Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Improving Physical, Physiological, and Psychological Health Outcomes in Patients with Diabetic Foot Ulcers – State of the Art
Authors Vas P , Chockalingam N
Received 30 April 2023
Accepted for publication 30 November 2023
Published 12 December 2023 Volume 2023:16 Pages 3547—3560
DOI https://doi.org/10.2147/CCID.S333660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Prashanth Vas,1– 3 Nachiappan Chockalingam2
1Department of Diabetes and Diabetic Foot, King’s College Hospital NHS Foundation Trust, London, UK; 2Centre for Biomechanics and Rehabilitation Technologies, Staffordshire University, Stoke on Trent, UK; 3Department of Diabetes and Endocrinology, Guy’s and St Thomas’ Hospitals NHS Foundation Trust, London, UK
Correspondence: Prashanth Vas, Consultant in Diabetes and Diabetes Foot Medicine, Department of Diabetes and Diabetic Foot, King’s College Hospital NHS Foundation Trust, Denmark Hill, London, SE5 9RS, UK, Email [email protected]
Abstract: Diabetic foot disease is a complex and challenging complication of diabetes mellitus, which imposes a significant burden of disease on patients, their carers, and the wider health systems. Recurrence rates are high, and current evidence indicates a high mortality associated with it. While management algorithms have primarily focused on the physical aspects of healing, there is increasing recognition of the critical role played by psychological and biomechanical factors in the development and resolution of diabetic foot disease. Therefore, in this paper, we aim to explore how diabetic foot outcomes can be improved by addressing not only the physical but also the psychological and biomechanical aspects that are integral to the development of this condition and its optimal resolution. We explore new technologies that allow for non-invasive objective assessment of the diabetic foot at risk, and we also explore the role of understanding biomechanics, which is essential to determining risk of foot disease, but also the potential for recurrence. In addition, we discuss the evidence linking depression and cognitive impairment to diabetic foot disease and offer our insight on the research direction required before implementing novel information into front-line clinics.
Keywords: diabetic foot disease, diabetic foot ulceration, biomechanics, technology, mental health and cognition
Burden of Diabetic Foot Ulceration
Diabetic foot disease is perhaps the most feared of all diabetes complications and is associated with significant morbidity and mortality.1 The lifetime risk of developing diabetic foot ulceration (DFU) is approximately 19–35% in individuals with diabetes.2 Despite several improvements in diabetic foot care over the years, many continue to progress to lower extremity amputation (LEA). The mortality in people with DFU can be higher than many common cancers, with a 5-year mortality rate in excess of 50% reported in those undergoing a major amputation secondary to DFU.3 Likewise, the economic burden from DFU can be staggering with direct treatment cost in England estimated to be in excess of £1 billion,4 while costs in the United States of America are in the region of $ 9–13 billion.5 The presence of DFU can double the cost of delivering diabetes care,5 increase visits to the emergency room and increase hospitalisation rates.6 The indirect costs to society, carers and to employers are more difficult to quantify but are likely to be substantial.1
Epidemiology
Studies assessing European diabetes populations have reported DFU incidence rates between 1.9% and 4.0% depending on the country surveyed.7–9 The global prevalence rate of DFU is greater, estimated at 6.3% in one recent meta-analysis.10 Charcot neuroarthropathy (CN), a condition that can cause deformity, ulcers, infections, and even amputation, is identified by damage to the bones and joints due to neuropathy. It commonly affects the midfoot but can also occur in the forefoot and hindfoot. However, the hindfoot is more vulnerable and may result in ankle instability, leading to more critical outcomes. CN is long perceived as an uncommon complication and has no precise epidemiological data. In the United States, a prevalence rate of 4.1–7.4 per 1000 population with diabetes has been reported, while estimates from the United Kingdom report that 4.3 per 10,000 population with diabetes have active CN.11 Another report from Ireland has noted a higher 7-year prevalence of approximately 3 per 1000 of the total diabetes population.12 Early reports from India13 and Africa14 indicate a much higher prevalence (10–18%) of established CN presenting with deformity and ulceration to specialist foot centres.
Risk Factors for Diabetic Foot Ulceration
The development of DFU underscores a complex interplay between various foot level factors such as neuropathy, arterial disease, abnormal biomechanics; systemic factors such as diabetes control, renal disease, cardiovascular disease, frailty, and extrinsic factors such footwear, and the quality of foot protection provision. Furthermore, as with any chronic condition, there is a significant psychological burden that is often aggravated by cognitive dysfunction.
Diabetic Neuropathy
Diabetic Sensorimotor Peripheral Neuropathy, or specifically, its most advanced presentation, loss of protective sensation (LOPS), has been a consistent risk predictor for foot ulceration. In the North-West Diabetes Foot Care study, insensitivity to the 10gm monofilament was associated with an increased risk of DFU development (relative risk RR 1.80, 95% CI 1.40–2.32),8 while in the Seattle Diabetic Foot Study it was higher (RR 2.2 95% CI 1.5–3.1).15 The development of small fibre neuropathy leads to abnormalities of pain and temperature perception. Cutaneous autonomic denervation will lead to abnormal sweating and dry skin. Such changes usually run alongside the involvement of the large sensory nerve fibre which mediates vibration, touch, and proprioception. Typically, motor neuropathy is a late development but when present, collectively with sensory neuropathy, may lead to the development of foot deformities, gait abnormalities and balance disorders.
The presence of diabetic neuropathy is also key to the development of CN. However, and importantly, current evidence suggests that any degree of neuropathy may trigger CN, not just LOPS.16 The neurological phenotype in CN is complex and is the subject of much debate. Indeed, studies have demonstrated differential involvement of neuronal markers such as abnormalities of vibration and small fibres concurrently17–19 but with preserved warm and light touch perception.17 Risk factors for the development of neuropathy in diabetes, in addition to age, gender, duration of diabetes, appear to be similar to those determining cardiovascular risk such as hypertension, dyslipidaemia, smoking history and the presence of other microvascular complications of diabetes.20,21 Using these factors in future clinical algorithm models and adding in social determinants such as educational level may allow us to identify those at risk of neuropathy development.21 Classical neuropathic DFU occurs over pressure areas or over deformity prominences (Figure 1).
Peripheral Vascular Disease
Diabetes significantly increases the risk of developing peripheral arterial disease (PAD), and vascular event rates are higher in diabetic individuals with PAD than in non-diabetes populations.22 Diabetes confers 2 to 4 times increased risk of incidence of PAD. It is estimated that PAD is present in nearly half of all DFUs and in certain countries such as China and the Far East, the proportion may be even higher. Furthermore, the clinical and anatomical presentation of PAD may be atypical. Those with diabetes are more likely to be asymptomatic because of concurrent neuropathy and a significant proportion will have diffuse, below the knee predominant disease and at times limited to the foot level.23,24 In addition to conferring risk for the development of DFU, the presence of PAD is also a marker for slower healing of DFU infection, a higher risk of lower extremity amputation (both major and minor), unplanned hospital admissions, cardiovascular events and mortality.25 We now recognise that DFU developing on the plantar/pressure regions can still have an additional element of PAD (neuroischaemic DFU) but classically ischaemic lesions, usually presenting with tissue loss and gangrene, are still commonly encountered (Figure 2).
Biomechanical Determinants
Previous research has explored the interplay between foot deformity, morphology, and mobility to demonstrate that diabetes affects the biomechanics of the tissues and the overall structure and function of the foot and ankle in several ways.26,27
One of the common complications of diabetes is limited joint mobility occurring in approximately 30–40% of patients.27 In addition, motor neuropathy also causes foot deformities due to alterations in the mechanical balance between the extrinsic and intrinsic muscles of the foot. Structural and functional foot deformities of the foot such as claw toes, hammertoes, bunions, or changes to midfoot and hindfoot shape induce significant biomechanical alterations during ambulation. The presence of sensorimotor neuropathy will further augment these deleterious effects leading to the development of DFU28 but may also serve as a trigger for CN. Formosa et al28 reporting in a Maltese population observed a Hallux valgus deformity in 49.4% and hammer toes in 39% of their sample. In addition, they also report that 24% and 44% of their patients presented with prominent metatarsal heads and other bony prominences, respectively. Despite these foot deformities, up to 56% of the patients used inappropriate footwear and on clinical examination a further 28% of the patients required prescription orthosis.27,28 In the North-West Diabetic Foot study, the presence of any deformity increased the relative risk of developing DFU by 1.57 (95% CI 1.22–2.02).8 In the Seattle Diabetes Foot Study, the presence of CN-related deformity rendered 3.5 times increased risk for DFU; in certain subgroups, a higher DFU risk was also noted for hammertoe/claw toes.15 Another prospective study, also from Seattle, reported a strong relationship hammer/claw toes (Odds Risk OR 3.91, p = 0.003) and hallux limitus (OR 3.02, p = 0.006) and the increased risk of any DFU incidence.29 Once joint mobility is compromised, the development of callus is common27 and possibly the most important pre-ulcerative sign.
There is a plethora of work which demonstrates that foot structure can affect peak pressures. During ambulation, peak plantar pressures in the diabetic foot are higher at the forefoot than the rearfoot27 and also depend on the walking speed.30 Therefore, repetitive and/or excessive forefoot plantar pressure, coupled with foot structural changes, can increase the risk of the development of forefoot plantar ulceration. A previous study by Lavery et al showed that patients with ankle equinus had higher peak plantar pressures than those without the deformity.31 Also, subtalar joint angles were found to be predictive of medial or lateral ulceration.31 Additionally, ankle and knee joint stiffening, prevalent in people with diabetes and peripheral neuropathy, may additionally contribute to the biomechanical impairment.32,33 This may be further worsened in individuals who have undergone a minor amputation leading to a higher frequency of prone feet.34
Whilst, the non-enzymatic glycation of type 1 collagen is said to be the cause of compromised mobility, abnormal loading and altered plantar pressure distribution due to these functional issues and deformities leading to hyperkeratosis. The effects of high levels of glucose in the blood are reportedly observed in the feet in which the skin thickness is known to decrease whilst skin hardness increases, tendons thicken, joints have limited mobility, fat pads decrease, and results in muscle atrophy. Additionally, previous studies on plantar soft tissue mechanics highlight the link between mechanical stiffness and ulceration.35 Whilst there are still questions on the causation and implications of mechanical changes at the tissue level, studies show that the assessment of mechanical properties can enhance the diagnosis and prognosis of DFU.35,36
It has been reported via a plethora of studies that diabetic footwear and insoles play an important role in the reduction of plantar pressure in people with diabetes.37,38 However, other work39 shows that the heel pad stiffness and thickness influence plantar pressure but not the optimum insole properties. Also, it has been reported that individuals with type-2 diabetes and high levels of triglycerides and fasting blood sugar are more likely to have stiffer heel-pads. This increased stiffness could limit the tissues’ ability to evenly distribute loads making them more vulnerable to trauma and ulceration even without any change in the plantar pressure.40 Another study which investigated if the parameters describing the mechanical properties of plantar soft tissue were associated with ulceration showed that these mechanical properties can be used to complement existing risk factors to improve the predictability of DFU incidence in moderate-to-high risk populations.41
In summary, a combination of several biomechanical factors and compromised mobility not only results in an altered gait but also severely affects balance and overall dynamic posture. A brief overview of the key risk factors, extrinsic precipitants, and associates of DFU development and suboptimal outcomes are shown in Figure 3.
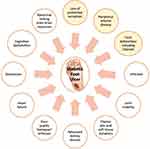 |
Figure 3 Key risk factors, precipitants, and associates of suboptimal outcomes in diabetic foot ulceration. Those in yellow circles are the key drivers of diabetic foot ulcer development. |
Systemic Risk Factors
In addition to neuropathy and PAD, other factors but not limited to contributing to the development of DFU include age, long duration of diabetes, suboptimal glycaemic control with HbA1C >9%, male gender and higher body mass index.8 Advanced chronic kidney disease is a strong risk factor for the development of ulceration. Dialysis treatment is strongly associated with prevalent DFU (OR 4.2 95% CI 1.7–10, p = 0.002).42 It is also a major risk factor for non-healing and amputation.43 The EURODIAB study, factors such as cigarette smoking, high-density lipoprotein cholesterol, elevated diastolic blood pressure, increased fasting triglycerides and presence of microalbuminuria were independently associated with diabetic neuropathy development.44 By extension, these risk factors and those driving the development of PAD will apply to DFU. Presence of cardiovascular and cerebrovascular events has been shown to confer additional risk42,45 with a lower probability of amputation free limb salvage, a higher risk of amputation and mortality.45,46 Extrinsic precipitants such as poorly fitting shoes/socks, acute mechanical trauma, and shear stress are also recognised as important triggers.47 Nail overgrowth and paronychia are also important drivers in some instances, especially the elderly.47 It is vital to acknowledge that DFU represents a complex setting, where several risk factors coexist and continuously interact within the same individual.
Cognitive and Psychological Impact in Diabetic Foot Disease
An association between diabetes and depression is well accepted, particularly in T2DM where a bidirectional relationship has been recognised [1–3]. The presence of depression can increase the risk of developing T2DM by 17–60%48–50 and conversely, a diagnosis of T2DM may double the odds of prevalent depression compared to those without diabetes.51 Therefore, it is perhaps not surprising that anxiety and depression are present at levels consistent with clinical depression in patients with DFU.52,53 In a community setting, the presence of depression may double the risk of new DFU in an individual with no history of diabetic foot problems.54 Nearly half of all individuals with DFU attending hospital-based clinics may meet the criteria for depression.55,56 In addition to increasing the risk of incident DFU,54,57 depression has also been shown to be associated with a two-fold increased risk of mortality during or after treatment for DFU.55
An increasing body of evidence suggests that abnormal cognitive function is prevalent in longstanding diabetes. Diabetes increases the risk of developing cognitive dysfunction which may progress to cognitive impairment.58,59 A decline in cognitive function may be manifested as diabetes self-care neglect, inability to follow through with recommendations and increased use of care resources.59 The risk factors associated with such cognitive decline60 are the same cluster that drives vascular complications, diabetic neuropathy and indeed depression. This signifies that the processes that underpin the development of these individually distinctive complications may be interlinked.60,61 Of note, these are also the same risk factors for diabetic foot ulceration. More recently, the presence of cognitive dysfunction complicating DFD has been recognised. In one study, while the premorbid cognitive abilities among diabetes individuals with and without DFU were estimated to be similar, the cognitive abilities of individuals with ongoing DFU were found to be significantly lower in all domains tested (memory, attention, executive function, reaction time and psychomotor skills).62 This is likely to pose significant challenges in the delivery of quality care that patients with DFU need. They are expected to understand and comply with many complex instructions, for healing and preventing recurrence and may struggle to do so. Concurrently, healthcare professionals may be unable to pick up the nuances of cognitive dysfunction or early impairment and may not appreciate the need for careful reiteration at every visit. It is unclear whether DFU occurs more frequently in individuals with cognitive abnormalities than in those without, or whether cognitive abnormalities precede the development of DFU or follow it.
The central nervous system (CNS) is involved in receiving sensory inputs, the perception of pain and coordinating motor responses. A growing body of evidence points to alterations in the CNS of individuals with diabetes, both functional and/or structural, which may lead to maladaptive responses even in the absence of cognitive impairment or depression. It is therefore possible that the development of LOPS may further complicate this milieu with the lack of consistent sensory feedback, leading to a disruption of centres involved in interception which is relevant to symptom generation and perception and in regions involved in emotional salience and threat avoidance, akin to central responses in hypoglycaemia.63 In Figure 4, we outline a schematic between depression, cognition and brain responses and their interaction with traditional risk factors which may potentially impact the development and persistence of DFU.
Improving the Outlook of People Living with DFU
Role of Screening
Foot-risk screening allows for the stratification of future DFU risk and the institution of appropriate prevention methods to reduce the risk of incident DFU. Typically, all individuals with diabetes receive an annual review and have their foot risk confirmed as low, medium, or high risk.64,65 Those deemed medium or high risk should be closely monitored, ideally in a foot protection service. However, presently, this aspect is poorly carried out and there is a wide variation in the quality of screening undertaken.66 Studies from Europe indicate that annual foot-risk screening in primary care is lower than expected, ranging between 20% and 65%,67–69 and much lower than, reciprocal rates for diabetic retinopathy screening.70 In addition, how the risk level is communicated to the individual patient needs to be improved71 and efforts made to improve any educational barriers. The provision of structured education to improve self-care knowledge (do’s and don’ts) and develop foot protective practices, reinforced at every visit, are important and recommended in guidance.72
Prompt Assessment and Treatment of DFD
Once a DFU is diagnosed, a prompt referral to a specialist foot unit is recommended. Delays in the referral pathway are common and a major factor in patients presenting to specialist units with severe grades of DFU resulting in suboptimal outcomes, including amputation. One recent survey of general practitioners’ attitudes to DFU in 5 European countries identified significant hesitation in primary care to diagnose a DFU and refer onwards with 48% of DFU being referred to a specialist unit after a 1-month delay.73 Specialist foot centres should be clearly identified, easily accessible to patients and provide a robust multi-disciplinary service.74 When limb-threatening features are present (“diabetic foot attack”, for eg, severe infection with or without systemic upset, signs of advanced limb threatening ischaemia, acute hot foot), individuals should be immediately escalated to the local hospital for rapid assessment and management.75 Many such individuals will require surgical debridement to control necrotising infection or urgent revascularisation and critical delays in the process may lead to amputation.76 Developing a regional referral framework from primary care into specialist foot service is vital in providing responsive DFU care, and there are fast-track pathways available to guide clinicians and health administrators.77
Focus on Maintaining Remission
Presently, the rates of DFU recurrence can approach almost 60% at 3 years – a statistic that absolutely needs to be improved over the next few years. One reason for this may be that current clinical systems and endeavour are focused primarily on DFU healing. The focus on post-healing care and management has been less than optimal with a dearth of quality evidence.64,78 To change the long-term outlook of individuals with DFU, we should recognise that DFU healing represents the halfway point in limb salvage and the entry point into addressing factors that led to ulceration in the first place (Figure 5). Only by doing so, we can hope to maintain the foot in remission. Furthermore, management of cardiovascular risk factors that often prevail in this group is vital as diabetic foot disease and even high-risk foot serve as a proxy for risk of mortality.
The triggers that led to DFU development are likely to be present at the point of healing, in particular serious foot deformities which are most likely to drive re-ulceration, at times, despite the best efforts from the patient and their clinical team. Surgical correction of significant deformity should be considered soon after DFU healing has occurred, and sometime, even to achieve healing.79 Likewise, it will be important to ensure any revascularisation undertaken during the initial phase of DFU care is monitored and pharmacotherapy adjusted to ensure long-term durability.80,81 The biomechanical considerations are perhaps the most vital, representing a dynamic process and are discussed further below.
Focus on Addressing Biomechanics
We are at the crossroads of technological advancements for clinical assessment and rehabilitation, and we currently have the right tools to eliminate the incidence of amputations due to complications resulting from diabetic foot disease. However, we still need to substantially improve our understanding of the consequences of disease progression along with the early changes to foot biomechanics. Whilst assistive technology, in the form of custom orthoses or therapeutic footwear, is pivotal in the clinical management of people with diabetic foot disease, there is a clear paucity of structured information and research evidence to inform the clinical management.82 Assistive technology to aid mobility is vital to prevent and treating DFUs. Without them, people can be isolated increasing the impact of disease on them, their families, and society.83 Good quality orthoses and therapeutic footwear have been shown to reduce plantar pressure84 and reduce the incidence of developing DFU.85 Although the use of orthoses or therapeutic footwear as therapy is becoming more ubiquitous, the process of diagnosing the foot problem and prescription of orthoses is still subjective and depends on several variables including the clinician’s knowledge and experience, poorly evidenced screening tools and non-scientific techniques used for a prescription (eg, casting/scanning techniques for orthoses). Data-driven optimisation of prescription footwear remains an important unmet need in front-line clinics globally,86 and is an issue even in advanced healthcare systems. Another challenge to overcome would be the adherence to therapeutic footwear – this can be notoriously insufficient87,88 and can trigger ulceration. Studies exploring the precise reasons behind this and best ways to promote and improve adherence are required.
Previous systematic synthesis of the recommendations included in the existing guidelines showcases substantial differences in the grading of these recommendations.37,89,90 Whilst this may not impact the overall policy development or the implementation of these guidelines, it is important to reflect on the current developments in footwear technology in the future revision of such guidelines.
Focus on Technology and Remote Monitoring in Diabetic Foot-Care
Interest in using technology to monitor at-risk feet is significant, although current studies on available technologies have mainly focused on secondary prevention. The potential of such technologies to provide non-invasive, rapid objective and at times continuous monitoring which could indicate the potential of DFU occurrence is attractive, both to the clinician and the patient, has received significant attention.91–94 A recent meta-analysis has reported that home monitoring of plantar foot skin temperature at least once daily using an infrared thermometer nearly halved the risk of developing a DFU risk of 0.51 (95% CI: 0.31–0.84) as long as preventative action was undertaken in response to abnormal readings.90 The recent IWGDF guidance on prevention now recommends using the technology in those considered moderate or high risk for DFU as a self-monitoring tool,95 furthermore, in the right healthcare setting, this may be cost-effective.96
Recently, there have been several reports on the use of foot thermal imaging, which can accurately capture the entire surface temperature of the foot rapidly, the facilitating foot-care self-management91,96 (Figure 6). A recent study has reported that a foot temperature monitoring system using a SmartMat™ was able to identify 97% of subsequently observed DFUs with an average lead time of 37 days; although there was a rather high (57%) false-positive rate.93 Likewise, the use of smart textile with flexible optical fibres may have a role in managing biomechanical risk factors such as pressure and gait.97–99 The use of an intelligent insole providing continuous monitoring of plantar pressure, coupled with audio-visual alerts delivered through a smartwatch, resulted in a 71% reduction in ulcer incidence when compared to a control group.94
The American Diabetes Association recommends that those with diabetes undertake at least 150 minutes per week of moderate activity per week.100 Wearable devices measuring physical activity, such as pedometers and accelerometers, have the potential to encourage individuals with diabetes to improve activity levels and work towards lifestyle goals of delaying diabetes-related complications.101 They also can be used for evaluating the adherence of therapeutic footwear and offloading devices.87,102
One challenge with new evolving technologies is the optimal individual deployment scenarios – whether in domiciliary or clinical settings – have yet to be determined. Additionally, various factors such as ambient temperature, location of at-risk site, distance of travel, and timing of the measurement may impact the validity of the readings. The perceived burden of regular measurement may limit adherence especially for home monitoring devices – the recent DIATEMP study using infrared thermography highlighted this “prevention paradox” – where participants at some time point become less responsive to taking evasive action when hot spots occurred or reduced frequency of regular monitoring.103
Another challenge is the accuracy of the results and setting of abnormal thresholds. While advanced modelling techniques employing patient-specific finite element (FE) analysis and artificial intelligence (AI) systems are being developed, recent reviews highlight that there are still fundamental challenges that must be addressed to achieve satisfactory accuracy.104 The accuracy of FE analysis and AI systems relies on our ability to accurately quantify patient-specific geometry and material properties. These type of approaches helps long-term patient monitoring in their own environment and enhances the effectiveness of clinical management.105 Combining technologies may be one way forward, for example, one suggestion is to integrate plantar pressure assessment and thermography for more refined prediction.106,107
The use of deep machine learning and AI has the potential to identify patients who are at risk of developing future diabetes complications including DFUs.108–110 The ability to prospectively identify such individuals, with the system flagging up any alarming developments, may help overcome the challenges related to LOPS. Further research in this area is greatly required, and healthcare systems without vertical integration may struggle to provide rich granular data. Telemedicine also has the potential to facilitate close monitoring and communication required to bridge routine clinic-based care. Digital photography can provide update on the trajectory of foot ulcers and alert multidisciplinary team members of pre-ulcerative lesions. Two recent meta-analyses have indicated statistically similar healing time of DFU with telemedicine compared to clinic only appointments and a trend towards less costs but could not differentiate between the various technologies.111,112 Therefore, the optimal telemedicine options (photography, video, telephone, or a combination), the wound care expertise required to deliver such a program, and the ideal number of interval clinic visits remain to be determined.
Addressing Psychological and Cognitive Health in DFU
Despite the evidence supporting suboptimal DFU outcomes and lower quality of life,113 limited clinical consensus on optimal management exists. Ideally, all new patients referred to specialist foot centres should be assessed for the presence of depression and cognitive impairment. This may help teams to provide more nuanced care and refer for psychological support. While longitudinal assessment of depression would seem intuitive during and after DFU healing, there are no high-quality observational or preferably interventional studies or current consensus statements to guide practitioners. Future research should also be focussed on ascertaining if there are any resting central CNS adaptions/maladaptations which may impair ability recognise or intuitively respond to DFU occurrence.
Addressing Education
Proper education can help patients understand the importance of proper foot care and self-management. Patients should be educated on proper foot hygiene, how to inspect their feet for cuts or sores, the importance of regular check-ups with a healthcare professional, and the use of proper footwear. According to a Cochrane review, educating patients about foot care can have a positive impact on their knowledge and self-reported behaviour in the short term. However, there is a lack of strong evidence from current studies to support the effectiveness of patient education alone in achieving significant reductions in the incidence of ulcers and amputations with clinical relevance.114
Education should also be provided to healthcare professionals on the management of diabetic foot disease, including early detection, prevention, and treatment. This includes education on the use of bespoke shoes and orthotics, high-quality wound care, and the importance of early referral to a specialist clinic and multidisciplinary care.
Conclusion
In summary, the development of diabetic foot ulcers is influenced by both intrinsic factors such as neuropathy, peripheral vascular disease, and biomechanical impairments, as well as extrinsic factors such as footwear, plantar pressure, and shear stress, all playing a significant role. Recent research indicates that the brain also contributes to the development and influences clinical outcomes in diabetic foot disease, with conditions such as depression and cognitive impairment having a significant impact. Therefore, improving not only physical but also psychological health should be considered in the management of diabetic foot disease. However, our understanding of the brain-periphery nexus remains limited, and further research is necessary to fully comprehend this relationship. Management of diabetic foot disease should be undertaken through a multidisciplinary team approach. As remote monitoring and other objective foot assessment-based technologies continue to develop, they may provide an interesting paradigm for early identification, help refine referral pathway and direct connect patients with the right teams. All these fields of enquiry are evolving rapidly, and we hope this brings an improved outlook for individuals with diabetic foot ulceration.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88–93. doi:10.1016/j.jcot.2021.01.017
2. Armstrong DG, Boulton AJM, Bus SA, Ingelfinger JR. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376(24):2367–2375. doi:10.1056/NEJMra1615439
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. doi:10.1186/s13047-020-00383-2
4. Kerr M, Barron E, Chadwick P, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019;36(8):995–1002. doi:10.1111/dme.13973
5. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651–658. doi:10.2337/dc13-2176
6. Skrepnek GH, Mills JL, Lavery LA, Armstrong DG. Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the US. Diabetes Care. 2017;40(7):936–942. doi:10.2337/dc16-2189
7. Crawford F, McCowan C, Dimitrov BD, et al. The risk of foot ulceration in people with diabetes screened in community settings: findings from a cohort study. QJM. 2011;104(5):403–410. doi:10.1093/qjmed/hcq227
8. Abbott CA, Carrington AL, Ashe H, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19(5):377–384.
9. Henriksson F, Agardh CD, Berne C, et al. Direct medical costs for patients with type 2 diabetes in Sweden. J Intern Med. 2000;248(5):387–396. doi:10.1046/j.1365-2796.2000.00749.x
10. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med. 2017;49(2):106–116. doi:10.1080/07853890.2016.1231932
11. Metcalf L, Musgrove M, Bentley J, et al. Prevalence of active Charcot disease in the East Midlands of England. Diabet Med. 2018;35(10):1371–1374. doi:10.1111/dme.13679
12. O’Loughlin A, Kellegher E, McCusker C, Canavan R. Diabetic charcot neuroarthropathy: prevalence, demographics and outcome in a regional referral centre. Irish J Med Sci. 2017;186(1):151–156. doi:10.1007/s11845-016-1508-5
13. Salini D, Harish K, Minnie P, et al. Prevalence of charcot arthropathy in type 2 diabetes patients aged over 50 years with severe peripheral neuropathy: a retrospective study in a Tertiary Care South Indian Hospital. Indian J Endocrinol Metab. 2018;22(1):107–111. doi:10.4103/ijem.IJEM_257_17
14. Abbas ZG, Lutale JK, Formosa C, Gatt A, Chockalingam N. The charcot foot: an emerging public health problem for African Diabetes Patients. Int J Low Extrem Wounds. 2021;15347346211066684. doi:10.1177/15347346211066684
15. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036–1042. doi:10.2337/diacare.22.7.1036
16. Vas PR, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. Lancet Diabetes Endocrinol. 2016;4(9):723–725. doi:10.1016/S2213-8587(16)30063-8
17. Stevens MJ, Edmonds ME, Foster AV, Watkins PJ. Selective neuropathy and preserved vascular responses in the diabetic Charcot foot. Diabetologia. 1992;35(2):148–154. doi:10.1007/BF00402547
18. Khan A, Petropoulos IN, Ponirakis G, et al. Corneal confocal microscopy detects severe small fiber neuropathy in diabetic patients with Charcot neuroarthropathy. J Diabetes Investig. 2018;9(5):1167–1172. doi:10.1111/jdi.12806
19. Dehghani C, Russell AW, Perkins BA, et al. A rapid decline in corneal small fibers and occurrence of foot ulceration and Charcot foot. J Diabetes Complication. 2016;30(8):1437–1439. doi:10.1016/j.jdiacomp.2016.07.004
20. Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi:10.1056/NEJMoa032782
21. Chicharro-Luna E, Pomares-Gómez FJ, Ortega-ávila AB, Marchena-Rodríguez A, Blanquer-Gregori JFJ, Navarro-Flores E. Predictive model to identify the risk of losing protective sensibility of the foot in patients with diabetes mellitus. Int Wound J. 2020;17(1):220–227. doi:10.1111/iwj.13263
22. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921–929. doi:10.1016/j.jacc.2005.09.065
23. Formosa C, Cassar K, Gatt A, et al. Hidden dangers revealed by misdiagnosed peripheral arterial disease using ABPI measurement. Diabetes Res Clin Pract. 2013;102(2):112–116. doi:10.1016/j.diabres.2013.10.006
24. Manu CA, Freedman B, Rashid H, Winkley K, Edmonds ME. Peripheral arterial disease located in the feet of patients with diabetes and foot ulceration demands a new approach to the assessment of ischemia. Int J Lower Extremity Wounds. 2020;21(4)1534734620947979.
25. Hinchliffe RJ, Brownrigg JR, Apelqvist J, et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):37–44. doi:10.1002/dmrr.2698
26. Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol. 2010;4(4):833–845. doi:10.1177/193229681000400411
27. Formosa C, Gatt A, Chockalingam N. The importance of clinical biomechanical assessment of foot deformity and joint mobility in people living with type-2 diabetes within a primary care setting. Prim Care Diabetes. 2013;7(1):45–50. doi:10.1016/j.pcd.2012.12.003
28. Formosa C, Gatt A, Chockalingam N. Diabetic foot complications in Malta: prevalence of risk factors. Foot. 2012;22(4):294–297. doi:10.1016/j.foot.2012.08.008
29. Ledoux WR, Shofer JB, Smith DG, Sullivan K, Assal M, Reiber GE. Relationship between foot type, foot deformity, and ulcer occurrence in the high-risk diabetic foot. J Rehabilitat Res Dev. 2005;42(5):665–672. doi:10.1682/JRRD.2004.11.0144
30. Segal A, Rohr E, Orendurff M, Shofer J, O’Brien M, Sangeorzan B. The Effect of Walking Speed on Peak Plantar Pressure. Foot Ankle Int. 2004;25(12):926–933. doi:10.1177/107110070402501215
31. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26(5):1435–1438. doi:10.2337/diacare.26.5.1435
32. Salsich GB, Mueller MJ, Sahrmann SA. Passive ankle stiffness in subjects with diabetes and peripheral neuropathy versus an age-matched comparison group. Phys Ther. 2000;80(4):352–362. doi:10.1093/ptj/80.4.352
33. Williams DB, Brunt D, Tanenberg RJ. Diabetic neuropathy is related to joint stiffness during late stance phase. J Appl Biomech. 2007;23(4):251–260. doi:10.1123/jab.23.4.251
34. Simon-Perez E, Simon-Perez C, Alonso-Pena D, et al. Stiffness degree of ankle range of motion in diabetic patients with atypical amputation. Revista da Associacao Medica Brasileira. 2020;66(2):216–221. doi:10.1590/1806-9282.66.2.216
35. Chatzistergos P, Naemi R, Chockalingam N. The role of tissue biomechanics in improving the clinical management of diabetic foot ulcers. In: The Science, Etiology and Mechanobiology of Diabetes and Its Complications. Elsevier; 2021:123–141.
36. Kwak Y, Kim J, Lee KM, Koo S. Increase of stiffness in plantar fat tissue in diabetic patients. J Biomech. 2020;107:109857. doi:10.1016/j.jbiomech.2020.109857
37. Lazzarini PA, Jarl G, Gooday C, et al. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3275. doi:10.1002/dmrr.3275
38. Healy A, Naemi R, Chockalingam N. The effectiveness of footwear and other removable off-loading devices in the treatment of diabetic foot ulcers: a systematic review. Curr Diabetes Rev. 2014;10(4):215–230. doi:10.2174/1573399810666140918121438
39. Chatzistergos PE, Naemi R, Chockalingam N. A method for subject-specific modelling and optimisation of the cushioning properties of insole materials used in diabetic footwear. Med Eng Phys. 2015;37(6):531–538. doi:10.1016/j.medengphy.2015.03.009
40. Chatzistergos PE, Naemi R, Sundar L, Ramachandran A, Chockalingam N. The relationship between the mechanical properties of heel-pad and common clinical measures associated with foot ulcers in patients with diabetes. J Diabetes Complication. 2014;28(4):488–493. doi:10.1016/j.jdiacomp.2014.03.011
41. Naemi R, Chatzistergos P, Suresh S, Sundar L, Chockalingam N, Ramachandran A. Can plantar soft tissue mechanics enhance prognosis of diabetic foot ulcer? Diabetes Res Clin Pract. 2017;126:182–191. doi:10.1016/j.diabres.2017.02.002
42. Ndip A, Rutter MK, Vileikyte L, et al. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care. 2010;33(8):1811–1816. doi:10.2337/dc10-0255
43. Lavery LA, Lavery DC, Hunt NA, La Fontaine J, Ndip A, Boulton AJ. Amputations and foot-related hospitalisations disproportionately affect dialysis patients. Int Wound J. 2015;12(5):523–526. doi:10.1111/iwj.12146
44. Tesfaye S, Selvarajah D. The Eurodiab study: what has this taught us about diabetic peripheral neuropathy? Curr Diab Rep. 2009;9(6):432–434. doi:10.1007/s11892-009-0070-1
45. Meloni M, Izzo V, Giurato L, Cervelli V, Gandini R, Uccioli L. Impact of heart failure and dialysis in the prognosis of diabetic patients with ischemic foot ulcers. Journal of Clinical and Translational Endocrinology. 2018;11:31–35. doi:10.1016/j.jcte.2018.01.002
46. Dietrich I, Braga GA, de Melo FG, da Costa Silva Silva ACC. The diabetic foot as a proxy for cardiovascular events and mortality review. Curr Atherosclerosis Rep. 2017;19(11):1–5. doi:10.1007/s11883-017-0680-z
47. Apelqvist J, Larsson J, Agardh CD. The influence of external precipitating factors and peripheral neuropathy on the development and outcome of diabetic foot ulcers. J Diabet Complication. 1990;4(1):21–25. doi:10.1016/0891-6632(90)90060-I
48. Pan A, Lucas M, Sun Q, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170(21):1884–1891. doi:10.1001/archinternmed.2010.356
49. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi:10.2337/dc08-0985
50. Demakakos P, Pierce MB, Hardy R. Depressive symptoms and risk of type 2 diabetes in a national sample of middle-aged and older adults: the English longitudinal study of aging. Diabetes Care. 2010;33(4):792–797. doi:10.2337/dc09-1663
51. Darwish L, Beroncal E, Sison MV, Swardfager W. Depression in people with type 2 diabetes: current perspectives. Diabetes Metab Syndr Obes. 2018;11:333–343. doi:10.2147/DMSO.S106797
52. Ismail K, Winkley K, Stahl D, Chalder T, Edmonds M. A cohort study of people with diabetes and their first foot ulcer: the role of depression on mortality. Diabetes Care. 2007;30(6):1473–1479. doi:10.2337/dc06-2313
53. Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi:10.2337/diacare.28.10.2378
54. Williams LH, Rutter CM, Katon WJ, et al. Depression and incident diabetic foot ulcers: a prospective cohort study. Am J Med. 2010;123(8):748–54 e3. doi:10.1016/j.amjmed.2010.01.023
55. Winkley K, Sallis H, Kariyawasam D, et al. Five-year follow-up of a cohort of people with their first diabetic foot ulcer: the persistent effect of depression on mortality. Diabetologia. 2012;55(2):303–310. doi:10.1007/s00125-011-2359-2
56. Jiang F-H, Liu X-M, H-R Y, Qian Y, Chen H-L. The incidence of depression in patients with diabetic foot ulcers: a systematic review and meta-analysis. Int J Lower Extremity Wounds. 2022;21(2):161–173. doi:10.1177/1534734620929892
57. Gonzalez JS, Vileikyte L, Ulbrecht JS, et al. Depression predicts first but not recurrent diabetic foot ulcers. Diabetologia. 2010;53(10):2241–2248. doi:10.1007/s00125-010-1821-x
58. Moran C, Beare R, Phan T, et al. Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. J Alzheimers Dis. 2017;59(2):405–419. doi:10.3233/JAD-161166
59. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8(6):535–545. doi:10.1016/S2213-8587(20)30118-2
60. Moran C, Than S, Callisaya M, Beare R, Srikanth V. New horizons-cognitive dysfunction associated with type 2 diabetes. J Clin Endocrinol Metab. 2022;107(4):929–942. doi:10.1210/clinem/dgab797
61. Rawlings AM, Sharrett AR, Albert MS, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC Study. Diabetes Care. 2019;42(7):1248–1254. doi:10.2337/dc19-0120
62. Natovich R, Kushnir T, Harman-Boehm I, et al. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care. 2016;39(7):1202–1207. doi:10.2337/dc15-2838
63. Amiel SA. The consequences of hypoglycaemia. Diabetologia. 2021;64(5):963–970. doi:10.1007/s00125-020-05366-3
64. Bus SA, Lavery LA, Monteiro‐Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metabol Res Rev. 2020;36(S1):e3269. doi:10.1002/dmrr.3269
65. Singh AK, Gillies CL, Singh R, et al. Prevalence of comorbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915–1924. doi:10.1111/dom.14124
66. Patel J, Zamzam A, Syed M, et al. A scoping review of foot screening in adults with diabetes mellitus across Canada. Can J Diabetes. 2022;46(5):435–440.e2. doi:10.1016/j.jcjd.2022.01.004
67. Chevreul K, Berg Brigham K, Bouche C. The burden and treatment of diabetes in France. Global Health. 2014;10(1):6. doi:10.1186/1744-8603-10-6
68. Alonso-Fernandez M, Mediavilla-Bravo JJ, Lopez-Simarro F, et al. Evaluation of diabetic foot screening in Primary Care. Endocrinol Nutr. 2014;61(6):311–317. doi:10.1016/j.endonu.2014.01.007
69. Alkhouli M, Alqahtani F, Elsisy MF, Kawsara A, Alasnag M. Incidence and outcomes of acute ischemic stroke following percutaneous coronary interventions in men versus women. Am J Cardiol. 2020;125(3):336–340. doi:10.1016/j.amjcard.2019.10.045
70. Lawrenson JG, Bourmpaki E, Bunce C, et al. Trends in diabetic retinopathy screening attendance and associations with vision impairment attributable to diabetes in a large nationwide cohort. Diabet Med. 2021;38(4):e14425. doi:10.1111/dme.14425
71. Walton DV, Edmonds ME, Bates M, Vas PRJ, Petrova NL, Manu CA. People living with diabetes are unaware of their foot risk status or why they are referred to a multidisciplinary foot team. J Wound Care. 2021;30(8):598–603. doi:10.12968/jowc.2021.30.8.598
72. Schaper NC, van Netten JJ, Apelqvist J, et al. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3266. doi:10.1002/dmrr.3266
73. Manu C, Lacopi E, Bouillet B, et al. Delayed referral of patients with diabetic foot ulcers across Europe: patterns between primary care and specialised units. J Wound Care. 2018;27(3):186–192. doi:10.12968/jowc.2018.27.3.186
74. Musuuza J, Sutherland BL, Kurter S, Balasubramanian P, Bartels CM, Brennan MB. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J Vasc Surg. 2020;71(4):1433–46. e3. doi:10.1016/j.jvs.2019.08.244
75. Vas PRJ, Edmonds M, Kavarthapu V, et al. The diabetic foot attack: “Tis Too Late to Retreat!”. Int J Low Extrem Wounds. 2018;17(1):7–13. doi:10.1177/1534734618755582
76. Vainieri E, Ahluwalia R, Slim H, et al. Outcomes after emergency admission with a diabetic foot attack indicate a high rate of healing and limb salvage but increased mortality: 18-month follow-up study. Exp Clin Endocrinol Diabetes. 2022;130(3):165–171. doi:10.1055/a-1322-4811
77. Meloni M, Izzo V, Manu C, et al. Fast-track pathway: an easy-to-use tool to reduce delayed referral and amputations in diabetic patients with foot ulceration. Diabetic Foot. 2019;22(2):39.
78. Bus SA, van Netten JJ, Lavery LA, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):16–24. doi:10.1002/dmrr.2696
79. Frykberg RG, Attinger C, Smeets L, Koller A, Bal A, Kavarthapu V. Surgical strategies for prevention of amputation of the diabetic foot. J Clin Orthop Trauma. 2021;17:99–105. doi:10.1016/j.jcot.2021.02.019
80. Hinchliffe RJ, Forsythe RO, Apelqvist J, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3276. doi:10.1002/dmrr.3276
81. Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994–2004. doi:10.1056/NEJMoa2000052
82. Healy A, Dunning DN, Chockalingam N. Effect of insole material on lower limb kinematics and plantar pressures during treadmill walking. Prosthet Orthot Int. 2012;36(1):53–62. doi:10.1177/0309364611429986
83. Healy A, Farmer S, Pandyan A, Chockalingam N. A systematic review of randomised controlled trials assessing effectiveness of prosthetic and orthotic interventions. PLoS One. 2018;13(3):e0192094. doi:10.1371/journal.pone.0192094
84. Burns J, Wegener C, Begg L, Vicaretti M, Fletcher J. Randomized trial of custom orthoses and footwear on foot pain and plantar pressure in diabetic peripheral arterial disease. Diabet Med. 2009;26(9):893–899. doi:10.1111/j.1464-5491.2009.02799.x
85. Rizzo L, Tedeschi A, Fallani E, et al. Custom-made orthesis and shoes in a structured follow-up program reduces the incidence of neuropathic ulcers in high-risk diabetic foot patients. Int J Lower Extremity Wounds. 2012;11(1):59–64. doi:10.1177/1534734612438729
86. Zwaferink JBJ, Custers W, Paardekooper I, Berendsen HA, Bus SA, Jan Y-K. Optimizing footwear for the diabetic foot: data-driven custom-made footwear concepts and their effect on pressure relief to prevent diabetic foot ulceration. PLoS One. 2020;15(4):e0224010. doi:10.1371/journal.pone.0224010
87. Waaijman R, Keukenkamp R, de Haart M, Polomski WP, Nollet F, Bus SA. Adherence to wearing prescription custom-made footwear in patients with diabetes at high risk for plantar foot ulceration. Diabetes Care. 2013;36(6):1613–1618. doi:10.2337/dc12-1330
88. Jarl G, Tranberg R, Johansson U, Alnemo J, Lundqvist LO. Predictors of adherence to wearing therapeutic footwear among people with diabetes. J Foot Ankle Res. 2020;13(1):45. doi:10.1186/s13047-020-00413-z
89. Formosa C, Gatt A, Chockalingam N. A Critical Evaluation of Existing Diabetic Foot Screening Guidelines. Rev Diabet Stud. 2016;13(2–3):158–186. doi:10.1900/RDS.2016.13.158
90. van Netten JJ, Price PE, Lavery LA, et al. Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(Suppl 1):84–98. doi:10.1002/dmrr.2701
91. Hernandez-Contreras D, Peregrina-Barreto H, Rangel-Magdaleno J, Gonzalez-Bernal J. Narrative review: diabetic foot and infrared thermography. Infrared Phys Technol. 2016;78:105–117. doi:10.1016/j.infrared.2016.07.013
92. Petrova NL, Donaldson NK, Tang W, et al. Infrared thermography and ulcer prevention in the high-risk diabetic foot: data from a single-blind multicentre controlled clinical trial. Diabet Med. 2020;37(1):95–104. doi:10.1111/dme.14152
93. Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a smart mat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973–980. doi:10.2337/dc16-2294
94. Abbott CA, Chatwin KE, Foden P, et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: a prospective, randomised, proof-of-concept study. Lancet Digit Health. 2019;1(6):e308–e318. doi:10.1016/S2589-7500(19)30128-1
95. Bus SA, Sacco ICN, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev;2023. e3651. doi:10.1002/dmrr.3651
96. Kurkela O, Lahtela J, Arffman M, Forma L. Infrared thermography compared to standard care in the prevention and care of diabetic foot: a cost analysis utilizing real-world data and an expert panel. Clinicoecon Outcomes Res. 2023;15:111–123. doi:10.2147/CEOR.S396137
97. El-Nahas M, El-Shazly S, El-Gamel F, Motawea M, Kyrillos F, Idrees H. Relationship between skin temperature monitoring with Smart Socks and plantar pressure distribution: a pilot study. J Wound Care. 2018;27(8):536–541. doi:10.12968/jowc.2018.27.8.536
98. Najafi B, Mohseni H, Grewal GS, Talal TK, Menzies RA, Armstrong DG. An Optical-Fiber-Based Smart Textile (Smart Socks) to manage biomechanical risk factors associated with diabetic foot amputation. J Diabetes Sci Technol. 2017;11(4):668–677. doi:10.1177/1932296817709022
99. Armstrong DG. Subscription prescription: remote patient monitoring using smart shoes, socks and insoles. J Wound Care. 2019;28(Sup9):S3. doi:10.12968/jowc.2019.28.Sup9.S3
100. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi:10.2337/dc16-1728
101. Dasanayake IS, Bevier WC, Castorino K, et al. Early detection of physical activity for people with type 1 diabetes mellitus. J Diabetes Sci Technol. 2015;9(6):1236–1245. doi:10.1177/1932296815592409
102. Armstrong DG, Lavery LA, Holtz-Neiderer K, et al. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27(8):1980–1984. doi:10.2337/diacare.27.8.1980
103. Bus SA, Aan de Stegge WB, van Baal JG, Busch-Westbroek TE, Nollet F, van Netten JJ. Effectiveness of at-home skin temperature monitoring in reducing the incidence of foot ulcer recurrence in people with diabetes: a multicenter randomized controlled trial (DIATEMP). BMJ Open Diabetes Res Care. 2021;9(1):e002392. doi:10.1136/bmjdrc-2021-002392
104. Filipe V, Teixeira P, Teixeira A. Automatic classification of foot thermograms using machine learning techniques. Algorithms. 2022;15(7):236. doi:10.3390/a15070236
105. Chatzistergos PE, Chockalingam N. A novel concept for low-cost non-electronic detection of overloading in the foot during activities of daily living. Royal Soc Open Sci. 2021;8(6):202035. doi:10.1098/rsos.202035
106. Perren S, Formosa C, Camilleri L, Chockalingam N, Gatt A. The thermo-pressure concept: a new model in diabetic foot risk stratification. Appl Sci. 2021;11(16):7473. doi:10.3390/app11167473
107. Walk With Path. Path Feel. An innovative solution for sensory deficit. Available from: https://www.walkwithpath.com/pages/path-feel-for-professionals.
108. Ipp E, Liljenquist D, Bode B, et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA network open. 2021;4(11):e2134254. doi:10.1001/jamanetworkopen.2021.34254
109. Ellahham S. Artificial Intelligence: the future for diabetes care. Am J Med. 2020;133(8):895–900. doi:10.1016/j.amjmed.2020.03.033
110. Shao H, Shi L, Lin Y, Fonseca V. Using modern risk engines and machine learning/artificial intelligence to predict diabetes complications: a focus on the BRAVO model. J diabet complicat. 2022;36(11):108316. doi:10.1016/j.jdiacomp.2022.108316
111. Tchero H, Noubou L, Becsangele B, Mukisi-Mukaza M, Retali G-R, Rusch E. Telemedicine in diabetic foot care: a systematic literature review of interventions and meta-analysis of controlled trials. Int J Lower Extremity Wounds. 2017;16(4):274–283. doi:10.1177/1534734617739195
112. Yammine K, Estephan M. Telemedicine and diabetic foot ulcer outcomes. A meta-analysis of controlled trials. Foot. 2022;50:101872. doi:10.1016/j.foot.2021.101872
113. Pedras S, Carvalho R, Pereira MG. Predictors of quality of life in patients with diabetic foot ulcer: the role of anxiety, depression, and functionality. J Health Psychol. 2018;23(11):1488–1498. doi:10.1177/1359105316656769
114. Dorresteijn JAN, Kriegsman DMW, Assendelft WJJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2012. doi:10.1002/14651858.CD001488.pub4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





