Back to Journals » International Journal of Nanomedicine » Volume 17
In situ Co-Delivery of Doxorubicin and Cisplatin by Injectable Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment
Authors Si M, Xia Y, Cong M, Wang D, Hou Y, Ma H
Received 30 December 2021
Accepted for publication 10 March 2022
Published 22 March 2022 Volume 2022:17 Pages 1309—1322
DOI https://doi.org/10.2147/IJN.S356453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Meng Si,1,* Yanni Xia,1,* Menglin Cong,1,* Dandan Wang,2 Yong Hou,1 Hecheng Ma1
1Department of Orthopedic Surgery, Qilu Hospital of Shandong University, Jinan, 250012, People’s Republic of China; 2Jinan Center hospital affiliated to Shandong University, Shandong University, Jinan, 250012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hecheng Ma, Department of Orthopedic Surgery, Qilu Hospital of Shandong University, Jinan, 250012, People’s Republic of China, Tel +86-531-82166551, Email [email protected]
Purpose: Osteosarcoma is considered as the most common primary malignant bone tumor in children and adolescents, and the treatments including chemotherapy and surgery were far from satisfactory. Localized tumor treatments by hydrogels incorporating combined chemotherapeutic drugs have recently emerged as superior approaches for enhanced anti-tumor effects and reduced systemic toxicity.
Methods: A novel injectable thermosensitive poly (lactide-co- glycolide)-poly (ethylene glycol)-poly(lactide-co-glycolide) triblock copolymer hydrogel containing doxorubicin and cisplatin for the localized chemotherapy of osteosarcoma were synthesized and characterized. The in vitro drug release properties of the drugs-loaded hydrogels were investigated. To study the anti-tumor efficacy of hydrogels depots in vitro, the cytotoxicity and apoptosis rate against Saos-2 and MG-63 cells were evaluated by MTT, Annexin V and PCR methods. The in vivo synergistic anti-tumor efficacy of the multi-drugs co-loaded hydrogels was investigated by human osteosarcoma xenografts. Additionally, the systemic toxic side effects were evaluated by ex vivo histological analysis of the major organs of the mice.
Results: The PLGA-PEG-PLGA copolymer solution underwent a sol-gel transition at appropriate temperature and degraded in the PBS, presenting a friendly biocompatibility in vitro. The in vitro cell viability tests demonstrated that DOX and CDDP co-loaded hydrogels exhibited synergistic anti-proliferation effect, due to the sustained release of drugs from the drugs-loaded hydrogel. The treatment with DOX and CDDP co-loaded hydrogel led to the highest efficiency in inhibiting the tumor growth, enhanced tumor necrosis rate and increased regulation of the apoptosis-related gene expressions, indicating a synergistic anti-tumor efficacy in vivo. Additionally, ex vivo histological analysis of the nude mice exhibited low systemic toxicity.
Conclusion: The combination treatment of osteosarcoma by localized, sustained co-delivery of DOX and CDDP by PLGA-PEG-PLGA hydrogel may serve as a promising strategy for efficient clinical treatment of osteosarcoma.
Keywords: hydrogels, localized delivery, synergistic therapy, combination therapy, osteosarcoma
Introduction
Osteosarcoma is considered as the most common primary malignant bone tumor in children and adolescents, and shows a peak incidence in the second and third decades, representing the second highest cancer-related death in this age group.1 The annual incidence rate is 10–26/1,000,000 per year in the Europe, and approximately 400 new cases of osteosarcoma are diagnosed in the United States each year.2 Osteosarcoma pathologically origins from mesenchymal cells, which was characterized by spindle cells and aberrant osteoid formation.3 Currently, the first-line standard treatments of osteosarcoma are consist of surgery and combination chemotherapy regimens (neoadjuvant and adjuvant chemotherapy).4 As a result, the long-term survival has dramatically increased to 60–70%, and limb-salvage come to a reality.5
In modern chemotherapy schedules, according to protocols by the Cooperative Osteosarcoma Study Group (COSS),6 EURAMOS,7 the European Osteosarcoma Intergroup’s (EOI),8 osteosarcoma patients are suggested to take a combination of chemotherapeutics drugs, based on doxorubicin (DOX) and cisplatin (CDDP), both before and after surgery.9,10 Doxorubicin is one of the most powerful and effective anti-neoplastic drugs, which presents excellent therapeutic efficacy against osteosarcoma.11 DOX inhibits cellular DNA, RNA and protein synthesis and deregulate the DNA damage responses, epigenome and transcriptome due to binding and intercalating to nucleic acids to inhibit the DNA polymerases.12,13 CDDP is sensitive to osteosarcoma, by interacting with cell DNA in several ways to regulate mitotic progression. In return, the damaged DNA repair mechanisms leads to the apoptosis of osteosarcoma.14
However, due to the systemic distribution and dose-related toxicity of chemotherapeutic drugs, chemotherapy schedules causes serious adverse effects, such as cardiotoxicity,15 nephrotoxicity, ulcerative stomatitis, myelosuppression.16,17 Over 30% of osteosarcoma patients were either resistant to current chemotherapy regimens or suffered from serious life-threatening complications, succumbing to grave consequences eventually.18
Nowadays, localized chemotherapy has attracted increasing attention. The multi-agent chemotherapeutics were assembled in a localized drugs delivery vehicle and controlled released at target pathological sites, with relatively high drug concentration and less non-specific distribution of drugs in normal organs, which eventually can reduce adverse effects of systemic chemotherapy.19,20 As a localized drug delivery system, injectable hydrogels have attracted increasing interest due to the superior advantages including mild gelation process, target injection, appropriate biodegradability, less systemic toxicity, as well as sustained drugs release profiles.21–23
The study by our group have investigated the local chemotherapy by DOX and CDDP loaded injectable hydrogels, demonstrated an improved anticancer efficacy for osteosarcoma treatments.24 However, a fly in the ointment was the release of CDDP from the hydrogel, where a fast CDDP release profile was noted. Over 70% of CDDP was released from the hydrogels in 2 days. In order to achieve sustained CDDP release from the hydrogel, we redesigned the construction of the PLGA-PEG-PLGA copolymer. Nevertheless, to-date, the investigation on localized co-delivery of DOX and CDDP by injectable thermosensitive hydrogels for osteosarcoma treatment are still limited.
In this study, we designed, synthesized and characterized a novel injectable thermosensitive poly (lactide-co- glycolide)-poly (ethylene glycol)-poly(lactide-co-glycolide) triblock copolymer (PLGA-PEG-PLGA) hydrogel containing DOX and CDDP for the localized chemotherapy of osteosarcoma (Scheme 1). The in vitro drug release properties of the drugs-loaded hydrogels were investigated. To study the anti-tumor efficacy of DOX and CDDP co-loaded hydrogels in vitro, the cytotoxicity and apoptosis rate were studied against Saos-2 and MG-63 cells. Furthermore, the in vitro synergistic anti-tumor efficacy of the multi-drug co-loaded hydrogels was investigated by peritumoral injection of the drug-containing hydrogels into nude mice bearing human osteosarcoma Saos-2 xenografts. Additionally, the systemic toxic side effects of the localized treatments were evaluated by ex vivo histological analysis of the major organs of the mice.
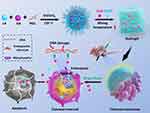 |
Scheme 1 (A) Schematic illustration for in situ co-delivery of doxorubicin and cisplatin by injectable thermosensitive PLGA-PEG-PLGA hydrogels for synergistic enhanced osteosarcoma treatment. |
Materials
Poly (ethylene glycol) (PEG, Mn = 1500), stannous 2-ethylhexanoate (Sn(Oct)2, 95%) and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (USA). Trizol was purchased from Invitrogen (USA). The PCR kit was purchased from Takara (Japan). Lactide (LA) and glycolide (GA) were purchased from Purac (the Netherlands). Doxorubicin hydrochloride (DOX·HCl) was bought from Zhejiang Hisun Pharmaceutical Co., Ltd (China). CDDP were purchased from Sigma-Aldrich (USA). The osteosarcoma cell lines Saos-2 and Mg-63 was purchased from ATCC.
Synthesis and Characterization of PLGA-PEG-PLGA Triblock Copolymer
The PLGA-PEG-PLGA triblock copolymer was synthesized by the ring-opening copolymerization of LA and GA using PEG (Mn = 1500) as the macro-initiator and Sn(Oct)2 as catalyst. Briefly, PEG, LA and GA were dissolved in anhydrous toluene in the presence of Sn(Oct)2 for 24h at 130°C in nitrogen protection environment. The weight ratio of (LA+GA)/PEG was 3:1, and the LA/GA molar ratio was 3:1. The crude copolymer was precipitated into diethyl ether, and collected by filtration. Then the copolymer was purified by dialysis and then collected.
A 300 MHz Bruker spectrometer (DMX300) with CDCl3 as solvent was used to detect the 1H nuclear magnetic resonance (NMR) of the copolymer to confirm the chemical structure and composition. Gel permeation chromatography (GPC, Waters), with tetrahydrofuran as eluent at a flow-rate of 1.0 mL/min at 35°C, was used to monitor the molecular weights (MW) and the polydispersities (PDI) of the copolymer.
Based on the 1H NMR result, the molecular weight of the triblock copolymer is 6200 with the LA/GA molar ratio of 2.7:1, which are coincident with the theoretical values.
Sol-Gel Phase Transition of Drugs Co-Loaded Hydrogel
The sol-gel phase transition behaviors of the drugs co-loaded PLGA-PEG-PLGA aqueous solutions were evaluated by a vial inverting approach with a temperature increment of 1°C per step. The samples include copolymer, DOX load copolymer, CDDP load copolymer and DOX, CDDP co-loaded copolymer at different polymer concentrations were tested. The sol-gel transition temperatures were recorded until the formulation did not flow within 30 sec after inverting the vials.
In vitro Gel Degradation
The PLGA-PEG-PLGA hydrogels were formed and incubated at 37°C in the vials with an inner diameter of 16 mm. Then 3 mL of phosphate buffer saline (PBS, pH 7.4) was added in the vials with continuous shaking at 50 rpm and 37°C. At predetermined time intervals, the remaining gels were accurately weighted after the PBS was removed.
In vitro Drug Release
The drug formulations were prepared by mixing DOX, CDDP in 20 wt% PLGA-PEG-PLGA copolymer solution in an ice-water bath. The drug concentration was 1 mg/mL. Then, the drug-loaded copolymer aqueous solutions were incubated at 37°C to form the hydrogel. At predetermined time intervals, the release behaviors of DOX and CDDP from the hydrogels were investigated and quantify. The amount of DOX was tested by fluorescence measurement (λex = 480 nm), while the amount of CDDP was evaluated by ICP-MS (Thermo XSERIES 2).
In vitro Biocompatibility
The in vitro biocompatibility of the PLGA-PEG-PLGA triblock copolymer against both a normal cell line (L929) and osteosarcoma cell lines (Saos-2 and MG-63) were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, the cells were seeded and incubated in 96-well plates at a density of 10,000 cells per well at 37°C for 24 h. After the culture medium was removed, the copolymer solutions were added to the wells at different concentrations. After incubation for additional 24 h, the cells were subjected to MTT assay. The absorbency of the solution was tested by a Bio-rad 680 microplate reader at 492 nm. Cell viability (%) was estimated by the equation:
Cell viability (%) = (Asample/ Asample) × 100% (1)
Asample and Asample are the absorbencies of the sample and control wells, respectively.
In vitro Cytotoxicities Efficacy
The in vitro cytotoxicities efficacy of the hydrogels loaded with single drug or multi-drugs against osteosarcoma cells (Saos-2 and MG-63) were calculated by MTT assay. The osteosarcoma cells were incubated with the drug-loaded hydrogels for 24h, 48h respectively. The concentrations of each drug (DOX and CDDP) loaded in the hydrogels ranged from 0.375–1.5 mg/mL. Finally, the cells were subjected to MTT assay and the cell viability was calculated according to equation (1).
The synergistic, additive or antagonistic effects of the multiple drugs for a certain inhibitory concentration on the cytotoxicity against the osteosarcoma cells were analyzed by calculating the combination index (CI) according to the Berenbaum method.24,25
Endocytosis of DOX
The Saos-2 cells and MG-63 cells were seeded in six-well plates at 1.0×105 cells per well in 2.0 mL of complete DMEM and cultured for 24h. Then cell were incubated at 37 °C with DOX, Gel+DOX, DOX+CDDP and Gel+DOX+CDDP at a final DOX and CDDP concentration of 5.0 mg /mL for additional 3 h respectively. Then, the culture medium was removed and cells were washed three times with PBS. Then the cells were fixed with 4% formaldehyde for 20 min at room temperature, followed by the cell nuclei stained with 4′, 6-diamidino-2-phenylindole (DAPI, blue) for 10 min and the cell membranes stained with Alexa Fluor 488 (green) for 30 min. The cells were observed by a Carl Zeiss LSM 780 confocal microscope.
In vitro Detection of Apoptosis in Osteosarcoma Cells
The apoptosis of osteosarcoma cells in vitro after the treatments of PLGA-PEG-PLGA hydrogels containing DOX and CDDP was assessed by flow cytometry. The collected osteosarcoma cells after each treatment were stained with Annexin V-APC for 15 min at room temperature. Then 7-AAD were added and incubated for another 15 min before being transferred to flow cytometry for analysis within 30 min.
The expression of apoptosis-related genes was evaluated by quantitative real-time PCR tests. The osteosarcoma cells were collected after treatments, and the total RNA was extracted according to the protocol by using Trizol reagent (Invitrogen). Then total RNA was converted to a complimentary DNA (cDNA) by reverse transcription kit (Takara, Japan). Quantitative real-time PCR was performed on a Mxpro3005 systems (Stratagene, USA) and SYBR Premix Ex TaqTM kit (Takara, Japan). Specific primers of apoptosis-related genes (Bcl-2, BAX, caspase-3) were designed (Table 1). Gene expression values were calculated using the ΔCt method, relative to housekeeping gene β-actin. Then the result subsequently was normalized by the respective genes of control group, and obtained gene expression fold values.
 |
Table 1 Characterization of the PLGA-PEG-PLGA Triblock Copolymers |
In vivo Antitumor Tests
The mice procedure was performed according to the protocols by Animal Care and Use, approved by the Animal Ethics Committee of Shandong University. About 5.0×106 Saos-2 cells suspension were inoculated subcutaneously into the armpit of right anterior limb of 5-week-old male BALB/c nu/nu nude mice (19–20 g). After the tumors reached to ~ 50 mm3, the mice were weighed and divided randomly into 5 groups (6 mice per group). Then, the mice were treated by single injection of 0.1 mL of drug solutions alone or drug-loaded hydrogels besides the tumors. The drug formulations include PLGA-PEG-PLGA hydrogel only (Gel), free DOX solution in PBS (DOX), mixed solution of DOX and CDDP (DOX+CDDP), DOX-loaded hydrogel (Gel+DOX), DOX and CDDP co-loaded hydrogel (Gel+DOX+CDDP). The concentrations of DOX and CDDP were 5.0 and 3.0 mg per kg mice weight, respectively.
The tumor volume and body weight of mice were measured every 2 days for up to 14 days, to estimate the anti-tumor efficacy and safety evaluation. The tumor volume was calculated according to equation (2):
V = L× W2/2 (2)
where L and W (mm) were the largest and smallest diameters of tumor mass, respectively. The mice were sacrificed at the end of the experiment. Moreover, the tumors and organs were collected for further analysis.
Ex vivo Analysis of Tumor Mass Apoptosis
The apoptosis of the tumors after drugs formulations were evaluated by TUNEL assay and real-time PCR, respectively.
The apoptosis-related genes in the tumors were assessed by real-time PCR. After the isolated tumor masses were frozen and grinded in liquid nitrogen, total RNA of 100 mg tumor mass was extracted. The real-time PCR was performed according to the protocol.
Furthermore, terminal nucleotidyl transferase-mediated nick end labeling (TUNEL, Roche, Switzerland) assay was used to evaluated the apoptosis of tumor tissues. The nicked DNA ends of apoptotic tumor sections were labeled and observed by fluorescence microscopy (Carl Zeiss).
Systemic Safety Evaluation by Histology Analysis
At the end of the animal test, the major organs of the sacrificed mice, including hearts, lungs, livers, spleens and kidneys were collected and sectioned and stained for histology analysis.
Statistical Analysis
The statistical difference was analyzed by the paired Student’s t-test. Statistically significance was reported if p value was less than 0.05. The data were showed as mean ± standard deviation.
Results and Discussion
Synthesis and Characterization of PLGA-PEG-PLGA Triblock Copolymer
According to our previous report,24,26 the PLGA-PEG-PLGA triblock copolymer was synthesized via ring-opening polymerization of L-lactide (L-LA) and glycolide (GA) with PEG and Sn(Oct)2 as the macroinitiator and catalyst, respectively (Figure 1A). In theoretical design of PLGA-PEG-PLGA copolymer, the weight ratio of PLGA(LA+GA) to PEG was 3.0:1 and the molar ratio of LA to GA was 3:1. The chemical structure of the resulting copolymer was analyzed by 1H NMR spectrum and GPC. The molecular weight calculated from 1H NMR was 6200, and the molar ratio of LA to GA was 2.7:1. Moreover, the molecular weight and polydispersity index (PDI) evaluated by GPC measurement was 9412 and 1.31 (Table 2) respectively, indicating a unimodal distribution of the resulting copolymer. All these indicated the PLGA-PEG-PLGA triblock copolymer was synthesized successfully and the structure was consistent with the theoretical design.
 |
Table 2 Sequences of Primers Used for Realtime PCR |
Sol-Gel Phase Transition, Degradation, and Biocompatibility
Triblock PLGA-PEG-PLGA copolymers had been proved to be able to self-assemble into micelles when the copolymer concentration reached or exceeded the critical micelle concentrations, with a strong hydrophobic PLGA chains and a hydrophilic shell of PEG chains.27 As the temperature increased, the number of aggregated micelles are increased abruptly and inter-micellar aggregations are formed, due to the increase in the hydrophobic interactions of PLGA blocks and dehydration of PEG shell. A phase transition (sol-to-gel) of the copolymer occurs when the concentration is high enough. However, further increase in temperature lead to serious dehydration of the PEG shell and dramatical shrinkage of the micelles, contributing to the precipitation of the copolymer.28,29 The thermosensitive properties of PLGA-PEG-PLGA copolymers can be modulated by the structure of the copolymer. The hydrophobicity of the PLGA-PEG-PLGA molecules increased by increasing of the LA/GA ratio and PLGA block length, leading to a higher sol-to-gel phase transition temperature30.
The sol-gel phase transition of PLGA-PEG-PLGA triblock copolymers with or without drugs were evaluated by a vial inverting approach, as shown in Figure 1B. In all drug formulations, the transition temperature was found to be dependent on the concentration and reversible. The sol-gel transition temperature of thermosensitive hydrogel suitable for clinical application should be around 25 ◦C, for the easy handling of injectables at room temperature and in situ gel formation after injection with a minimal extent of shrinkage to avoid initial rapid release of the drugs.28 Furthermore, drugs incorporated PLGA-PEG-PLGA copolymers do have an effect on the transition temperature. DOX incorporated hydrogels exhibited a higher transition temperature, while CDDP incorporated hydrogels had a lower temperature than the copolymer itself. DOX and CDDP co-incorporated hydrogels showed a similar transition temperature with hydrogel alone. The changes on the transition temperature of different drug formulations were attributed to the hydrophilic DOX·HCl and hydrophobic CDDP respectively. Eventually, the hydrogels and multi-drugs co-incorporated hydrogel demonstrated an appropriate transition temperature for an injection.
In vitro Biocompatibility and Degradation
The biodegradability and biocompatibility are the key property for biomedical applications. The in vitro biocompatibility of the copolymers was tested by MTT method, against a normal fibroblast cell line (L929 cells) and human osteosarcoma cells (Saos-2 and MG-63 cells). As shown in Figure 1D, the viability the cells were over 90% after incubated with polymer concentrations up to 4 mg/mL, indicating an excellent biocompatibility of the PLGA-PEG-PLGA copolymers.
As shown in Figure 1C, over 70% of PLGA-PEG-PLGA hydrogels were degraded gradually after incubated with PBS for 40 days. PLGA-PEG-PLGA copolymers can self-assemble into micelles and transform to hydrogels by the interaction force between hydrophobic segments. Studies have shown that degradation of the hydrogels were driven mainly by surface erosion and the fast break of copolymer chains,31 while copolymer chains degradation is mainly accomplished by hydrolysis of ester bond. The degradation profiles of PLGA-PEG-PLGA hydrogel were closely related to the molecular structure of the hydrogels. The higher molar ratio of LA/GA and the stronger hydrophobic effect of PLGA segment lead to slower degradation rate of the hydrogel.32–34
Drugs Release Form the PLGA-PEG-PLGA Hydrogel
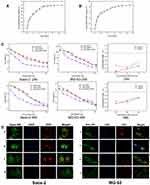
The drugs release behaviors of the drugs (DOX, CDDP) loaded 20 wt% PLGA-PEG-PLGA hydrogels were evaluated in vitro. The drugs release from the PLGA-PEG-PLGA hydrogels via two principal mechanisms: drug diffusion process in the initial burst release stage and the diffusion/degradation-mediated process in the plateau release stage.35 As shown in Figure 2A and 2B, the release behaviors of DOX and CDDP from the hydrogels demonstrated an initial burst release in the first two days, followed by a sustained drug release profile for over 10 days. Yu et al36 reported that DOX was released from the hydrogel in a sustained manner following an initial burst. With the increased hydrophobic property of the new synthesized PLGA-PEG-PLGA hydrogels, compare to our previous report,24 we achieved the sustained release profile of CDDP from the hydrogel for up to 10 days.
Cellular Uptake and DOX Release
DOX and CDDP are powerful and effective anti-neoplastic drugs, which presents excellent therapeutic efficacy against osteosarcoma in clinical work.11,37 DOX and CDDP inhibits cellular DNA, RNA and protein synthesis by binding and intercalating to nucleic acids.38,39 The cellular uptake and DOX release behavior of the DOX and CDDP co-incorporated PLGA-PEG-PLGA hydrogels were evaluated by the confocal laser scanning microscopy (CLSM). After incubation with DOX and CDDP for 3h, we found some osteosarcoma cells was shrinked and deformed irregularly, leading to the pyknotic, karorrhexis and karyolysis. The fluorescence characteristics of DOX can be detected and used to demonstrate the anti-tumor mechanism. DOX was distributed in the nucleus after endocytosis by osteosarcoma cells, and the nucleus presented obvious red fluorescence. As shown in Figure 2D, osteosarcoma cells incubated with free DOX and free DOX+CDDP exhibited the strongest fluorescence intensity. Meanwhile, when the DOX and CDDP were co-incorporated into the hydrogels, the fluorescence intensity of DOX was much weaker, meaning less DOX was uptaked by the osteosarcoma cells exposure to drug for 3h. The reason to the lower DOX uptake may be attributed to the sustained and slow release of DOX from the hydrogel depot, which is in coincidence with the DOX release test in vitro. It implies that CDDP did not decrease the uptake of DOX in osteosarcoma cells and confirms the synergistic anti-tumor effect of doxorubicin and cisplatin on osteosarcoma.
In vitro Synergistic Cytotoxicity
The in vitro cytotoxicites of the DOX, CDDP co-incorporated PLGA-PEG-PLGA hydrogels against osteosarcoma cells were evaluated by MTT assay at 24h, 48h, 72h respectively. The drugs co-incorporated hydrogels exhibited an effective antitumor effect. As shown in Figure 2C, it should be noted that multiple drugs in hydrogels displayed cytotoxicities after incubation for 24h, 48h in vitro, due to the controlled release profiles of the drugs from the drug-loaded hydrogels. Moreover, it is noteworthy that the hydrogels loaded with DOX and CDDP showed the higher cytotoxicities against Saos-2 and MG-63 cells compared to the hydrogels loaded with single drugs, and the combination index of the Saos-2, MG-63 was range from 0–0.6, implying a synergistic effect of the multiple drugs on the anti-proliferation of the osteosarcoma cells.
The drug co-incorporated hydrogel was formed as a drug depot in situ. The drugs were sustained release from the drug depot, maintaining a relative high drug concentration for a long time and exhibiting a synergic antitumor effect.
In vivo Anti-Tumor Effects
The in vivo antitumor efficacies of DOX and CDDP co-loaded hydrogels were investigated on human osteosarcoma xenografts model by transplantation of human osteosarcoma Saos-2 cell into the armpit of right anterior limb of nude mice. As shown in Figure 3A, C and D, the tumor mass of the control groups treated with hydrogels alone increased rapidly. Moreover, treatments with DOX or CDDP loaded hydrogels displayed enhanced tumor inhibition efficacies compared to the treatments with free drugs for up to 14 days. It was believed that the sustained release behaviors of the drugs from the hydrogels led to prolonged tumor suppression. Notably, DOX and CDDP co-loaded hydrogels presented the highest anti-tumor efficacy compared to the hydrogels loaded with single drug or free drugs, suggesting a synergistic anti-tumor effect of the combination of DOX and CDDP on osteosarcoma. As a result of improvement of release profile of CDDP, we found the combination of DOX and CDDP can achieved almost the same anti-tumor effect as DOX, CDDP and MTX co-loaded hydrogels in our previous study.24
Additionally, the body weights of the mice were monitored for the systemic safety evaluation of the treatments. As shown in Figure 3B, no obvious body weight loss was observed during the experimental period for all the groups, suggesting no systemic toxicity of the localized treatments. A large number of multicentric randomized controlled clinical studies have found that regular combination chemotherapy based on DOX and CDDP can prolong the survival time of patients with osteosarcoma.37,40–42 The in vivo anti-tumor test result also proved the synergistic effect of DOX and CDDP on osteosarcoma.
Furthermore, the tumor masses from the mice were dissected into sections and H&E and TUNEL stained for further pathology analysis. As shown in Figure 3E, we found osteosarcoma cells was shrinked and deformed irregularly in the treatment groups, leading to the pyknotic, karorrhexis and karyolysis. Osteosarcoma treated with the hydrogel containing DOX and CDDP exhibited the largest tumor necrosis areas, indicating the synergistic anti-tumor effects. Furthermore, the tumor treated with DOX and CDDP co-loaded hydrogel exhibited highest green fluorescence, demonstrating the highest apoptosis ratio.
Mechanism of Doxorubicin and Cisplatin Inducing Apoptosis Against Osteosarcoma
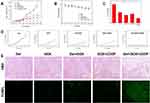
The apoptosis of human osteosarcoma cells was evaluated by Annexin V-APC/7-AAD assay in vitro. For the Annexin V test, the osteosarcoma cells were double stained for viability (7-AAD) and apoptosis (Annexin V-APC). Annexin V is considered as the symbol of early apoptosis, which can transfer from the intracellular membrane to external leaflet and specifically binds to phosphatidylserine.43 As shown in Figure 4A, the cell apoptosis rate showed a significant increase in treatments groups compared with the control group. Moreover, the treatment with DOX and CDDP co-incorporated hydrogels leading to higher apoptosis than DOX or CDDP alone. The result was consistent with the cell proliferation inhibition test in vitro, due to sustained controlled release of drugs from the hydrogels.
Additionally, the expression of apoptosis gene on osteosarcoma cells and tumor mass treated by different formulations were measured by quantitative Real-Time PCR. As shown in Figure 4B and C, the combination of DOX and CDDP co-loaded hydrogels increased the expression of caspase-3, and BAX significantly, whereas the expression of anti-apoptosis gene Bcl-2 was reduced obviously in both Saos-2 and tumor mass. The expression of the apoptosis genes was in accordance with the Annexin V test.
Endogenous and exogenous apoptotic pathways are two classical apoptotic way. They can activate caspase-3, Caspase-9 and BAX pathways and inhibit Bcl-2, and regulate a variety of substrate proteins, resulting in protein inactivation and cell apoptosis. Caspase-3 can activate Caspase-9 through endogenous apoptotic pathways, which are released from mitochondria and eventually induce the apoptosis of osteosarcoma cells.44 The Bcl-2 protein family can regulate the membrane permeability, and stabilize the mitochondria by inhibiting the insertion of BAX into the mitochondrial outer membrane, presenting an anti-apoptotic effect.45,46 The present study showed that in Saos-2 cell lines and tumor mass, the expression of Bcl-2 decreased significantly after DOX and CDDP co-loaded hydrogels treatment compared with the control group, and the decreased expression of Bcl-2 was more obviously in the combination chemotherapy group than in the single treatment group. At the same time, the expressions of BAX, caspase-3 were increased in the treatment groups, especially in the combination chemotherapy group. The combination of doxorubicin and cisplatin co-loaded hydrogels can enhance apoptosis and inhibit tumor growth through endogenous and exogenous pathways.
Systemic Safety Evaluation
At the end of the experiment, the key organs of mice were sectioned and stained to evaluated the systemic toxicities of local treatments with different formulations. As shown in Figure 5, no obvious abnormality was observed in the organs after different treatment, suggesting the low systemic toxicity to the normal organs by localized injection treatments.
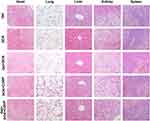 |
Figure 5 Histological H&E staining of the mice organs after different treatments: Gel, DOX, Gel+DOX, DOX+CDDP and Gel+DOX+CDDP. |
Conclusions
In this study, we designed, synthesized and characterized a novel injectable thermosensitive PLGA-PEG-PLGA hydrogel containing DOX and CDDP for the localized treatment of osteosarcoma. The PLGA-PEG-PLGA solution underwent a sol-gel transition at appropriate temperature and degraded in the PBS, presenting a friendly biocompatibility in vitro. The in vitro cell viability tests demonstrated that the DOX and CDDP co-loaded hydrogels exhibited synergistic anti-proliferation effect on osteosarcoma Saos-2 and MG-63 cells, due to the sustained release of drugs from the drugs-loaded hydrogel. The in vivo anti-tumor effects were investigated by single injection of the drug-loaded hydrogel in the human osteosarcoma nude mice xenografts model, presenting a synergistic anti-tumor effect. The treatment with DOX and CDDP co-loaded hydrogel led to the highest efficiency in inhibiting the tumor growth, enhanced tumor necrosis rate and increased regulation of the apoptosis-related gene expressions, compare with the free drugs or the single drug loaded hydrogel, indicating a synergistic anti-tumor efficacy of DOX and CDDP co-loaded hydrogel in vivo. Additionally, ex vivo histological analysis of the nude mice exhibited no obvious abnormality was observed in the organs after different treatment, suggesting the low systemic toxicity to the normal organs by localized injection treatments. Thus, the combination treatment of osteosarcoma by localized, sustained co-delivery of DOX and CDDP by PLGA-PEG-PLGA hydrogel may serve as a promising strategy for efficient clinical treatment of osteosarcoma.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (81902741), the Science Foundation of Shandong Province (project ZR2019BH077).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–285. doi:10.1016/j.ctrv.2010.02.003
3. Dahlin DC. Pathology of osteosarcoma. Clin Orthop Relat Res. 1975;111(111):23–32. doi:10.1097/00003086-197509000-00004
4. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21:vii320–5. doi:10.1093/annonc/mdq276
5. Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5–a population-based study. Lancet Oncol. 2014;15(1):35–47. doi:10.1016/s1470-2045(13)70548-5
6. Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23(3):559–568. doi:10.1200/jco.2005.04.063
7. Marina N, Bielack S, Whelan J, et al. International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res. 2009;152:339–353. doi:10.1007/978-1-4419-0284-9_18
8. McTiernan A, Jinks RC, Sydes MR, et al. Presence of chemotherapy-induced toxicity predicts improved survival in patients with localised extremity osteosarcoma treated with doxorubicin and cisplatin: a report from the European Osteosarcoma Intergroup. Eur J Cancer. 2012;48(5):703–712. doi:10.1016/j.ejca.2011.09.012
9. Anninga JK, Gelderblom H, Fiocco M, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer. 2011;47(16):2431–2445. doi:10.1016/j.ejca.2011.05.030
10. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi:10.1016/j.ctrv.2013.11.006
11. Carter SK, Blum RH. New Chemotherapeutic Agents. Bleomycin and Adriamycin. CA Cancer J Clin. 1974;24(6):322–331. doi:10.3322/canjclin.24.6.322
12. Pang B, Qiao X, Janssen L, et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nature Communications. 2013;4(1):1908. doi:10.1038/ncomms2921.
13. Swift LP, Rephaeli A, Nudelman A, Phillips DR, Cutts SM. Doxorubicin-DNA adducts induce a non-topoisomerase II–mediated form of cell death. Cancer Res. 2006;66(9):4863–4871. doi:10.1158/0008-5472.CAN-05-3410.
14. Loehrer PJ. Drugs five years later. Cisplatin. Ann Intern Med. 1984;100(5):704–713. doi:10.7326/0003-4819-100-5-704
15. Hayek ER, Speakman E, Rehmus E. Acute doxorubicin cardiotoxicity. New Engl J Med. 2005;352(23):2456–2457. doi:10.1056/NEJM200506093522321.
16. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. The Oncologist. 2006;11(6):694–703. doi:10.1634/theoncologist.11-6-694.
17. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419–437. doi:10.3322/caac.21204.
18. Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. The Lancet Oncology. 2007;8(9):773–783. doi:10.1016/S1470-2045(07)70245-0
19. Li Y, Yang HY, Lee DS. Advances in biodegradable and injectable hydrogels for biomedical applications. J Control Release. 2021;330:151–160. doi:10.1016/j.jconrel.2020.12.008
20. Ma H, He C, Chen X. Injectable Hydrogels as Local Depots at Tumor Sites for Antitumor Immunotherapy and Immune-Based Combination Therapy. Macromol Biosci. 2021;21(6):e2100039. doi:10.1002/mabi.202100039
21. Rizzo F, Kehr NS. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv Healthc Mater. 2021;10(1):e2001341. doi:10.1002/adhm.202001341
22. Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21(11):1835–1849. doi:10.1016/j.drudis.2016.07.006
23. Oliva N, Conde J, Wang K, Artzi N. Designing Hydrogels for On-Demand Therapy. Acc Chem Res. 2017;50(4):669–679. doi:10.1021/acs.accounts.6b00536
24. Ma H, He C, Cheng Y, et al. Localized Co-delivery of Doxorubicin, Cisplatin, and Methotrexate by Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. ACS Appl Mater Interfaces. 2015;7(49):27040–27048. doi:10.1021/acsami.5b09112
25. Berenbaum MC. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137(2):122–130. doi:10.1093/infdis/137.2.122
26. Ma H, He C, Cheng Y, et al. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials. 2014;35(30):8723–8734. doi:10.1016/j.biomaterials.2014.06.045
27. Deng H, Dong A, Song J, Chen X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J Control Release. 2019;297:60–70. doi:10.1016/j.jconrel.2019.01.026
28. Wei P-S, Chen Y-J, Lin S-Y, Chuang K-H, Sheu M-T, Ho H-O. In situ subcutaneously injectable thermosensitive PEG-PLGA diblock and PLGA-PEG-PLGA triblock copolymer composite as sustained delivery of bispecific anti-CD3 scFv T-cell/anti-EGFR Fab Engager (BiTEE). Biomaterials. 2021;278:121166. doi:10.1016/j.biomaterials.2021.121166
29. Park MH, Joo MK, Choi BG, Jeong B. Biodegradable thermogels. Acc Chem Res. 2012;45(3):424–433. doi:10.1021/ar200162j
30. Yu L, Zhang Z, Ding J. Influence of LA and GA sequence in the PLGA block on the properties of thermogelling PLGA-PEG-PLGA block copolymers. Biomacromolecules. 2011;12(4):1290–1297. doi:10.1021/bm101572j
31. Cheng Y, He C, Ding J, Xiao C, Zhuang X, Chen X. Thermosensitive hydrogels based on polypeptides for localized and sustained delivery of anticancer drugs. Biomaterials. 2013;34(38):10338–10347. doi:10.1016/j.biomaterials.2013.09.064
32. Moon HJ, Ko Y, Park MH, Joo MK, Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem Soc Rev. 2012;41(14):4860–4883. doi:10.1039/c2cs35078e
33. Hu J, Chen Y, Li Y, Zhou Z, Cheng Y. A thermo-degradable hydrogel with light-tunable degradation and drug release. Biomaterials. 2017;112:133–140. doi:10.1016/j.biomaterials.2016.10.015
34. Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA–PEG–PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1–2):103–112. doi:10.1016/j.ijpharm.2005.01.017
35. Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG–PLGA–PEG triblock copolymers. J Control Release. 2000;63(1–2):155–163. doi:10.1016/s0168-3659(99)00194-7
36. Yu L, Ci T, Zhou S, Zeng W, Ding J. The thermogelling PLGA–PEG–PLGA block copolymer as a sustained release matrix of doxorubicin. Biomater Sci. 2013;1(4):4. doi:10.1039/c2bm00159d
37. Kudawara I, Aoki Y, Ueda T, et al. Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide, doxorubicin, cisplatin and high-dose methotrexate in non-metastatic osteosarcoma of the extremities: a Phase II trial in Japan. J Chemother. 2013;25(1):41–48. doi:10.1179/1973947812y.0000000055
38. Cores EP. Doxorubicin in disseminated osteosarcoma. JAMA. 1972;221(10):1132–1138. doi:10.1001/jama.221.10.1132
39. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
40. Iwamoto Y, Tanaka K. The activity of the Bone and Soft Tissue Tumor Study Group of the Japan Clinical Oncology Group. Jpn J Clin Oncol. 2012;42(6):467–470. doi:10.1093/jjco/hys059
41. Tanaka K, Kawamoto H, Saito I, Yoshimura K, Fukuda H, Iwamoto Y. Preoperative and postoperative chemotherapy with ifosfamide and Adriamycin for adult high-grade soft-tissue sarcomas in the extremities: japan Clinical Oncology Group Study JCOG0304. Jpn J Clin Oncol. 2009;39(4):271–273. doi:10.1093/jjco/hyn153
42. Bacci G, Ferrari S, Delepine N, et al. Predictive factors of histologic response to primary chemotherapy in osteosarcoma of the extremity: study of 272 patients preoperatively treated with high-dose methotrexate, doxorubicin, and cisplatin. J Clin Oncol. 1998;16(2):658–663. doi:10.1200/jco.1998.16.2.658
43. Van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V‐affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1–9. doi:10.1002/(sici)1097-0320(19980101)31:1<::aid-cyto1>3.0.co;2-r
44. Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9(7):532–542. doi:10.1038/nrm2434
45. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi:10.1038/nrc883
46. Cheng EHYA, Wei MC, Weiler S, et al. BCL-2, BCL-XL Sequester BH3 Domain-Only Molecules Preventing BAX- and BAK-Mediated Mitochondrial Apoptosis. Mol Cell. 2001;8(3):705–711. doi:10.1016/S1097-2765(01)00320-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.