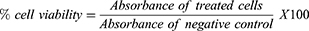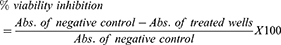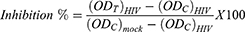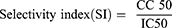Back to Journals » Journal of Experimental Pharmacology » Volume 13
In Vitro Cytotoxicity and Anti-HIV Activity of Crude Extracts of Croton macrostachyus, Croton megalocarpus and Croton dichogamus
Authors Terefe EM , Okalebo FA, Derese S , Muriuki J, Batiha GES
Received 30 August 2021
Accepted for publication 2 November 2021
Published 22 December 2021 Volume 2021:13 Pages 971—979
DOI https://doi.org/10.2147/JEP.S335104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
Ermias Mergia Terefe,1 Faith Apolot Okalebo,2 Solomon Derese,3 Joseph Muriuki,4 Gaber El-Saber Batiha5
1Department of Pharmacology and Pharmacognosy, United States International University-Africa, Nairobi, Kenya; 2Department of Pharmacology and Pharmacognosy, University of Nairobi, Nairobi, Kenya; 3Department of Chemistry, University of Nairobi, Nairobi, Kenya; 4Centre for Virus Research, Kenya Medical Research Institute, Nairobi, Kenya; 5Department of Pharmacology and Therapeutics, Damanhour University, Damanhour, AlBeheira, 22511, Egypt
Correspondence: Ermias Mergia Terefe Tel +254 746272742
Email [email protected]
Introduction: Human immunodeficiency virus (HIV) affects the body’s defense mechanisms and leads to a number of opportunistic infections which later cause fatality as a result of an acquired immunodeficiency syndrome (AIDS). More than half a million individuals have lost their life in 2020 due to this disease. Antiretroviral drugs have played a great role in improving the quality of life of HIV infected individuals. The side effects of these drugs coupled with resistance of the virus to the various regimens, necessitates the search for potentially new and effective antiretroviral medication. The objective of this study is to evaluate anti-HIV activity of crude extracts of three Croton plants.
Methods: As part of our effort in screening anti-HIV medications, we evaluated the cytotoxicity and anti-HIV activity of three Croton species used as herbal medicine in Africa. Crude extracts of Croton macrostachyus, Croton megalocarpus and Croton dichogamus were tested for their replication inhibition activity against laboratory adapted strains HIV-1IIIB in Human T-lymphocytic MT-4 cell line.
Results: Based on our findings, the crude aerial part extract of C. dichogamus displayed the highest anti-HIV activity by inhibiting 73.74% of viral induced cytopathic effect (CPE) at IC50 value of 0.001 + 0.00 μg/mL giving a selectivity index (SI) of 3116.0. In addition, the crude leaf extract of C. megalocarpus showed higher anti-HIV activity by inhibiting 74.65% of CPE at IC50 value of 0.05 + 0.03 μg/mL giving an SI of 571.3.
Conclusion: Out of five extracts from three Croton species screened for anti-HIV activity using human T-lymphocytic MT-4 cells, the leaf extract of Croton megalocarpus and aerial part extract of Croton dichogamus could be considered as promising extracts as they display high antiviral activity with low toxicity and high selectivity index values. To investigate the active constituents responsible for the anti-HIV activity, chemical identification of the active constituents is now in progress in our laboratory. Since there is no previously reported anti-HIV activity for these plants, there is a great need to isolate the compounds responsible for the noted activity.
Keywords: HIV, MT-4 cells, Croton macrostachyus, Croton megalocarpus, Croton dichogamus, cytotoxicity, antiviral activity
Background
Human immunodeficiency virus (HIV) infection causes serious immunosuppression that leads to a decline in the ability of the body to fight infection and progresses to acquired immunodeficiency syndrome (AIDs). Currently 38 million individuals are living with the virus. Among them 21 million (54%) live in southern and east Africa. Still 10 million people do not have access to the antiretroviral treatment.1 Therefore, there is a need to identify alternative, safe and less expensive drugs for treatment of HIV. As herbs are the major source of drugs, the search for new antiretroviral compounds can start from herbal medicines used by traditional healers as remedy for HIV and related opportunistic infections.
More than 80% of the world population relies on use of herbal medicine in the prevention and treatment of diseases2 because of their accessibility, availability, effectiveness and affordability.3 The screening of ethno medicines and other natural products against HIV has been suggested by the World Health Organization.4,5 Phytochemicals isolated from natural products are important sources of lead-compounds for the treatment of HIV/AIDs and other viral diseases. Among the promising compounds isolated from natural products with significant efficacy against HIV include Calanolides (Coumarins), Betulinic acid (triterpene), Baicalin (flavonoid), polycitone A (alkaloid), and lithospermic acid (phenolic compound).4
Recently the Croton genus has gained the attention of many researchers for their potential source of bioactive compounds against HIV. Previous studies have shown that alkaloids isolated from Croton echinocarpus leaves have significant in vitro anti-HIV activity by inhibiting the HIV-1 reverse transcriptase enzyme.6
Croton plants have wider traditional uses including STIs, HIV, cancer, analgesic, laxative, and as cardioprotectives.7–10 In this study we explored the cytotoxicity and anti-HIV activity of crude extracts of C. macrostachyus, C. megalocarpus and C. dichogamus against human immunodeficiency virus type 1 (HIV-1). Since there is no previously reported anti-HIV activity for these plants, the findings of this study will be novel and plays a significant role in the discovery of new pharmaceutically active ingredients from natural origins.
Materials and Methods
Preparation of Crude Extract and Phytochemical Screening
The leaves and stem bark of Croton macrostachyus and Croton megalocarpus were collected from USIU botanical garden and Murema Primary school, Mwiki, Nairobi, Kenya. The aerial parts of Croton dichogamus were collected from Mwala constituency, Machakos County, Kenya in June 2020. The collection of these medicinal plants was done after obtaining the required ethical approval from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UON ERC), approval number P992/12/2019.
Taxonomic identification was done by Ms. Lucy Wambui (botanist) and voucher specimen TEREFE E. /044, TEREFE E. /045 and TEREFE E. /046 was deposited for C. megalocarpus, C. macrostachyus, and C. dichogamus respectively at the United States International University herbarium for future reference.
The powdered plant materials were extracted with dichloromethane and methanol (1:1) using the cold maceration technique. The extract was concentrated using a rota-vapor (Buchi RE) at not more than 40 °C. Then phytochemical screening was done as per standard procedures.11,12
Sample Preparation
The concentrated dried extracts were dissolved in dimethylsulphoxide (DMSO) (not more than 1%) and then diluted with RPMI 1640 medium. Each extract was reconstituted to prepare stock of 4 mg/mL. The stock solutions were filtered through a 0.22 µm membrane filter (Sigma, South Africa) and stored at 4 °C until further use.13
Cytotoxicity Test
In this study, Human T-lymphocytic MT-4 cells (ARP-120) were obtained from the National Institute of Health (NIH) HIV Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH: MT-4 cells, ARP-120, contributed by Dr. Douglas Richman. Human T-lymphocytic MT-4 cells (ARP-120) cells expresses CD4, CXCR4, and CCR5 and are very useful for cytotoxicity inhibition assays for antiviral drugs.
The cytotoxicity test was conducted using MTT colorimetric assay.14,15 The assay was carried out on 96 well, flat bottomed microtitre plates (Cellstar, Greiner, Germany). 200 µL of MT-4 (1 ×105) cells were added to each well. The plates were pre-incubated for 24 h at 37 °C to allow stabilizations. Then fifty microliters (50 µL) of various concentrations (800–8.192×105 μg/mL) of the test compounds and the positive control (zidovudine, tenofovir, abacavir and nevirapine), were added to the wells in triplicate. The negative control wells contained 50 µL of MT-4 cells in 0.5% DMSO.16 The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 5 days then 20 μL of MTT reagent (5 mg/mL MTT in phosphate-buffered saline) was added to each well. After 4 hrs. of incubation, 100 μL of DMSO was added to dissolve the dark-blue formazan crystals from surviving cells.17 The resulting optical density (OD) readings from three independent experiments were measured relative to the controls on ELISA plate reader at 540 nm with a reference wavelength of 620 nm.18 The percentage viability was determined using the formula below:
A dose-response curve was plotted to enable the calculation of the concentrations that reduced the number of viable cells by 50% (CC50). The 50% cellular cytotoxicity concentration (CC50) was defined as the concentration of test compound that reduces the viability (absorbance) of the negative control by 50%. The concentration that determined a cell viability above 80% was chosen as the maximum non-toxic concentration (MNTC).
The maximum cytotoxic effect (EmaxC) determined by percentage inhibition of cell growth was calculated as
Anti-HIV Activity Test
Human immunodeficiency virus type 1 (HIV-1IIIB) (also referred to as HTLV-IIIB) laboratory adapted strain, isolated from peripheral blood of human patients with AIDS was obtained through the NIH HIV Reagent Program, Division of AIDS, NIAID, NIH: Human Immunodeficiency Virus-1 IIIB, ARP-398, contributed by Dr. Robert Gallo.19,20
HIV-1IIIB laboratory adapted viral strain was cultured with Human T-lymphocytic MT-4 cells according to the procedure outlined by Pauwels and his co-workers.18 The endpoint dilution of HIV-1 to determine tissue culture dose for 50% tissue culture infective dose (TCID50) was performed to determine the infectious titer of the virus which can cause cytopathic effects (CPE) in tissue culture.21,22
The effects of the test compounds in preventing a cytopathic effect which occurs as a result of HIV-1 replication was evaluated by MTT colorimetric assay described above. HIV-infected MT-4 cells were seeded in 96-well flat-bottomed microtitre culture plates with 50 µL of different concentrations (800–8.192×105 μg/mL) of the test compounds. The test compounds were dissolved in 0.5% DMSO and assayed at varying concentrations as described above. Each dilution was tested in triplicate. The microtitre plates were incubated at 37 °C in a 5% CO2 incubator for 5 days. Two negative controls, untreated HIV infected cells and uninfected untreated (mock) cells, and the positive control (zidovudine, tenofovir, abacavir and nevirapine) were also included. After 5 days of incubation, cell viability was determined by MTT assay.18,23 From three independent experiments, the antiviral activity was calculated as percent cell protection or inhibition % for viral induced cytopathic effect (CPE inhibition %).15,24–26
Where:
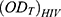 : is the optical density of the test compounds in HIV-infected cells.
: is the optical density of the test compounds in HIV-infected cells.
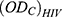 : is the optical density of the negative control-infected untreated HIV-infected cells.
: is the optical density of the negative control-infected untreated HIV-infected cells.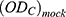 : is the optical density for the control-uninfected untreated (with no virus) cells.
: is the optical density for the control-uninfected untreated (with no virus) cells.
The effective inhibitory concentration at 50% (IC50) values were calculated from the percent cell protection results by non-linear regression analysis. The IC50 is defined as the concentration of the test compound that achieves 50% protection in infected cultures.
The selectivity index (SI) of the test compounds were calculated as the ratio of 50% cytotoxic concentration (CC 50) to 50% effective concentration (IC50).
Statistical Analysis
The CC50 and IC50 values were calculated with GraphPad Prism v9, using the equation for sigmoidal dose-response (variable slope). Statistical significances in comparison between control drugs and extracts cytotoxicity and antiviral activity parameters (CC50, EmaxC, IC50 and EmaxAV) were determined by one-way ANOVA followed by Dunnett’s post-hoc tests, a difference was considered significant when p < 0.05.
Results
Yield of Plant Extraction
Table 1 depicts the percentage yield obtained from the leaves and stem bark of Croton macrostachyus and Croton megalocarpus, and the aerial parts of Croton dichogamus.
 |
Table 1 Summary of Plants Collected and the Weight of Extracts |
Phytochemical Screening
Phytochemical screening of the crude extracts of Croton macrostachyus, Croton megalocarpus, and Croton dichogamus had revealed the presence of different secondary metabolites (Table 2).
 |
Table 2 Phytochemical Analysis for Crude Extracts of Croton macrostachyus and Croton megalocarpus, and Croton dichogamus |
Cytotoxicity Assay
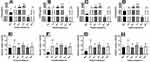
The cytotoxicity and anti-HIV-1 activity of the tested extracts and control using MT4 cell line are summarized in Table 3. Among the tested extracts, the crude bark extract of Croton macrostachyus (AC) has the highest CC50 value of 45.9 ± 0.12 µg/mL followed by crude leaf extract of Croton megalocarpus (ELC) with a CC50 value of 27.7 ± 0.65 µg/mL. Among the tested extracts, highest value for maximum non-toxic concentration (MNTC) was achieved by the ELC extract at 20.56 ± 0.00 µg/mL indicating its relative safety on the cell lines. The efficacy (Emaxc) of the crude leaf extracts of Croton macrostachyus (ALC) and ELC on cell viability showed that they have less effect (Emaxc < 40%) in suppressing cell viability as compared to the other extracts. AC was found to have higher inhibition of cell growth (Emaxc = 49.96%) as compared to the other extracts.
 |
Table 3 Cytotoxicity and Anti-HIV-1 Activities of Control Drugs and Extracts Using MT4 Cell Line |
Among the FDA approved antiretreoviral drugs, zidovudine (AZT) and nevirapine (NVP) showed higher cytotoxicity (EmaxC > 35%) with CC50 value of 0.53 ± 0.29 and 0.82 ± 0.0 µg/mL respectively as compared with tenofovir (TDF) and abacavir (ABC).
Based on our findings, all the tested extracts showed maximum cytotoxic effect (EmaxC) that is non-significantly higher than that of the control drugs; on the other hand, the CC50 was not significantly different between the extracts and control drugs, except for extract AC which showed significantly higher (P < 0.01) CC50 as compared with the four control drugs and ELC which showed significantly higher (P < 0.05) CC50 values as compared to AZT, ABC, and NVP (Figure 1). From the results, the extracts with the closest cytotoxic effect to the control drugs were extracts ELC and ALC (Emaxc < 40%), the other three extracts EC, AC and CDC showed higher toxicity (Emaxc > 42%).
Anti-HIV Assay
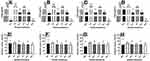
Based on our antiviral assay results, higher anti-HIV activity was observed by crude leaf extracts of C. megalocarpus (ELC) and crude aerial part extracts of C. dichogamus (CDC) extracts. CDC displayed the highest anti-HIV activity by inhibiting 73.74% of viral induced cytopathic effect at an IC50 value of 0.001 + 0.00 μg/mL giving a selectivity index of 3116.0. Similarly, ELC displayed a higher anti-HIV activity by inhibiting 74.65% of CPE at IC50 value of 0.05 + 0.03 μg/mL giving a selectivity index (SI) of 571.3. The crude bark extract of Croton megalocarpus (EC) showed lower potency with IC50 value of 3.73 + 1.20 µg/mL, while the crude leaf extract of Croton macrostachyus (ALC) displayed very narrow selectivity index (Table 3).
As displayed in Figure 2, the crude bark extract of C. megalocarpus (EC) has a significantly higher (P < 0.01) IC50 value as compared to the positive controls. However, comparing the antiviral efficacy (EmaxAV), all the tested extracts showed similar efficacy to the control drugs as they showed non-significantly different maximum inhibitions of viral induced CPE when compared to the control drugs.
It was found that the percentage of growth inhibition increased with increasing concentration of test compounds. Figure 3 depicts the concentration – response curve for the cell viability % and the inhibition % of the viral induced cytopathic effect associated with the tested substances. Despite its relatively higher cytotoxicity (42% inhibition of cell viability), the crude extract of Croton dichogamus has displayed strong activity with IC50 of 0.001 μg/mL giving an SI of 3115.97. Among the FDA approved standard drugs, zidovudine displayed the highest antiviral activity with 83% CPE inhibition at IC50 value of 0.001 µg/mL giving an SI of 279.4.
Selectivity index reflects both antiviral activity and eventual toxicity of the test compounds. The high SI value indicates the low toxicity of the test compound and high activity against the virus. A dose-response curve was plotted to enable the calculation of the concentrations that reduced the viral replication by 50% (IC50).15,16,27
Discussion
Currently available anti-HIV drugs are chemically synthesized and are often limited by side effects and emergence of drug resistance.28 On top of this, still over 5 million people do not have access to the treatments.1 Therefore, all possible approaches towards the development of new anti-HIV drugs should be pursued. This calls for a need to identify local, alternative, less expensive and less toxic drugs for treatment of HIV. A potential source of thiese demands is of natural products.29
Natural products are the major source of new active pharmaceutical ingredients for treatment of infectious diseases including HIV/AIDs.30–32 In order to find potential anti-HIV agents from natural sources, we evaluated the cytotoxicity and anti-HIV activities of three Croton species including Croton macrostachyus, Croton megalocarpus and Croton dichogamus against laboratory adapted strains of HIV (HIV-1IIIB) in Human T-lymphocytic MT-4 cells.
In this study, human T-lymphocytic MT-4 cells were used. Lymphocyte cell line, MT-4, which carries the HTLV-I genome is highly susceptible to HIV infection.33 The MT-4 cells were inoculated with HIV showed a markedly different cell growth and viability pattern when compared with mock infected cells. The number of viable cells rapidly decreased at 24 hours post infection, and by day 4 only 2–4% of the infected MT-4 cells were viable. In contrast, the mock-infected cells grew well, reaching a plateau between days 2 and 4. After 4 days, the viability of these cells began to decline appreciably. The infected cells became round, lost their surface characteristics, became refractile and diminished in size. By day 3, many infected cells developed a balloon-like, cytoplasmic swelling, a morphological observation which later disappeared. The dose of virus influenced the number of viable cells and the time course of appearance of these cytopathic effects. These observations were in agreement with previous reports of many scholars who used the MT-4 cell line to evaluate anti-HIV activity of various compounds.16,18,34,35
The effects of the test compounds in preventing cytopathic effect which occurs as a result of HIV-1 replication was evaluated by MTT colorimetric assay. Human immunodeficiency virus type 1 (HIV-1IIIB) laboratory adapted strain, was obtained from the NIH HIV Reagent Program. HIV-infected MT-4 cells were seeded in 96-well flat-bottomed microtitre culture plates with 50 µL of different concentrations (800–8.192×105 μg/mL) of the test compounds.
To ensure the safety of the extracts on human T-Lymphocytes, a cytotoxicity test was conducted using MT-4 cell lines. Based on our results, Croton megalocarpus leaf extracts (ELC) and Croton macrostachyus leaf extracts (ALC) were relatively less toxic as compared to the other extracts and are with the closest cytotoxic effect to the control drugs, while the other three extracts EC, AC and CDC showed higher toxicity.
The phytochemical analysis in the current study confirmed the presence of flavonoids, saponins, phenolic compounds and terpenes in these crude extracts (Table 2). Previous phytochemical analysis studies from these plants revealed the presence of phytosterols,36 terpenes,37 triterpenoids,37–40 diterpenoids41–43 and phenolic compounds36,38 and fatty acids.44 The difference in the cytotoxicity in these extracts could be attributed to the phytochemical ratios of tannins, alkaloids, flavonoids, phenols, and terpenoids in them.45
The higher potency of Croton megalocarpus (ELC) extract (CC50= 27.73 µg/mL, IC50= 0.05 µg/mL and SI = 571.3) to inhibit the cytopathic effect by the virus could be due to the saponins in the leaf of the plant. Previous studies have shown the efficacy of saponins isolated from soybean seeds in inhibiting HIV-1 replication in MT-4 cells.46 In another study acetin, a tetracyclic saponin isolated from the rhizome of Cimicifuga racemosa (black cohosh), showed potent anti-HIV activity.47
The observed efficacy could also be attributed to the diterpenes in the plant. Previous studies have shown the presence of terpenes, diterpenoids and triterpenoids in the stem bark and roots of Croton megalocarpus and Croton macrostachyus.41,42,48 Moderate anti-HIV activity was reported from cyanthiwigin B, a diterpene isolated from the Jamaican sponge Myrmekioderma styx in cell based in vitro assay. Similarly other diterpenes like betulinic acid, platanic acid and oleanolic acid isolated from the leaves of Syzygium claviflorum have exhibited anti-HIV activity.49
Furthermore, the efficacy of the plants in this study could also be due to the flavonoids, as some flavonoids have been shown to have HIV inhibitory potential and also in reducing oxidative stress.50 Polyphenols and flavonoids are known to stabilize membranes and to prevent lipid peroxidation, a key process in the onset, progression and complication of many pathologies.
This study is important because it served as a starting point in the discovery of new cytotoxic agents and the unveiling of the potent extracts from three Croton species with tremendous traditional use in Africa.
Conclusion
To conclude, out of five extracts from three Croton species screened for anti-HIV activity using Human T-lymphocytic MT-4 cells, the leaf extract of Croton megalocarpus and aerial part extract of Croton dichogamus could be considered as promising extracts as they display high antiviral activity with low toxicity and high SI values. Further studies to determine the mechanism of action of these plants as anti-HIV agents are needed. Moreover, research is needed to isolate and identify the active phytoconstituents responsible for the cytotoxic and anti-HIV efficacy in these plants. Therefore, currently we are working on isolating pure compounds from the different solvent fractions so as to determine the mechanism of action of isolated compounds on different viral protein.
Ethical Approval
The research was approved by Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UON ERC), approval number P992/12/2019.
Acknowledgments
The authors would like to extend their gratitude to the United States International University - Africa Internal grant no. 10-2854, and University of Nairobi, Kenya Medical Research institute and the Institute of Primate Research for their support toward the successful completion of the research work.
Disclosure
The authors declare that they do not have any conflicts of interest for this work.
References
1. UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet | UNAIDS; 2020. Available from: https://www.unaids.org/en/resources/fact-sheet.
2. Hedberg I, Hedberg O, Madat PJ, Mshigeni KE, Mshiu EN, Samuelsson G. Inventory of plants used in traditional medicine in Tanzania. II. Plants of the families dilleniaceae-Opiliaceae. J Ethnopharmacol. 1983;9(1):105–127. doi:10.1016/0378-8741(83)90030-2
3. Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Altern Med. 2005;2(4):465–473. doi:10.1093/ecam/neh140
4. Kurapati KRV, Atluri VS, Samikkannu T, Garcia G, Nair MPN. Natural products as Anti-HIV agents and role in HIV-associated neurocognitive disorders (HAND): a brief overview. Front Microbiol. 2016;6(JAN):1–14. doi:10.3389/fmicb.2015.01444
5. Matsuse IT, Lim YA, Hattori M, Correa M, Gupta MP. A search for anti-viral properties in Panamanian medicinal plants. The effects on HIV and its essential enzymes. J Ethnopharmacol. 1998;64(1):15–22. doi:10.1016/S0378-8741(98)00099-3
6. Ravanelli N, Santos KP, Motta LB, Lago JHG, Furlan CM. Alkaloids from Croton echinocarpus Baill.: anti-HIV potential. South African J Bot. 2016;102:153–156. doi:10.1016/j.sajb.2015.06.011
7. Campos AR, Albuquerque FAA, Rao VSN, Maciel MAM, Pinto AC. Investigations on the antinociceptive activity of crude extracts from Croton cajucara leaves in mice. Fitoterapia. 2002;73(2):116–120. doi:10.1016/S0367-326X(02)00004-7
8. Murillo RM, Jakupovic J, Rivera J, Castro VH. Diterpenes and other constituents from Croton draco (Euphorbiaceae). Rev Biol Trop. 2001;49(1):259–264. doi:10.1016/0031-9422(92)80479-X
9. Rodrigues GR, Di Naso FC, Porawski M, et al. Treatment with aqueous extract from croton cajucara benth reduces hepatic oxidative stress in streptozotocin-diabetic rats. J Biomed Biotechnol. 2012;2012:1–7. doi:10.1155/2012/902351
10. Vigor C, Fabre N, Fourasté I, Moulis C. Three clerodane diterpenoids from Croton eluteria Bennett. Phytochemistry. 2001;57(8):1209–1212. doi:10.1016/S0031-9422(01)00183-2
11. Evans WC. Alkaloids. Chem Biol. 2009:1–77.
12. Tyler VE The recent history of pharmacognosy.
13. Rege AA, Ambaye RY, Deshmukh RA. In-vitro testing of anti-HIV activity of some medicinal plants. Indian J Nat Prod Resour. 2010;1(2):193–199.
14. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
15. Pauwels R, Balzarini J, Baba M, et al. Rapid and automated tetrazolium-based calorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi:10.1016/0166-0934(88)90134-6
16. Weislow OS, Kiser R, Fine DL, Bader J, Shoemaker RH, Boyd MR. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81(8):577–586. doi:10.1093/jnci/81.8.577
17. Bahuguna A, Khan I, Bajpai VK, Kang SC. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J Pharmacol. 2017;12(2):115–118. doi:10.3329/bjp.v12i2.30892
18. Szucs G, Melnick JL, Hollinger FB. A simple assay based on HIV infection preventing the reclustering of MT-4 cells. Bull World Health Organ. 1988;66(6):729–737.
19. Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224(4648):497–500. doi:10.1126/science.6200935
20. Ratner L, Haseltine W, Patarca R, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313(6000):277–284. doi:10.1038/313277a0
21. Gao Y, Nankya I, Abraha A, et al. Calculating HIV-1 infectious titre using a virtual TCID50 method. Methods Mol Biol. 2009;485:27–35. doi:10.1007/978-1-59745-170-3_3
22. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;28(2):244–270. doi:10.1016/j.jvs.2011.05.096
23. Rashid M, Gustafson K, Kashmani Y, Cardellina J, Mcmahon J, Boyd M. Anti-Hiv alkaloids from toddalia asiatica^. Nat Prod Lett. 1995;6(2):153–156. doi:10.1080/10575639508044104
24. Kiani P, Scholey A, Dahl TA, McMann L, Iversen JM, Verster JC. In Vitro Assessment of the Antiviral Activity of Ketotifen, Indomethacin and Naproxen, Alone and in Combination, against SARS-CoV-2. Viruses. 2021;13(4):558. doi:10.3390/V13040558
25. Betancur-Galvis LA, Morales GE, Forero JE, Roldan J. Cytotocxic and antiviral activties of Colombian medicinal plant exatracts of the Euphorbia genus. Mem Inst Oswaldo Cruz. 2002;97(4):541–546. doi:10.1590/S0074-02762002000400017
26. Latronico T, Pati I, Ciavarella R, et al. In vitro effect of antiretroviral drugs on cultured primary astrocytes: analysis of neurotoxicity and matrix metalloproteinase inhibition. J Neurochem. 2018;144(3):271–284. doi:10.1111/jnc.14269
27. Gustafson KR, McKee TC, Bokesch HR. Anti-HIV cyclotides. Curr Protein Pept Sci. 2004;5(5):331–340. doi:10.2174/1389203043379468
28. De Clercq E. New developments in anti-HIV chemotherapy. Biochim Biophys Acta Mol Basis Dis. 2002;1587(2–3):258–275. doi:10.1016/S0925-4439(02)00089-3
29. Bedoya LM, Sanchez-Palomino S, Abad MJ, Bermejo P, Alcami J. Anti-HIV activity of medicinal plant extracts. J Ethnopharmacol. 2001;77(1):113–116. doi:10.1016/S0378-8741(01)00265-3
30. Jung M, Lee S, Kim H, Kim H. Recent Studies on Natural Products as Anti-HIV Agents. Curr Med Chem. 2012;19(11):1731–1737. doi:10.2174/0929867003374822
31. Matthée G, Wright AD, König GM. HIV reverse transcriptase inhibitors of natural origin. Planta Med. 1999;65(6):493–506. doi:10.1055/s-1999-14004
32. Yang SS, Cragg GM, Newman DJ, Bader JP. Natural product-based anti-HIV drug discovery and development facilitated by the NCI Developmental Therapeutics Program. J Nat Prod. 2001;64(2):265–277. doi:10.1021/np0003995
33. Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229(4713):563–566. doi:10.1126/science.2992081
34. Asres K, Bucar F. Anti-HIV activity against immunodeficiency virus type 1 (HIV-I) and type II (HIV-II) of compounds isolated from the stem bark of Combretum molle. Ethiop Med J. 2005;43(1):15–20.
35. Harada S, Yamamoto N. Quantitative Analysis of Aids-Related Virus-Carrying Cells by Plaque-Forming Assay Using an Htlv-I-Positive Mt-4 Cell Line. Japanese J Cancer Res GANN. 1985. doi:10.1016/j.joms.2006.09.031
36. Tala MF, Tan NH, Ndontsa BL, Tane P. Triterpenoids and phenolic compounds from Croton macrostachyus. Biochem Syst Ecol. 2013;51:138–141. doi:10.1016/j.bse.2013.08.001
37. Aldhaher A, Langat M, Ndunda B, et al. Diterpenoids from the roots of Croton dichogamus Pax. Phytochemistry. 2017;144:1–8. doi:10.1016/j.phytochem.2017.08.014
38. Addae-Mensah M, Karanja W, Waibel A. Constituents of the stem bark and twigs of Croton macrostachyus. J Fitoterapia. 1992;81.
39. Langat MK, Crouch NR, Pohjala L, Tammela P, Smith PJ, Mulholland DA. Ent-kauren-19-oic acid derivatives from the stem bark of Croton pseudopulchellus Pax. Phytochem Lett. 2012;5(3):414–418. doi:10.1016/j.phytol.2012.03.002
40. Maroyi A. Croton megalocarpus Hutch. in Tropical Africa: phytochemistry, Pharmacology and Medicinal Potential. Res J Med Plants. 2017;11(4):124–133. doi:10.3923/RJMP.2017.124.133
41. Kapingu MC, Guillaume D, Mbwambo ZH, Moshi MJ, Uliso FC, Mahunnah RLA. Diterpenoids from the roots of Croton macrostachys. Phytochemistry. 2000;54(8):767–770. doi:10.1016/S0031-9422(00)00166-7
42. Alqahtani A. Phytochemical investigation of members of the Asparagaceae and Euphorbiaceae families. Univ Surey, PhD Disertation. 2015;1(Volume 1):458.
43. Liu CP, Xu JB, Zhao JX, et al. Diterpenoids from croton laui and their cytotoxic and antimicrobial activities. J Nat Prod. 2014;77(4):1013–1020. doi:10.1021/np500042c
44. Kafuku G, Mbarawa M. Biodiesel production from Croton megalocarpus oil and its process optimization. Fuel. 2010;89(9):2556–2560. doi:10.1016/j.fuel.2010.03.039
45. Nguta JM, Mbaria JM, Gakuya DW, Gathumbi PK, Kabasa JD, Kiama SG. Cytotoxicity of antimalarial plant extracts from Kenyan biodiversity to the brine shrimp, Artemia salina L. (Artemiidae). Drugs and Therapy Studies. 2012;27:e12. doi:10.4081/dts.2012.e12
46. Singh IP, Bharate SB, Bhutani KK. Anti-HIV natural products. Curr Sci. 2005;82(2):269–290.
47. Sakurai N, Wu JH, Sashida Y, et al. Anti-AIDS Agents. Part 57: actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorganic Med Chem Lett. 2004;14(5):1329–1332. doi:10.1016/j.bmcl.2003.12.035
48. Zhang ZX, Wu PQ, Li HH, et al. Norcrassin A, a novel C16 tetranorditerpenoid, and bicrotonol A, an unusual dimeric labdane-type diterpenoid, from the roots of: croton crassifolius. Org Biomol Chem. 2018. doi:10.1039/c7ob02991h
49. Fujioka T, Kashiwada Y, Kilkuskie RE, et al. Anti-aids agents, 11. betulinic acid and platanic acid as anti-HIV principles from Syzygium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57(2):243–247. doi:10.1021/np50104a008
50. Cos P, Maes L, Vanden Berghe D, Hermans N, Pieters L, Vlietinck A. Plant Substances as Anti-HIV Agents Selected According to Their Putative Mechanism of Action. J Nat Prod. 2004;67(2):284–293. doi:10.1021/np034016p
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.