Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
In a Preliminary Study on Human Subjects, a Cosmetic Cream Containing a Harungana madagascariensis Plant Extract Induces Similar Anti-Aging Effects to a Retinol-Containing Cream
Authors Gibielle C , Bousseksou L, Guéhenneux S, Vié K
Received 13 December 2022
Accepted for publication 8 February 2023
Published 19 April 2023 Volume 2023:16 Pages 1051—1058
DOI https://doi.org/10.2147/CCID.S401290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Clara Gibielle,1 Lydia Bousseksou,2 Sabine Guéhenneux,1 Katell Vié1
1Laboratoires Clarins, Pontoise, 95300, France; 2IEC France, Lyon, 69006, France
Correspondence: Clara Gibielle, Laboratoires Clarins, 5 rue Ampère, Pontoise, 95300, France, Tel +33 1 34 35 15 15, Fax +33 1 30 38 41 48, Email [email protected]
Abstract: Retinoids are the most effective anti-aging ingredients. Yet, their use can result in adverse reactions. Even bakuchiol, a natural functional analog, can lead to contact dermatitis. We previously showed that a Harungana madagascariensis (Lam. ex Poir.) plant extract (HME) presents retinol-like properties in vitro. Therefore, we performed a preliminary analysis of the anti-aging potential of a cream containing HME on 46 subjects. The participants applied a HME cream on half of their face and one forearm. The effects induced were compared to those produced by a retinol cream applied to the contralateral side. Clinical evaluations indicate that the two creams rapidly (28 days) improve wrinkles underneath the eyes, ptosis, color homogeneity, smoothness, plumpness, firmness, and elasticity of the facial skin. The improvement of crow’s feet is only significant after 56 days. For all clinical signs, the effects of both creams are indistinguishable. Instrumental measurements of silicon replica from the eye contour region indicate that the wrinkle surface reduction is already noticeable after 28 days with the HME and retinol cream, while it takes 56 days to have a significant depth decrease. Only the retinol cream improves wrinkle length after 56 days. Ultrasound assessment of forearm skin revealed that the HME cream improves superficial dermis density as early as 28 days with further improvement at day 56, timepoint at which improvement is at the limit of significance with the retinol cream. These preliminary results indicate that HME has similar in vivo functional properties to retinol for reducing the severity of aging signs. Future works, including a bona fide clinical study, are needed to confirm these findings.
Keywords: retinol, Harungana madagascariensis, anti-aging, cosmetics, in vivo, scoring
Introduction
Age and chronic sun exposure alter the skin, leading to the appearance of visible signs.1 Because they prevent, even reverse, these signs, retinoids became a staple in dermo-cosmetics.2,3 Indeed, topically applied retinoids stimulate epidermal differentiation and keratinization, leading to epidermis thickening, reinforcement of the stratum corneum, and enhanced barrier function.3,4 In the dermis, they improve the extracellular matrix, resulting in better skin elasticity and fine wrinkle reduction.1,5
Although natural, retinol’s widespread use revealed side effects.1,6 This is also the case with retinoids, even recent ones. There is a need for functional analogs, and there is also a growing demand for natural products.
Bakuchiol, a meroterpene isolated from Psoralea corylifolia seeds, is such a compound. Inducing retinol-like genes/proteins expressions, it reduces aging signs.7–9 Yet, its growing use led to identify contact dermatitis cases.10,11 Such adverse effects are not unexpected, as intolerance to botanical compounds is frequent.12 Therefore, finding natural retinoid functional analogs is still of interest.
We previously reported that a Harungana madagascariensis (Lam. ex Poir.) extract (HME) could be such an alternative.13 It induces a retinol-like gene induction profile in isolated fibroblasts and a retinoid-like protein expression in isolated fibroblasts and photoaged skin explants. These results suggested that HME targets pathways similar to those of retinoids. To further investigate the HME potential, we performed a preliminary randomized, double-blind, comparative study to determine if an HME-containing cream could have comparable effects to a retinol containing cream.
Materials and Methods
Study Design
After a 14-day washout period during which subjects applied (morning and evening) a neutral cream on their face, they used for 56 days (morning and evening) a cream containing HME on half of their face and a retinol-containing cream on the contralateral side. They also applied one of the creams on each forearm. The side onto which each cream was applied was randomly selected, and the application was blind as both creams were similar and provided in identical vials (labeled A or B). Clinical evaluations and data analysis were performed blindly.
During the entire study, make-up was restricted to eyes and lips. The only cosmetics allowed were neutral cleansers or make-up removers. Assessments were performed blindly on days 0 (D0), 28 (D28), and 56 (D56) in a dedicated research facility (IEC France, Lyon, France).
A diagram of the main steps of the study is presented in Figure 1.
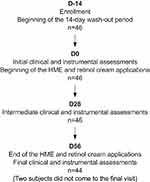 |
Figure 1 Flow chart of the study steps and the number of subjects (n) involved at each stage. Days are indicated in bold. |
Ethic
This cosmetic, non-invasive study being conducted in France, local and EU regulations require no approval by an ethics committee. The principles of the Declaration of Helsinki were strictly followed. All subjects received detailed information and gave their written informed consent.
Intervention
The neutral cream used during the washout period served as a base for the HME and retinol creams. It is an oil-in-water emulsion consisting of aqua, glycerin, caprylic/capric triglyceride, propanediol, cetearyl alcohol, hydrogenated coco-glycerides, glyceryl stearate, PEG-100 stearate, pentylene glycol, cetearyl glucoside, steareth-21, dimethicone, carbomer, chlorphenesin, ethylhexylglycerin, disodium EDTA, dimethiconol, and sodium hydroxide. This cream base was supplemented with 0.02% (w/w) of dry HME. This extract was prepared by water extraction of Harungana madagascariensis crushed dry leaves. After filtration, the water is evaporated, and the dry matter is resuspended in glycerin to produce a 20% dry matter solution. Alternatively, the cream base was supplemented with 0.05% (w/w) retinol (with the addition of antioxidants – BHA and BHT – and a surfactant – Polysorbate 20).
Subjects
The number of subjects included relied on guidelines recommending 10 to 40 participants for a pilot study.14 Therefore, a group of 46 women was recruited. Their age ranges from 50- to 66-year-old (mean±SD: 60.5 ± 5.2), and all presented wrinkles, inhomogeneous complexion, pigmented spots, and skin slackening. Exclusion criteria were the use of anti-aging, anti-wrinkle, or firming products (including oral/topical retinoid treatments). Additional exclusion criteria were the use of possibly interfering treatments.
Tolerability
Participants had to report any adverse effects immediately. Their nature, location, intensity, duration, and the cream involved were recorded.
Assessment Conditions
All evaluations were performed under controlled conditions (21 ± 2°C and 45 ± 5% relative humidity). Clinical scorings were carried out after 15 minutes of acclimation and instrumental measures after 30 minutes.
Clinical Evaluations
A trained cosmetic expert scored the severity of crow’s feet (0 to 6 scale), wrinkles underneath the eyes (0 to 5 scale), and lower face ptosis (0 to 5 scale) according to Bazin and Doublet photographic scales.15 For more accurate scoring, 0.4 and 0.6 intermediate grades were used. The other characteristics evaluated using a 0 (slight) to 9 (marked) scale were skin color homogeneity, smoothness, plumpness, firmness, and elasticity.
Instrumental Measurements
Negative silicon (SifloTM) replicas of the eye outline region were illuminated with a 35° incident light. Analysis of light intensity with the QuantiridesTM software (Monaderm, Monaco) enabled quantification of wrinkles parameters: number, total depth, total length, and total surface.
Superficial dermis density and dermis thickness at the level of forearms were evaluated using a high-resolution ultrasound scanner (Dermcup, Atys Médical, France).
Statistical Analysis
Among the 46 participants recruited, all were analyzed on D0 and D28. Two did not come to the D56 visit, leaving 44 subjects for this time point (Figure 1). Data analysis was performed blindly on all available participants at each time point.
Results from clinical evaluations are expressed as mean ± standard deviation (SD). Instrumental measurements are presented as mean ± standard error of the mean (SEM). After checking data distribution (Shapiro–Wilk test), statistical analyses were performed using Student’s paired t-tests or Wilcoxon signed-rank tests. P-values < 0.05 were considered significant, while values between 0.05 and 0.1 were considered trends.
Results
Tolerability
Two subjects reported prickling, itching, heating sensations, or skin redness during the study. For the first one, a slight sensation occasionally occurred throughout one day on the whole upper part of the face. It was therefore considered as not related to cream applications. The second subject reported four episodes of slight to marked sensations and skin redness on the facial side onto which the retinol cream was applied. These episodes appeared rapidly after cream application and lasted from a few minutes to the entire day.
Clinical Evaluation
Compared to D0, clinical evaluation of wrinkles underneath the eyes, lower face ptosis, color homogeneity, smoothness, plumpness, firmness, and elasticity show improved conditions for both the retinol and HME creams as early as 28 days and further improvement at D56 (Table 1). Crow’s feet severity is the only sign requiring 56 days to show a positive effect by both creams. Furthermore, except for skin firmness that could be better improved by the retinol cream than the HME one, both creams present no significant difference in the effects they induce.
 |
Table 1 Clinical Scoring of Facial Features |
Instrumental Evaluation of Wrinkles
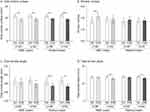
Analysis of the silicon replicas (Figure 2A–D) from the eyes’ outline region shows that the retinol and HME creams decrease wrinkles’ total surface. Compared to D0, the decrease is already significant at D28 and maintained at D56. If the HME cream could increase the wrinkle number at D28, this is transient, and no change in wrinkle number is evidenced at D56. Only the retinol cream reduces wrinkle total length, an effect that is significant at D56. However, both creams reduce wrinkle total depth at D56.
Ultrasound Analysis of the Skin
Skin layers were evaluated by high-resolution ultrasound (Table 2). Compared to D0, the HME cream improves the superficial dermis density at D28 and D56. The effect of the retinol cream is only at the significance limit at D56. Despite this different time effect of both creams, a comparison of dermis density at a time point revealed no difference between both creams. Finally, none of the creams influence dermis thickness.
 |
Table 2 Ultrasound Analysis of the Skin |
Discussion
A previous study showed that HME leads to an increased expression of many genes induced by retinol.13 In isolated fibroblasts and UV-aged skin explants, it also leads to the over-expression of dermal and epidermal proteins, which synthesis is also activated by retinoids. Results from this in vivo comparative study of creams containing either retinol or HME show that both have similar and undistinguishable positive effects on clinically evaluated wrinkles and skin properties affected by age: ptosis, color homogeneity, smoothness, plumpness, firmness, and elasticity. A certain number of these results are comforted by instrumental measurements. If the number of wrinkles around the eyes does not really change, their surface rapidly decreases while depth reduction takes longer, and only the retinol cream reduces their length. In agreement with what is known for retinol and our previous work on HME-induced collagen expression, epidermis density increases, even if only a trend for retinol, indicating a strengthened extracellular matrix. All these outcomes are consistent with previously published retinol effects and in vitro results of HME.2,3,13
Yet, this preliminary in vivo analysis of the effects of HME suffers limitations. One is its duration. If histological changes induced by retinol and retinoids can occur within a few weeks explaining why we could evidence an increased dermis density, improving the overall skin condition requires more time: several weeks, even months.2 We limited ourselves to 56 days. Even if these were enough to evidence mild yet noticeable effects, this relatively short period might be why we could not highlight some known effects of retinoids. This could be the case with some instrumentally quantified wrinkle parameters. This could also be the case for the increased dermis thickness. Therefore, negative results on such points should not be considered definitive, as the retinol cream showed moderate to no effect. A longer duration of evaluation could have revealed an improvement.
Another limitation is the lack of a placebo-control cream. This absence of placebo results from the choice to compare the effects of retinol and HME on the hemiface of a single subject. Such an approach presents the advantage of a reduced variability. It also offers a more robust comparison as it is rare that a subject presents very different skin characteristics on both facial sides and that both hemifaces evolve differently along the study. Yet, this approach prevented us from testing the possible effects of the vehicle cream. If we were careful to use as few ingredients as possible during formulation, two emollients entered its composition: hydrogenated coco-glycerides and glyceryl stearate. We cannot rule out their influence on improved skin smoothness.16 Nevertheless, as the cream base was used 15 days before the beginning of the study, emollient-induced skin smoothness should have mainly occurred during this period. Therefore, emollients seem unlikely to explain the evidenced wrinkle reduction. Furthermore, HME and retinol positively influence other age-related skin parameters, which cannot be explained by any ingredient of the vehicle cream. Therefore, the anti-aging action evidenced by retinol and HME can only relate to these two active ingredients.
Assessing the tolerability of HME on 46 subjects is also not enough, even if that was sufficient to reveal an obvious case of an adverse reaction to topical retinol application. It took some time and widespread use of bakuchiol to identify a few instances of contact dermatitis.10,11 Nevertheless, HME already enters the composition of several cosmetics, and, to our knowledge, no adverse reaction has been attributed to the extract. It, therefore, seems a safer option than retinol or retinoids. It is also of natural origin, which users are increasingly looking for.
Conclusion
In this preliminary study, results from clinical and instrumental assessments of the evolution of aging signs upon applications of a HME- or retinol-containing cream revealed similar results for most features evaluated. Further work will be required to confirm our findings. If performing a bona fide clinical trial seems essential, preliminary results indicate that HME has similar functional properties to retinol, reducing the severity of aging signs while offering a new natural alternative.
Acknowledgment
The authors wish to thank Ingrid Aimé and her team (IEC France) for their technical help, as well as Yves Chipier and his team for data management. They are also thankful to Anaïs Gaungoo for formulating the retinol and HME creams. They are grateful to Jean-Pierre Nicolas, Ethnobotanist from the Ingredient R&D Laboratory, for providing HME and their advice to study it. They also wish to thank Dr. Philippe Crouzet, Estium-Concept, for scientific writing services.
Funding
The present work was funded by Clarins, France.
Disclosure
CG, SG and KV are full-time employees of Clarins. LB is a full-time employee of IEC France, an independent CRO dedicated to cosmetic evaluation. The authors report no other conflicts of interest in this work.
References
1. Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370. doi:10.1101/cshperspect.a015370
2. Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–348. doi:10.2147/ciia.2006.1.4.327
3. Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019;36:392–397. doi:10.5114/ada.2019.87443
4. Törmä H. Regulation of keratin expression by retinoids. Dermatoendocrinol. 2011;3:136–140. doi:10.4161/derm.15026
5. Shao Y, He T, Fisher GJ, Voorhees JJ, Quan T. Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int J Cosmet Sci. 2017;39:56–65. doi:10.1111/ics.12348
6. David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273–288. doi:10.1007/BF03259940
7. Chaudhuri RK, Bojanowski K. Bakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int J Cosmet Sci. 2014;36:221–230. doi:10.1111/ics.12117
8. Dhaliwal S, Rybak I, Ellis SR, et al. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br J Dermatol. 2019;180:289–296. doi:10.1111/bjd.16918
9. Greenzaid J, Friedman A, Sodha P. The use of bakuchiol in dermatology: a review of in vitro and in vivo evidence. J Drugs Dermatol. 2022;21:624–629. doi:10.36849/JDD.6740
10. Malinauskiene L, Linauskiene K, Černiauskas K, Chomičiene A. Bakuchiol - A new allergen in cosmetics. Contact Dermatitis. 2019;80:398–399. doi:10.1111/cod.13211
11. Raison-Peyron N, Dereure O. A new case of contact dermatitis to bakuchiol in a cosmetic cream. Contact Dermatitis. 2020;82:61–62. doi:10.1111/cod.13387
12. Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by topical herbal remedies: importance of patch testing with the patients’ own products. Contact Dermatitis. 2018;78:177–184. doi:10.1111/cod.12939
13. Fitoussi R, Branchet MC, Garnier N, et al. A Harungana madagascariensis extract with retinol-like properties: gene upregulations and protein expressions in human fibroblasts and skin explants. Int J Cosmet Sci. 2022;44:201–215. doi:10.1111/ics.12768
14. Herzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–191. doi:10.1002/nur.20247
15. Bazin R, Doublet E. Skin Aging Atlas. In: Caucasian Type. Vol. 1. Paris, France: Med’Com; 2007.
16. Draelos ZD. The science behind skin care: moisturizers. J Cosmet Dermatol. 2018;17:138–144. doi:10.1111/jocd.12490
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.