidele AI, Sedoten AH, Stella N, Olabisi O, Phillip UA. Evaluation of the possible mechanisms of antihypertensive activity of Loranthus micranthus: an African Mistletoe. Biochem Res Int. 2011;2011:159439. | ||
Sivagnanam S, Singh MK, Kumar SM, Rao MRK. Preliminary phytochemical analysis of Amaranthus polygonoides. Res J Pharm Biol Chem Sci. 2014;5(3):82–87. | ||
Brindha V, Sugunabai J, Jayaraj M, Karpagam T. Antidiabetic efficiency of Moringa oleifera and Solanum nigrum. Int J Pharm Pharm Sci. 2014;6(1):40–42. | ||
Onyekaba TC, Chinedu OG, Fred AC. Phytochemical screening and investigations of antibacterial activities of the ethanol leaves extract of Moringa oleifera. J Pharm Chem Biol Sci. 2013;3(3):962–973. | ||
Denen A, Ejike DE, Moses DA, Seriki SA, Chiamaka NU. Hypolipidaemic effect of ethanol leaf extract of Moringa oleifera Lam. in experimentally induced hypercholesterolemic wistar rats. Int J Nutr Food Sci. 2014;3(4):355–360. | ||
Ojiako EN. Phytochemical analysis and antimicrobial screening of Moringa oleifera leaves extract. Int J Eng Sci. 2014;3(3):32–35. | ||
Nweze NO, Felix N. Phytochemical, proximate and mineral composition of leaf extracts of Moringa oleifera Lam. from Nsukka, South-eastern Nigeria. IOSR J Pharm Biol Sci. 2014;9(1):99–103. | ||
Aziz N, Mehmood MH, Siddiqi HS, et al. Antihypertensive, antidyslipidemic and endothelial modulating activities of Orchis mascula. Hypertens Res. 2009;32(11):997–1003. | ||
Gilani HA, Aftab K, Suria A, et al. Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother Res. 1994;8:87–91. | ||
Dangi SY, Jolly CI, Narayanan S. Antihypertensive activity of the total alkaloids from the leaves of Moringa oleifera. Pharm Biol. 2002;40(2):144–148. | ||
Jansakul C, Wun-noi A, Croft K, Byrne L. Pharmacological studies of thiocarbamate glycosides isolated from Moringa oleifera. J Sci Soc Thailand. 1997;23:335–346. | ||
Klabunde R. Cardiovascular Physiology Concepts. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2011. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.