Back to Journals » Journal of Inflammation Research » Volume 12
In vitro expression of NLRP inflammasome-induced active Caspase-1 expression in normal human epidermal keratinocytes (NHEK) by various exogenous threats and subsequent inhibition by naturally derived ingredient blends
Received 15 May 2019
Accepted for publication 17 July 2019
Published 26 August 2019 Volume 2019:12 Pages 219—230
DOI https://doi.org/10.2147/JIR.S215776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
James V Gruber,1 Robert Holtz2
1Research and Development, BotanicalsPlus, Little Falls, NJ 07424, USA; 2Research and Development, Bioinnovation Laboratories, Inc, Lakewood, CO 80235, USA
Correspondence: James V Gruber
BotanicalsPlus, 163 East Main Street, Suite #273, Little Falls, NJ 07424, USA
Email [email protected]
Background: The discovery of the nod-like receptor protein (NLRP) inflammasomes in 2002 has led to the rapid identification of these unique cellular proteins as key targets for studies on innate inflammation pathways. The NLRP inflammasomes have been shown to be expressed in normal human epidermal keratinocytes (NHEK) and human dermal fibroblasts (HDF). NLRP inflammasomes in keratinocytes are interesting as these skin cells are the first living cells in the skin to contact external exogenous threats such as UV energy, chemicals, physical trauma, and bacteria and viruses. Activation of the NLRP Inflammasomes by exogenous threats results in the release of active Caspase-1 (ACasp-1), a key protease enzyme, which targets inactive forms of IL-1β, IL-18 as well as IL-1α and IL-33.
Purpose: This article discusses efforts to examine the release of active Caspase-1 from NHEKs activated by various exogenous threats including UVB energy, ATP, Nigericin and Urban Dust. The work further examines if, after inflammasome activation and Caspase-1 release, certain naturally derived botanical ingredients known to have anti-inflammatory effects can function to inhibit upregulation of active Caspase-1.
Methods: NHEK were treated with various doses of UVB, ATP and Nigericin and with a single dose of Urban Dust. ACasp-1 expression was measured after 3 and 20 hours using the Promega Caspase Glo-1 bioluminescent assay. After confirmation that 60 mJ/cm2 of UVB and 5mM of ATP were effective to activate NHEK ACasp-1 release after 20 hrs, these conditions were employed to examine the influence of three botanical blends of ingredients on their ability to inhibit ACasp-1 expression.
Results: Initial results demonstrate that NHEKs can be activated to release active Caspase-1 by ATP and UVB, but not by Nigericin or Urban Dust. In addition, it was unexpectedly found that, while ATP and UVB activated NHEKs, the release of ACasp-1-did not happen within the first 3 hours after exposure but did become significant after 20 hours. Additional results indicate that a blend of polysaccharides and two blends of antioxidants, one oil-soluble and the other water-soluble, known for their anti-inflammatory effects, can reduce expression of active Caspase-1 in activated NHEKs when applied extracellularly.
Conclusion: Expression of NLRP activated release of ACasp-1 was found to be influenced by UVB and ATP but not by Nigericin or Urban Dust. The effects were also time dependent. Several botanical extract blends were found to reduce ACasp-1 expression in previously activated NHEKs. Links between these inflammatory effects and processes of cellular inflammaging are discussed.
Keywords: inflammation, inflammasome, NLRP3, NLRP1, polysaccharide, antioxidant, Caspase-1
Introduction
It is interesting that regardless of how well a person protects his/her skin or avoids sunlight, eats well, exercises, and gets plenty of sleep, the body continues to age. The aging process is inherent in life and progresses regardless of the steps often taken to avoid the gradual body diminishments. There are various theories as to why our bodies age that have captured the scientific and commercial cosmetic and therapeutic imagination. These include, for example, telomers which are the endcaps of our DNA and seem to shorten with aging.1 The possibility that caloric restriction turns on various primitive defense mechanisms such as Sirtuin proteins that can also be influenced by ingredients such as resveratrol has become an important target for longevity studies.2 The theory of free radical aging posits the body, burning oxygen and being exposed to external radiation like UV, creates countless free radicals that attack key cellular targets, like DNA, thereby resulting in aging.3 The idea that free radicals can be controlled by the use of antioxidants remains a popular antiaging concept.4 Control of key biological cellular defense pathways, such as p53 or mTOR, that are responsible for controlling cellular defensive processes and cellular senescence is also a key target of antiaging strategies.5 And yet, aging remains (and will likely remain) a mysterious process that can be influenced but never completely overcome.
Inherent in all the examples above is the body’s effort to fight aging that occurs through the cellular control of inflammation.6 Inflammation is the yin and yang of the body’s effort to fight adverse threats that diminish the proper functioning of the body’s normal defensive systems, and the aging processes associated with inflammation are known as “inflammaging”.7 Inflammation occurs when the body’s cells are attacked by external threats such as bacteria or viruses, or chemicals (like pollution). Inflammation also occurs when external energies, like UV or visible light, cause cellular damage.8 Inflammation upregulates body defenses that work to diminish the damage caused by the external attacks and brings the body back into homeostasis. But, when the body’s defenses over-compensate, the effects can be equally detrimental. A cytokine storm is an example of inflammation overcompensation to an allergen or microbial assault and often can be more dangerous than the actual initial aggressor.9
The cellular cascades that lead to inflammation and healing are complex. They begin with attack by an exogenous external agent that tries to change the natural balance of the body. In 2002, it was discovered that initiation of inflammation begins with the body’s recognition of an external aggressor through nod-like receptor proteins (NLRPs).10 The NLRPs activate the process of inflammation through recognition of the external threats and creation of cellular protein structures called Inflammasomes.11,12 Inflammasomes are the gatekeepers for the processes of inflammation.13
The NLRP inflammasomes: inflammation’s gatekeepers
In 2002, Martinon et al, published their initial findings on the discovery of cellular inflammasomes.10 They initially referred to inflammasomes as “molecular platforms that triggered activation of inflammation”. In a later publication, they focused attention on the defensive influences of inflammasomes, calling them the “guardians of the body”.13 Inflammasome activation has been linked to various skin abnormalities such as acne, dandruff, contact dermatitis, atopic dermatitis, rosacea, photoaging, and skin cancer.14–21
Inflammasome activation has been directly linked to the process of inflammaging.22,23 It has been demonstrated that in aging human fibroblasts, inflammasomes accumulate and that control of inflammasome activation can help improve cell survivability.14 Inflammasome function is associated with the processes of autophagy and has also been shown to be influenced by the body’s circadian fluctuations.24–27 In addition, while it appears that there have been correlations noted between the microbiome of the gut and inflammasome activation, solid links between the skin microbiome and inflammasome activation or suppression are lacking.28,29
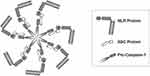
The activated nod-like receptor proteins, or NLRPs, create circular Inflammasome oligomers, Figure 1. The NLRPs are comprised of three key molecular components that include 1) Nucleotide-binding domain and Leucine-rich repeat Receptors (NLR), 2) Apoptosis-associated Speck-like protein (ASC), and 3) inactive Caspase-1 (Pro-Caspase-1).30 Formation of these protein fragments is controlled through transcription from nuclear signals arising from nuclear factor-kappa β (NF-ƙβ) within the cell’s nucleus.
These three protein components assemble to form a cohesive molecular unit called an NLRP Inflammasome. External threats cause the NLRP Inflammasomes to change structure and to oligomerize into unique structures called Inflammasome Complexes.31 The fully assembled Inflammasome Complex cleaves Pro-Caspase-1, forming the active form of the protease, Caspase-1. Caspase-1 acts on available inactive cytokine precursors, Pro-IL-1β and Pro-IL-18 always present in defensive body cells, creating active interleukin cytokines, IL-1β and IL-18. Activated IL-1β and IL-18 then initiate a process of inflammation response that ultimately leads to accumulation of cellular and biochemical protective and curative moieties.32 There are several Inflammasomes whose primary protein structures have been essentially determined. However, the lion’s share of work in skin has been done looking at the nod-like receptor protein-1 (NLRP1) and nod-like receptor protein-3 (NLRP3).
The interaction of NLRP and toll-like receptor (TLR) proteins via NF-ƙβ signaling
The surface of cells that can respond and make inflammasomes are typically studded with TLRs proteins which can and do become activated by external stimulants. The TLRs then upregulate expression of key inflammasome proteins necessary for creation of the inflammasome complex which is critical for the formation of active Caspase-1 (ACasp-1) via upregulation of genomic control through the NF-ƙβ nuclear pathways. TLRs communicate to create these proteins through upregulation of NF-ƙβ that transcribes the formation of the inert NLR, ASC, and Pro-Caspase-1 proteins that ultimately comprise the inflammasome complex. Skin cells that are first responders to danger associate molecular patterns (DAMPs) and pathogen associated molecular patterns (PAMPs) likely always have some reservoir of necessary inflammasome components residing intracellularly. It makes biological sense that keratinocytes would have the ability to create inflammasomes as these cells are essentially first-responders to exogenous threats like bacteria, UV, particles, etc. The inflammasomes can respond directly to exogenous threats by forming inflammasome complexes and so can react directly to exogenous threats like the TLRs. However, unlike the TLRs, once the inflammasome proteins assemble to form the inflammasome complex, the complex can activate Pro-Caspase-1 which then functions to activate Pro-IL-1β and Pro-IL-18, starting the inflammation cascades.
Caspase-1: a measure of NLRP inflammasome activation
Caspase proteins are cysteine proteases that initiate or carry out cellular processes involving protein cleavage.33 They help injured (activated) cells “decide” if they should repair themselves or die through cellular mechanisms such as apoptosis and pyroptosis.34 As noted, release of Caspase-1 through canonical immune pathways results in upregulation of critical inflammatory cytokines such as IL-1β, IL-18, but Caspase-1 can also cleave Pro-IL-1α and Pro-IL-33 through non-canonical immune pathways.35 Regardless of the pathway, expression of Caspase-1 is linked directly to formation and activation of NLRP Inflammasomes. If the injured cells are creating Caspase-1, it is via an inflammasome-mediated pathway.35
In 2017, O’Brien et al, reported on a new bioluminescence assay that directly detects the release of ACasp-1 from NLRP inflammasomes.36 Activation of the NLRP inflammasomes can occur via various agonists such as UV energy or chemical initiators.37 The assay has been used to examine Caspase-1 expression in skin fibroblasts and keratinocytes.38,39 The assay has also had some initial use in in vivo models on mice.40 Normal human epidermal keratinocytes (NHEK) have been shown to express Caspase-1 through NLR protein cascades.35,41–43 However, there is ongoing examination and discussion if the NLRP1 or NLRP3 inflammasome pathways are the most critical to skin health.42,43 This paper will discuss efforts to examine the expression of Caspase-1 from externally stressed NHEKs. In addition, the paper will examine the ability of some selected polysaccharide- and antioxidant-based topical treatments to inhibit Caspase-1 expression in Inflammasome-activated keratinocytes.
Materials and methods
Human keratinocyte cell culture
Human adult epidermal keratinocytes (C0055C, Thermofisher) were grown using EpiLife Media (60 µM calcium) supplemented with the HKGS kit (S001K, ThermoFisher) as recommended by the manufacturer. The cells were cultured at 37±2°C and 5±1% CO2 in T-75 flasks and then seeded into white, clear bottom 96-well plates and grown until confluent. Once confluent the cells were cultured overnight in hydrocortisone free EpiLife Media to minimize any potential anti-inflammatory effect from this component of the HKGS kit.
Screening of potential Caspase 1 activators: UVB, ATP, Nigericin, and Urban Dust
Initial pilot work for this study was conducted to determine what type of cellular insults would activate Caspase-1. To determine this, cultured keratinocytes were exposed to UVB, ATP (Sigma Aldrich, Catalog#A6419), Nigericin (Sigma Aldrich, Catalog#N7143), and Urban Dust (NIST SRM1649b). For the UVB exposure, the cell culture media were removed and replaced with 100 µL of PBS. The cells were then irradiated with 0, 15, 30, 45, or 60 mJ/cm2 of UVB using a UVB lamp (Model #UVLM-26, Fisher Scientific, MA, USA). After the irradiation, the PBS was removed and 100 µL of hydrocortisone free EpiLife media was added. Caspase-1 activity was then determined at 3 and 20 hrs post-irradiation. For the remaining insults, the materials were added to the hydrocortisone free EpiLife media at the following concentrations: 1 and 5 mM ATP, 20 and 40 µM Nigericin, and 100 µg/mL Urban Dust. Caspase-1 activation was then determined after 3 and 20 hrs of incubation using the Caspase-Glo 1 Inflammasome Assay (G9952, Promega).
Prevention of Caspase-1 activation
Based on the levels of Caspase-1 activation observed at the 20-hr incubation time point in the initial screening of potential activators, 5 mM ATP and 60 mJ/cm2 UVB were chosen as the two best activators to use for screening test materials for their ability to influence Caspase-1 activation. Test materials were prepared in hydrocortisone free EpiLife media. Stock solutions of non-water-soluble materials were prepared in dimethyl sulfoxide (DMSO), with dilutions also made in DMSO such that, when the stocks were added to hydrocortisone free EpiLife media, the final concentration of DMSO was 0.5% regardless of the final concentration of the non-water-soluble test material. A 0.5% DMSO-treated group served as a control for the effects of the DMSO vehicle.
ATP treatment: treatment of keratinocytes
The cell culture media for the cultured keratinocytes were removed and replaced with the media containing the test materials and 5 mM ATP. One set of cells was treated with media alone (no ATP added) to serve as a baseline control, while another set of cells was treated with media +5 mM ATP (no test materials) to serve as the stimulated control. The cells were then incubated for 20 hrs after which Caspase-1 activation was determined.
UVB exposure
Media for the Keratinocytes were exposed to a 60 mJ/cm2 dose of UVB as described above. A set of non-UVB keratinocytes was prepared and used as a non-stimulated control, while another set of keratinocytes was exposed to UVB and treated with media alone to serve as the untreated group. At the end of the incubation periods, Caspase-1 activity was then determined.
Caspase-1 assay (Promega Caspase-Glo 1 inflammasome assay kit)
Prior to the assay, the Caspase-1 luminescent substrate solution was prepared and equilibrated to room temperature. After the respective incubation periods, 100 µL of the substrate solution was added to each well of the 96-well plate, the plates were mixed for 30 s and then incubated at room temperature for 1.5 hrs to allow the luminescent signal to stabilize. The plate was then read using a Fluoroskan Ascent Luminescence Plate Reader. All Caspase-1 activation assays were conducted in triplicate with the results analyzed using an ANOVA to determine statistically significant treatment differences (p<0.05).
Test materials
The polysaccharide extract blend comprised of Opuntia ficus cladodes, Schyzophillan mushroom mycelium, Western Larch tree pulp, and brown algae was provided by BotanicalsPlus [Little Falls, NJ] and was used as is. The antioxidant blends including the oil-soluble blend comprised of Lithospermum erythrorhizon Roots, Haematococcus pluvialis algae, Camellia sinensis Leaf, and Glycyrrhiza glabra root extracts and the water-soluble antioxidant blend comprised of Lycium barbarum Fruit, O. ficus-indica Stem, C. sinensis Leaf, and Citrus sinensis Fruit extracts were sourced from BotanicalsPlus and were used as is. The MCC955 was sourced from Sigma Aldrich (PZ0280).
Results
Initiating NLRP inflammasome activation: energy sources
To examine the influence of external topical treatment’s ability to inhibit Caspase-1 expression, it was necessary to first examine various established inflammasome activators. Epidermal keratinocytes are the first cells in the skin that meet external threats. As such, it is not unexpected that they would be able to express innate immune responses. The literature suggests that UV energy, bacterial surface proteins like Nigericin, and ATP will cause activation of the NLR Pyrin inflammasomes and commence the release of ACasp-1.44 We examined various published and unpublished activators for their ability to stimulate the expression of Caspase-1 as measured via the O’Brien assay. Figure 2 shows the results of exposure of NHEK to various dosages of UVB radiation. It was found that at all energy levels, expression of Caspase-1 did not happen within the first 3 hrs of exposure. However, within 20 hrs, statistically significant expression of Caspase-1 occurred at 45 and 60 mJ/cm2 of UVB exposure.
Initiating NLRP inflammasome activation: chemical sources
We examined three potential chemical activators of keratinocyte Caspase-1 release. Two were known to have an influence on inflammasome activation in various cell types and included ATP, Nigericin and one, Urban Dust, had not been examined, Figure 3. As can be seen, statistically significant upregulation of ACasp-1 again did not happen within the first 3 hrs of treatment with any activators. However, ATP was found to be a potent stimulator of Caspase-1 expression within the 20-hr time frame at concentrations of 1 and 5 mM. Interestingly, in this study, Nigericin, a bacterial surface protein known to stimulate inflammasome activation, did not show a significant effect on NHEKs. It is possible that different concentrations may be required to activate NHEKs with this protein, but in the parameters reported here, it was ineffective. Also, Urban Dust, a marker not known to be examined for its impact on inflammasome expression in keratinocytes, also did not appear to have a significant impact on inflammasome activation at a dose of 100 µg/mL.
Treatment with polysaccharide-based blend with UVB and ATP activation
Having established that application of both UVB radiation and ATP caused statistically significant upregulation of Caspase-1 expression, a blend of polysaccharides comprising extracts taken from O. ficus cladodes, Schyzophillan mushroom mycelium, Western Larch tree pulp and brown algae was examined for its influence on Caspase-1 expression, Figures 4 and 5. These unique polysaccharides were selected because they were suggested to be effective at impacting either skin inflammation or direct expression of IL-1β in keratinocytes.45–48 Prior to examining the polysaccharide blend, they were examined for their cytotoxicity to keratinocytes [data not shown]. The blend was then developed by carefully mixing the polysaccharides such that topical applications on keratinocytes in the range of 1–3% would provide each polysaccharide at a dose below its demonstrated effective but non-cytotoxic level.
Treatment with oil-soluble antioxidant-based blend with UVB and ATP activation
Antioxidants are known to impact the NLRP inflammasome.49,50 Of special interest were extracts of L. erythrorhizon roots, H. pluvialis algae, C. sinensis leaf and Glycyrrhiza glabra root which are known to show anti-inflammatory effects on skin or skin cells.51–54 All four antioxidants are commercially available as oil-soluble extracts and were blended to provide a fully soluble oil-based mixture. The antioxidant blend was examined for its cytotoxicity to keratinocytes [data not shown] to establish baseline levels which could be tested in the Caspase-1 assay. The oil was dissolved into DMSO prior to treatment. The blend was examined for its ability to reduce ACasp-1 expression in UVA and ATP activated keratinocytes, Figures 6 and 7.
Treatment with water-soluble antioxidant-based blend with UVB and ATP activation
Similar to the details described above for the oil-based antioxidant blend, a water-soluble blend of antioxidants known to impact skin inflammation that included extracts of L. barbarum fruit, O. ficus-indica stem, C. sinensis leaf, and Citrus sinensis fruit was examined using both UV and ATP activation, Figures 8 and 9.53,55–57
Discussion
The discovery of the NLRP Inflammasomes as a source of cellular inflammation was a significant event in better understanding the innate immune response. Further discovery that NLRP1 and NLRP3 inflammasomes are both expressed in skin keratinocytes and respond to skin wounding and UV radiation damage demonstrates that these key skin cells, creating the first line of living defense against exogenous attack, are critical for maintaining the health of the skin.42,43
In this work, the activation of keratinocytes by two known Inflammasome activators, ATP and UVB, was confirmed via testing for direct expression of ACasp-1 in exposed cells. It was surprising that MCC950, a known inflammasome inhibitor, had little impact on either ATP or UVB expression of ACasp-1 in these assays. MCC950 is a known NLRP3 inhibitor and it may be that the assays discussed here are having a greater impact of the NLRP1 inflammasomes as suggested recently by various research groups.43,58,59
Nigericin is a known activator of inflammasomes in immune response cells such as dendritic cells and Langerhans cells and was recently examined in CRISP-modified keratinocytes as a potential activator of inflammasome response in keratinocytes.60 However, in the assays presented here, activation of the NHEKs with Nigericin failed to show an anticipated effect. We are not certain why the Nigericin failed to show an effect in this assay and continue to investigate possible reasons. To the best of our knowledge, Urban Dust, a component often suggested to be an inflammatory component of urban smog also failed to have an influence on inflammasome activation in this assay. 100 µg/mL was employed in this work because in early work done at BioInnovation Laboratories, Urban Dust had shown effects at stimulating the release of IL-6, IL-8, and PGE2 in keratinocytes and so was selected as a reasonable concentration to examine the release of ACasp-1 in the present assay. However, as noted, at 100 µg/mL, there appears to be no effect on activation of NLRP inflammasomes as measured by release of ACasp-1. There is continued interest to see if, perhaps, activation may require smaller particle-sized dust. The dust used in the present experiments had an average particle size between 10 and 100 microns. Dust of a particle size in the range of 2.5 microns (PM2.5) might be able to elicit an inflammatory response via the NLRP inflammasomes, but this was not tested here as this sized dust can be difficult to obtain commercially.
It must be noted as well that there was a time lag from the time of exposure to the activator and the expression of measurable ACasp-1 in NHEKs. The reason for the time lag is not immediately apparent, but recent discussion in the literature has suggested that it is not unknown that cells may experience a time lag between the time of exposure and the actual expression of inflammation cytokines.19
It is possible with the testing protocols described here to examine the effects of topically applied ingredients to diminish NLRP Inflammasome-induced ACasp-1 expression. Both water-soluble ingredients, including botanically isolated polysaccharides and antioxidants, as well as a botanically isolated oil-soluble antioxidant have potential influence on ACasp-1 expression when post-added after activation of the keratinocyte inflammasomes. While the ingredients had statistically significant impacts on reductions in ACasp-1 expression, there was not always a clear dose-response apparent in these effects. Statistical significance was measured against untreated controls, not within ingredient test groups so it is difficult to say if the ingredients simply all worked or did not work vs untreated controls and not whether they performed statistically in a dose-dependent fashion. Interestingly, while the polysaccharide blend appeared to influence both UVB and ATP-induced ACasp-1 expression, both the water-soluble and oil-soluble antioxidant blends appeared to only influence the UVB-induced ACasp-1 expression. This suggests, perhaps, that the polysaccharides may influence a different aspect of the ACasp-1 expression compared to the influence of the antioxidants which may be affecting free radical formation driven by mitochondrial dysfunction.61 Elucidating the mechanisms by which the ingredients impact the ACasp-1 expression via the current test methods, however, is difficult.
Conclusion
The discovery of the innate immune response inflammasomes has significantly expanded the understanding of how the body responds to attacks and damages. The fact that NLRP inflammasomes are expressed in both keratinocytes and fibroblasts suggests that the skin, being a primary contactor of the outside environment, is well suited to rapidly bring the body’s defenses to bear on damaging insults. In the work described here, it was found that NHEKs are well activated to express ACasp-1 via NLRP-induced cellular activation. However, while it was noted that ATP and UVB energy did activate NHEKs to express increased levels of ACasp-1, the effects were not immediate or even apparent at the 3-hr time point after exposure. However, within 20 hrs, both ATP and UVB did elicit an increase in expression of ACasp-1 indicating that the NLRP inflammasomes had been activated. Surprisingly, a known NLRP1 inhibitor, MCC950, did not have an inhibitory effect on the release of ACasp-1 as was anticipated to happen. The reasons for this apparent lack of functional activity on these cells are presently unknown but should be noted for others working with this molecule as a control in skin research. Also, another somewhat surprising finding in these studies was that Nigericin, a bacterial lipopolysaccharide known to induce activation of macrophages, did not seem to impact the activation of the NLRP inflammasomes in NHEKs. This finding as well should be considered cautionary for others working with this NLRP activator. The examination of Urban Dust as a potential activator of NHEK inflammasome activation has not been reported. While more work should be done to see if Urban Dust can activate NLRP inflammasome inflammatory pathways, it may be that particle size plays a strategic and critical role in this effect. The Urban Dust used here had a particle size between 10 and 100 microns. It may be worth examining if Urban Dust of a smaller particle size, perhaps around 2.5 microns, might have a more profound impact on inflammasome activation. The ability of various naturally derived ingredients to inhibit the expression of ACasp-1 is important. It suggests that these ingredients may have the potential to reduce skin inflammatory responses via topical treatments. However, this will need to be demonstrated in clinical work which is presently ongoing.
Disclosure
Funding for the research described here was provided by Jeen International and BotanicalsPlus. The study was conducted in BioInnovation Laboratories, Inc. The authors report no other conflicts of interest in this work. Human Epidermal Keratinocytes used in the studies reported were purchased commercially as noted in the Materials section.
References
1. Mensa E, Latini S, Ramini D, Storci G, Bonafè M, Olivieri F. The telomere world and aging: analytical challenges and future perspectives. Ageing Res Rev. 2019;50:27–42.
2. Wang Y, He J, Liao M, et al. An overview of sirtuins as potential therapeutic target: structure, function and modulators. Eur J Med Chem. 2019;161:48–77. doi:10.1016/j.ejmech.2018.10.028
3. Tan BL, Norhaizan ME, Liew WP, Sulaiman-Rahman H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol. 2018;9:1162.
4. Pomatto LCD, Davies KJA. Adaptive homeostasis and the free radical theory of ageing. Free Radic Biol Med. 2018;124:420–430. doi:10.1016/j.freeradbiomed.2018.06.016
5. de Magalhaes JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Ageing Dev. 2018;170:2–9. doi:10.1016/j.mad.2017.07.001
6. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Adv Gerosci. 2014;69:S4–S9.
7. Fulop T, Witkowski JM, Olivieri F, Larbi A. The integration of inflammaging in age-related diseases. Semin Immunol. 2018;40:17–35. doi:10.1016/j.smim.2018.09.003
8. Yap WN. Tocotrienol-rich fraction attenuates UV-induced inflammaging: a bench to bedside study. J Cosmet Dermatol. 2018;17:555–565. doi:10.1111/jocd.12421
9. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi:10.1128/MMBR.05015-11
10. Martinon F, Burns K, Tschopp J. The Inflammasome: a molecular platform triggering activation of inflammatory Caspases and processing of proIL-1β. Mol Cell. 2002;10:417–426.
11. de Sa DC, Neto CF. Inflammasomes and dermatology. An Bras Dermatol. 2016;91:566–578. doi:10.1590/abd1806-4841.20165577
12. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi:10.1016/j.cell.2014.04.007
13. Martinon F, Meyer A, Tschoop J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi:10.1146/annurev.immunol.021908.132715
14. Fabbrocini G, Galliano MF, Aries MF, et al. Fragility of the epidermis, a common pathophysiological mechanism of acne vulgaris, rosacea and reactive skin involving inflammasome activation. Inflamm Cell Signal. 2015;2:1–9.
15. Martin SF. The role of the innate immune system in allergic contact dermatitis. Allergologie. 2017;1:39–43. doi:10.5414/ALX01274E
16. Jang HY, Koo JH, Lee SM, Park BH. Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Exp Mole Med. 2018;50:73–81. doi:10.1038/s12276-018-0104-3
17. Kistowska M, Fenini G, Jankovic D, et al. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp Dermatol. 2014;23:884–889. doi:10.1111/exd.12552
18. Awad F, Assrawi E, Louvrier C, et al. Photoaging and skin cancer: is the inflammasome the missing link? Mech Ageing Dev. 2018;172:131–137. doi:10.1016/j.mad.2018.03.003
19. Faustin B, Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18:4–8. doi:10.1016/j.tcb.2007.10.004
20. Oyewole AO, Birch-Machin MA. Sebum, inflammasomes and the skin: current concepts and future perspective. Exp Dermatol. 2015;24:651–654. doi:10.1111/exd.12774
21. Liang N, Yang YP, Li W, et al. Overexpression of NLRP3, NLRC4 and AIM2 inflammasomes and their priming-associated molecules (TLR2, TLR4, Dectin-1, Dectin-2 and NFκB) in Malassezia folliculitis. Mycoses. 2018;61:111–118. doi:10.1111/myc.12711
22. Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73. doi:10.1016/j.smim.2018.09.001
23. Mejias NH, Martinez CC, Stephens ME, de Rivero Vaccari JP. Contribution of the inflammasome to inflammaging. J Inflamm. 2018;15:23–33. doi:10.1186/s12950-018-0198-3
24. Krakauer T. Inflammasomes, autophagy and cell death: the trinity of innate host defense against intracellular bacteria. Mediator Inflamm. 2019;2019:Article ID 2471215. doi:10.1155/2019/2471215
25. Chen RJ, Lee YH, Yeh YL, Wang YJ, Wang BJ. The roles of autophagy and the inflammasome during environmental stress-triggered skin inflammation. Int J Mol Sci. 2016;17(12):E2063. doi:10.3390/ijms17122063
26. Pourcet B, Zecchin M, Ferri L, et al. Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to reduce the severity of fulminant hepatitis in mice. Gastroenterology. 2018;154:1449–1464. doi:10.1053/j.gastro.2017.12.019
27. Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:1–11. doi:10.3389/fphys.2014.00001
28. Yao X, Meng G. Inflammasomes in the gut mucosal homeostasis. Adv Exp Med Biol. 2017;1024:133–151. doi:10.1007/978-981-10-5987-2_6
29. Fenini G, Contassot E, French LE. Potential of IL-1, IL-18 and inflammasome inhibition for the treatment of inflammatory skin diseases. Front in Pharmacol. 2017;8:1–20. doi:10.3389/fphar.2017.00278
30. Groslambert M, Py BF. Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res. 2018;11:359–374. doi:10.2147/JIR.S141220
31. Schroder K, Tschopp J. The inflammsomes. Cell. 2010;140:821–832. doi:10.1016/j.cell.2010.01.040
32. Nasti TH, Timares L. Inflammasome activation of IL-1 family mediators in response to cutaneous photodamage. Photochem Photobiol. 2012;88:1111–1125. doi:10.1111/j.1751-1097.2012.01154.x
33. Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi:10.1038/sj.cdd.4402038
34. Wang S, Yuan YH, Chen NH, Wang HB. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol. 2018;67:458–464. doi:10.1016/j.intimp.2018.12.019
35. Strittmatter GE, Garstkiewicz M, Sand J, Grossi S, Beer HD. Human primary keratinocytes as a tool for the analysis of Caspase-1-dependent unconventional protein secretion. Methods Mol Biol. 2016;1459:135–147. doi:10.1007/978-1-4939-3804-9_9
36. O’Brien M, Moehring D, Muñoz-Planillo R, et al. A bioluminescent Caspase-1 activity assay rapidly monitors inflammasome activation in cells. J of Immunol Meth. 2017;447:1–13. doi:10.1016/j.jim.2017.03.004
37. Ahn H, Kwon HM, Lee E, Kim PH, Jeung EB, Lee GS. Role of inflammasome regulation on immune modulators. J Biomed Res. 2018;32:401–410. doi:10.7555/JBR.32.20170120
38. Kühbacher A, Henkel H, Stevens P, et al. Central role for dermal fibroblasts in skin model protection against Candida albicans. J Infect Dis. 2017;215:1742–1752. doi:10.1093/infdis/jix153
39. Olivier E, Dutot M, Regazzetti A, Laprévote O, Rat P. 5-Hydroxycholesterol induces both P2X7-dependent pyroptosis and caspase-dependent apoptosis in human skin model: new insights into degenerative pathways. Chem Phys Lipids. 2017;207(Pt B):171–178. doi:10.1016/j.chemphyslip.2017.06.001
40. Castelblanco M, Lugrin J, Ehirchiou D, et al. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J Biol Chem. 2018;293:2546–2557. doi:10.1074/jbc.M117.806869
41. Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The Inflammasome mediates UVB-induced activation and secretion of Interleukin-1β by keratinocytes. Curr Biol. 2007;17:1140–1145. doi:10.1016/j.cub.2007.05.074
42. Ito H, Kanbe A, Sakai H, Seishima M. Activation of NLRP3 signaling accelerates skin wound healing. Exp Dermatol. 2018;27:80–86. doi:10.1111/exd.13441
43. Burian M, Yazdi AS. NLRP1 is the key inflammasome in primary human keratinocytes. J Invest Dermatol. 2018;138:2507–2510. doi:10.1016/j.jid.2018.08.004
44. Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory disease. Redox Biol. 2015;4:296–307. doi:10.1016/j.redox.2015.01.008
45. Petruk G, Di Lorenzo F, Imbimbo P, et al. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg Med Chem Lett. 2017;27:5485–5489. doi:10.1016/j.bmcl.2017.10.043
46. Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol. 2008;5:47–57. doi:10.1080/15476910802019045
47. Dion C, Chappuis E, Ripoll C. Does larch arabinogalactan enhance immune function? A review of mechanistic and clinical trials. Nutr Metab (Lond). 2016;13:28. doi:10.1186/s12986-016-0093-y
48. Florez N, Gonzalez-Munoz MJ, Ribeiro D, Fernandes E, Dominguez H, Freitas M. Algae polysaccharides’ chemical characterization and their role in the inflammatory process. Curr Med Chem. 2017;24:149–175. doi:10.2174/0929867323666161028160416
49. Tozser J, Benko S. Natural compounds as regulators of NLRP3 inflammasome-mediated IL1β production. Mediators Inflamm. 2016;2016:5460302. doi:10.1155/2016/5460302
50. Dutartre P. Inflammasomes and natural ingredients towards new anti-inflammatory agents. Molecules. 2016;21:1492. doi:10.3390/molecules21111492
51. Zorman J, Susjan P, Hafner-Bratovic I. Skikonin suppresses NLRP3 and AIM2 Inflammasomes by direct inhibition of Caspase-1. PLoS One. 2016;11(7):e0159826. doi:10.1371/journal.pone.0159826
52. Yoshihisa Y, Rehman MU, Shimizu T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp Dermatol. 2014;23:178–183. doi:10.1111/exd.12347
53. Ellis LZ, Liu W, Luo Y, et al. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem Biophys Res Commun. 2011;414:551–556. doi:10.1016/j.bbrc.2011.09.115
54. Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998;11:355–361.
55. Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F, So KF. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol Macromol. 2014;69:73–78. doi:10.1016/j.ijbiomac.2014.05.034
56. Antunes-Ricardo M, Gutiérrez-Uribe JA, Martínez-Vitela C, Serna-Saldívar SO. Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica. Biomed Res Int. 2015;2015:847320.
57. Khan RA, Mallick N, Feroz Z. Anti-inflammatory effects of Citrus sinensis L., Citrus paradisi L. and their combinations. Pak J Pharm Sci. 2016;29:843–852.
58. Lee JS, Robertson AAB, Cooper MA, Khosrotehrani K. The small molecule NLRP3 inflammasome inhibitor MCC950 does not alter wound healing in obese mice. Int J Mol Sci. 2018;19(11):E3289. doi:10.3390/ijms19113289
59. Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi:10.1038/nm.3806
60. Fenini G, Grossi S, Contassot E, et al. Genome editing of human primary keratinocytes by CRISPR/Cas9 reveals an essential role of the NLRP1 inflammasome in UVB sensing. J Invest Dermatol. 2018;138:2644–2652. doi:10.1016/j.jid.2018.07.016
61. Sanchez MC, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants. 2018;7:98. doi:10.3390/antiox708009
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.