Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
Improvement of skin conditions by ingestion of Aspergillus kawachii (Koji) extract containing 14-dehydroergosterol in a randomized, double-blind, controlled trial
Authors Sugihara Y, Ikushima S, Miyake M, Kirisako T, Yada Y, Fujiwara D
Received 30 September 2017
Accepted for publication 15 December 2017
Published 9 March 2018 Volume 2018:11 Pages 115—124
DOI https://doi.org/10.2147/CCID.S152979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yoshihiko Sugihara,1 Shigehito Ikushima,1 Mika Miyake,1 Takayoshi Kirisako,2 Yukihiro Yada,3 Daisuke Fujiwara2
1Research Laboratories for Health Science and Food Technologies, 2Central Laboratories for Key Technologies, Research and Development Division, Kirin Company, Limited, Yokohama, 3Doctorate Program in Human Biology, School of Integrative and Global Majors, University of Tsukuba, Tsukuba, Japan
Purpose: The present study examined the effect of ingestion of Koji extract containing 14-dehydroergosterol (14-DHE), prepared from Aspergillus kawachii NBRC4308, on improvement of skin conditions among healthy volunteers.
Subjects and methods: In a randomized, double-blind, placebo-controlled, parallel-group study, 70 healthy adult women who felt that their skin was dry ingested either a placebo dietary supplement or Koji extract (200 mg/day) supplement containing 0.1% 14-DHE for 12 weeks. Throughout the treatment period and for 4 weeks afterward, objective indicators – including moisture content of the stratum corneum, trans-epidermal water loss (TEWL), and skin wrinkles – were evaluated; in addition, the subjects answered a questionnaire on their skin conditions with ratings on a visual analog scale. Statistical analysis was conducted on the basis of differences from baseline scores.
Results: Compared with the placebo group, the Koji extract group showed significantly increased forearm moisture at 4, 8, and 16 weeks (p < 0.05 on unpaired t-test). The questionnaire survey showed a marked improvement in skin conditions, particularly crow’s feet, in the Koji extract group versus the placebo group at 8 weeks (p < 0.05 by unpaired t-test). Furthermore, the Koji extract group showed a trend (p < 0.10) toward improvement in skin moisture (at 4 weeks), dryness around the eyes/mouth (at 4 weeks), and overall skin condition (at 8 weeks) versus the placebo group.
Conclusion: Ingestion of Koji extract containing 14-DHE was demonstrated to have positive effects toward improving skin conditions – in particular, on increasing skin moisture in the stratum corneum.
Keywords: 14-dehydroergosterol, Aspergillus, Koji, skin, skin moisture, TEWL
Introduction
Skin plays a significant role as a barrier that prevents water loss from the body, and is continually exposed to a variety of stimuli such as dryness, ultraviolet (UV) radiation, and chemical agents, all of which can damage both the skin surface and internal structures. Notably, the aging process is known to lead to upregulation of inflammation,1–5 which occurs in response to the abovementioned stimuli and can result in disturbed turnover and roughness of skin related to decreased amounts of cellular components, including glycerolipids, natural moisturizing factors, and specific salts.6,7 Compared with internal organs, it is relatively easy to self-diagnose skin conditions such as dryness and wrinkles, and these conditions can have a huge impact on quality of life. As a result, skin dryness along with wrinkles represents one of the strongest areas in the skincare market,8,9 and there remains a huge demand for more effective ways to improve these conditions, despite numerous commercially available skincare products containing compounds such as hyaluronan, glucosylceramide, and collagen.10–12
Recently, a traditional Japanese cuisine called Washoku has been gathering interest internationally as well as in Japan, and has been certified as UNESCO’s Intangible Cultural Heritage of Humanity owing to its outstanding principles – namely, Washoku is an exceptionally well-balanced, respectful, and healthy way to produce and consume food.13 Notably, the cuisine incorporates fermented foods and seasonings such as miso, soy source, and sake, which are commonly made with Koji – the Japanese name for the fungus Aspergillus.14 Industrially, Aspergillus has great value because of its prominent characteristics, such as the capability to produce useful protease and carbohydrase enzymes in large quantities. Therefore, many studies have been conducted worldwide on Aspergillus at the molecular level;15,16 however, there are only a limited number of reports on the health benefits of Koji and its metabolites. For instance, some of the active metabolites of Aspergillus or Monascus fungus include β-glucan for immune activation,17 pyranonigrin A for antioxidation,18 and 4-aminobutanoic acid for anti-stress effects.19 Recently, we identified 14-dehydroergosterol (14-DHE, C28H42O) from Aspergillus awamori as a novel anti-inflammatory agent that induces regulatory T cells.20,21 14-DHE was initially isolated as by-product in cultures of Aspergillus niger;22 however, no biological activity was reported. In a previous study, injection of 14-DHE in mice significantly improved the clinical score and inflammatory response in a model of multiple sclerosis (experimental autoimmune encephalopathy).20,21 Additionally, 14-DHE suppressed the inflammatory phenotype in dendritic cells derived from human peripheral blood mononuclear cells in response to lipopolysaccharide and interferon-γ stimulation.21 Furthermore, 14-DHE has been shown in vitro to protect keratinocytes against UVA radiation.23
The aim of the present study was to assess the effects of the oral administration of Koji extract containing 14-DHE in humans for the first time. To this end, the effects of 14–DHE-containing Koji extract on skin conditions were investigated by a randomized, double-blind, placebo-controlled, parallel-group experiment.
Materials and methods
This clinical trial was conducted in accordance with the principles of the Declaration of Helsinki, and followed Japanese Ministry of Health and Welfare Ordinance No. 28, Standards of Implementation of the Clinical Trial of a Pharmaceutical (March 27, Heisei 9). Thus, the trial obtained approval from the Ethics Committee of the Oriental Ueno Detection Center, General Incorporated Association Oriental Occupational Health Association Tokyo Branch. Informed consent was obtained in writing from each volunteer prior to enrollment in the study. The study received no specific grant or funding from external organizations.
Samples used in the trial
Two kinds of dietary samples in soft gelatin capsules were prepared for the study – one was a placebo containing 200 mg rapeseed oil, and the other contained Koji extract comprising 0.1% 14-DHE dissolved in 200 mg rapeseed oil. The cultivated fungus body of Aspergillus kawachii was first extracted with 59% ethanol; then, rapeseed oil was added, and the oil fraction was collected as Koji extract. The concentration of 14-DHE in the Koji extract was determined by high-performance liquid chromatography (Shimadzu, Kyoto, Japan) using a C30-UG-5 column (Develosil, 10 mm × 250 mm; Nomura Chemical Co. Ltd., Aichi, Japan) with an acetonitrile–isopropanol (99:1) gradient as described by Ano et al.21 The nutritional content of this sample was: energy, 1.8 kcal; lipids, 0.2 g; and carbohydrate, protein, alcohol, moisture, and ash, 0 g per capsule.
Subjects
The study enrolled healthy Japanese female volunteers (aged 30–49 years) who were conscious of dry skin and fine wrinkles. The volunteers were recruited as follows (Figure 1). First, 157 potential candidates were recruited from a sample of healthy female volunteers; 23 candidates were then excluded after screening on the basis of the exclusion criteria (Table 1). Lastly, 70 subjects from the remaining 134 were selected for the study after additional screening on the basis of low moisture content in the stratum corneum, high trans-epidermal water loss (TEWL) of their left cheek, grade 1–3 wrinkles by clinical diagnosis,24 and high score related to moisture via a visual analog scale (VAS).
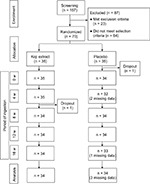  | Figure 1 Summary flow chart of the study design. Notes: “Missing data” means a subject was absent for the evaluation. Abbreviation: w, week. |
  | Table 1 Exclusion criteria in the study |
Study design
The trial was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (no. UMIN000019758). The present study was designed as a randomized, double-blind, placebo-controlled, parallel-group trial, and was planned and conducted by TES Holdings Co., Ltd. Stratified block randomization was carried out to allocate the 70 study subjects to either the placebo group (n = 35) or the Koji group (n = 35). Specifically, the subjects were divided into two strata based on median age and median value of moisture in the stratum corneum and TEWL, and were then allocated to a group randomly with the use of computer-generated randomization numbers. Then, the trial was implemented in a double-blind and placebo-controlled manner. Both the data monitor and data analyst were blinded to the treatment assignment until the analyses were completed.
The two kinds of dietary capsules used in the study appeared identical. The personnel managing test foods in Kirin Company printed a mark (A or B) on the respective outer boxes enclosing the capsules, and delivered both the capsules and the identifying code table to the assignment manager, Akiyoshi Sawabe (Department of Food Science and Nutrition, Faculty of Agriculture, Kindai University). The assignment personnel confirmed that there were no differences in appearance between the two types of capsules, and then delivered them to the testing agency. The assignment list was not opened until the data had been entered and analyzed to ensure effective blinding.
The subjects ingested one capsule of either the placebo or Koji extract containing 14-DHE per day for 12 weeks. During the study, the 70 subjects were instructed to visit the hospital to have an evaluation of skin conditions by a medical doctor five times: weeks 0 (baseline), 4, 8, and 12 during the ingestion period, as well as week 16, corresponding to 4 weeks after the period of ingestion. As primary outcomes, skin moisture, TEWL, wrinkle grade, and skin conditions by VAS were evaluated. Before all measurements and skin examinations, subjects washed their face and then rested for 20 min in a waiting room kept under mild environmental conditions (room temperature, 21 ± 1°C; relative humidity, 50 ± 10%) in order to maintain homogeneous environmental and measurement conditions as far as possible. Subjects were instructed to comply with the following points during the trial: (1) avoid excessive eating and exercise; (2) have enough sleep; (3) use cosmetics and supplements in the same manner as they did before the trial; and (4) avoid outdoor activities under sunlight without protecting the measurement areas against UV radiation, such as by wearing clothes, a hat, a sunscreen, etc. The trial was carried out between November, 2015 and May, 2016 at the laboratories of TES Holdings Co., Ltd.
Subjective evaluations
A VAS was used to subjectively evaluate how the subjects felt about their skin conditions. The objective score was obtained by a scaled length ranging from 0 to 100 mm (0, highest possible outcome; 100, lowest possible outcome). The following criteria were evaluated by the subjects through the VAS: overall skin condition, skin moisture, dryness of area around the eyes and mouth, crow’s feet, skin smoothness, skin brightness, skin dullness, skin redness, skin spots, and skin elasticity and firmness.
Skin moisture
Skin moisture in the stratum corneum was measured using a Corneometer® CM825 instrument (Courage + Khazaka Electronic GmbH, Cologne, Germany) at three points: left cheek bone area, left mouth corner, and left forearm. Each value recorded in this test was taken as the average of three measurements. Note that the Corneometer outputs a score in arbitrary units (a.u.) because the instrument measures variation in electrostatic capacity, which depends on the moisture content of the stratum corneum.
Trans-epidermal water loss
TEWL is a suitable indicator to evaluate barrier function in the stratum corneum. TEWL scores (g/h/m2) were obtained by using a Tewameter® TM300 instrument (Courage + Khazaka Electric GmbH) at three points: left cheek bone area, left mouth corner, and left forearm.
Wrinkle evaluation
The study evaluated wrinkles – defined as crevices extending into the dermal and subcutaneous layers25 – in the eye area by using the method of a skin replica at the inferolateral margin, 5 and 10 mm from the corner of the right eye. More specifically, a limited area of 10 mm × 10 mm in each replica sample was scanned by using an image analysis system ASA-03RXD (Asahibiomed Co., Ltd, Tokyo, Japan), which yielded the following parameters: area rate, volume rate, average depth (total wrinkle), average depth (maximum wrinkle), maximum depth (maximum wrinkle), maximum depth (total wrinkle), and number of wrinkles.
Statistical analysis
The sample size was calculated under the assumption that TEWL values would be similar to those in previous reports.26–28 It was calculated that, for a size of 29 in each group, statistical differences would be observed between the two groups, especially in TEWL values at 12 weeks as compared with the baseline period, assuming an average difference of 3.8 and a standard deviation of 5. Thus, it was considered sufficient to have 35 subjects per group for precise evaluation.
All data values were reported as the mean ± SE. Unpaired t-test was used to compare the analytical data (with baseline values subtracted) between the two groups (i.e., placebo and Koji extract). All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) or SPSS 19 (IBM, Armonk, NY, USA). A p-value of <0.05 was considered to be statistically significant, whereas a value between 0.05 and <0.10 was considered to be a trend toward significance.
Results
Baseline data
No adverse event related to the test supplement was reported throughout the course of the study. With respect to the number of subjects during the trial (Figure 1), one subject in the placebo group left the trial just before it began for health reasons. In addition, data for two subjects in the placebo group were missing at 4 weeks and for one subject at 16 weeks due to private matters. In the Koji group, one subject left the trial after 8 weeks due to personal reasons. Thus, 68 subjects (age [mean ± SD] 41.1 ± 5.5 years) completed the trial (per-protocol set), and with a mean age of 40.9 ± 5.6 years (placebo, n = 34) and 41.3 ± 5.5 years (Koji, n = 34).
Questionnaire survey to determine subjective skin symptoms
The volunteers evaluated their symptoms of skin conditions subjectively through the VAS system by responding to a questionnaire (Table 2). As a result, crow’s feet at 8 weeks were significantly decreased in the Koji group as compared with the placebo group (p < 0.05, Table 2). In addition, the Koji group showed a trend (p < 0.10) toward better skin moisture (4 weeks), dryness around eyes/mouth (4 weeks), and overall skin condition (8 weeks) as compared with the placebo group. This subjective evaluation, which was scored by the volunteers themselves, was followed by an objective evaluation with instruments as described further.
  | Table 2 Changes in the subjective evaluation of skin conditions with time following ingestion of Koji extract and placebo Note: *p < 0.05 (unpaired Student’s t-test between the two groups). |
Skin moisture in the stratum corneum and TEWL
Skin moisture content in the stratum corneum was determined by a Corneometer (Figure 2). No significant difference was observed between the two groups in the left cheek bone area or the left mouth corner. However, moisture (variance from the baseline score) in the left forearm was significantly improved at 4, 8, and 16 weeks in the Koji group as compared with placebo group (p < 0.05).
The same three skin areas were subjected to TEWL evaluation (Figure 3). No differences in values were observed between the two groups.
Evaluation of wrinkle with replica analysis
Fine wrinkles were evaluated by measurements made at 5- and 10-mm distances from the corner from the right eye (Table 3). Statistical analysis did not detect convincing differences in most loci evaluated by the replica test, but a significant difference (p < 0.05) in average wrinkle depth was found at a spot situated 10 mm from the corner of the eye at 12 weeks. This finding was inconsistent with the VAS data, which indicated that wrinkles improved in the Koji group.
Discussion
The present study has revealed, for the first time, that Koji extract containing 14-DHE is beneficial for maintaining healthy skin conditions in humans. Briefly, ingestion of a supplement containing Koji extract for 12 weeks significantly improved the skin conditions of healthy females (aged 30–49 years) who were conscious of dry skin and wrinkles. On the other hand, there was no difference in the TEWL score, indicative of barrier function between the placebo and the Koji groups (Figure 3). However, the TEWL score provides valuable information because the skin of the subjects in the Koji group was considered to be in a healthy condition at baseline. While skin moisture content was maintained or increased in the Koji group, the placebo group showed decreasing skin moisture content and their skin was considered to have become dry, which might lead to increased TEWL – in other words, disruption of the barrier function of the skin. On the other hand, the study enrolled healthy female volunteers with a healthy skin barrier; as a result, the TEWL score did not change considerably from baseline and no difference was statistically detected between the two groups, which is reasonable and supports the examination design used in the study.
As compared with the placebo group, the Koji group showed a trend toward significance (p < 0.10) in the VAS score of skin moisture or dryness around the eyes and mouth (Table 2), and a significant increase (p < 0.05) in the skin moisture content of the forearm (Figure 2). These consistent results indicate that the subjects confidently found an improvement in skin dryness. In general, only topical external applications to the forearm have an effect on the forearm. Here, however, ingestion of Koji extract had a moisturizing effect on forearm skin. Therefore, oral administration of 14-DHE might induce a systemic reaction and have an effect on the whole body. Further studies on the kinetics of 14-DHE in blood will be necessary to verify such an effect. This result can be rationalized because 14-DHE has been shown to protect cells against UVA radiation in vitro with increased transcription of genes involved in ceramide formation from sphingolipids/cholesterol-rich lipid rafts,6 whereas excess inflammation is known to have an unfavorable impact on the skin – for example, by reducing natural moisturizing factors and skin turnover.7 Indeed, ingestion of anti-inflammatory substances from aloe vera has been reported to increase skin moisture.29,30 These observations reasonably lead us to consider that the anti-inflammatory effect of 14-DHE contributed to the significant improvement in skin moisture in the present study. Furthermore, as an anti-inflammatory substance, 14-DHE might improve age-related phenotypes other than skin conditions.31–35
Previously, glucosylceramide from A. kawachii was shown to have a beneficial effect on skin conditions.36 However, an analysis of Koji extract did not detect the presence of glucosylceramide (data not shown); thus, it is likely that 14-DHE contributed to the improvements in skin conditions observed in this study. Although we did not elucidate the pharmacokinetics of 14-DHE, it seems reasonable to consider that the skin moisture content was improved owing to the anti-inflammation competence of 14-DHE in the Koji extract.
It is necessary to consider why the subjects in the Koji group showed a tendency toward better skin conditions (Table 2). Taking the result of the VAS questions into consideration, the improvement in score might be attributed to an improved feeling, not only on skin dryness but also on wrinkles. Indeed, the VAS score on crow’s feet was significantly improved in the Koji group after 8 weeks of consumption (Table 2). However, inconsistent with the VAS results, the replica analysis did not show a convincing improvement in wrinkles (Table 3). The results showed that there are substantial difficulties in evaluating improvements in wrinkles by replica analysis, which might be due to the following two reasons: (1) the replica analysis was not powerful enough to detect the depth and width of wrinkles as compared with a contactless optical tomographic imaging method; and/or (2) measurements of the roughness and wrinkles of the skin surface may have been smoothed because two replica samples are collected.37 Thus, it is necessary to further examine wrinkle conditions in a different manner, such as through an optical tomographic imaging method. Nevertheless, it was noted that only a single item in the replica analysis showed a worse score from the baseline in the Koji group (Table 3); a possible reason for this was skin irritation, which one of the subjects reported due to the replica patch. In future studies, exclusion of subjects with sensitive skin might provide a more stable replica evaluation of fine wrinkles.
It is important to consider the limitations of this study including the sample size (n = 35 per group), which might not be sufficient to assert a definitive conclusion as compared with previous studies.26–28 In other words, further studies should be carried out to statistically validate the effects of the Koji extract. However, it was important to set solid criteria for the subjects to conduct a convincing trial. In general, women pay more attention to their skin conditions as compared with men, and women younger than 30 years are less conscious of skin troubles, such as dry skin and wrinkles, than women aged 30 years or older, who were considered suitable for this trial. On the other hand, it was considered unreasonable to enroll females older than 50 years, who are more likely to have deteriorating skin conditions due to menopause. Nonetheless, there are many healthy people with skin concerns such as dryness even between the ages of 30 and 50 years, and the effect of Koji extract on improving skin moisture content is expected to be widely applicable to people in general.
In summary, this study has given new insight into the health benefits of metabolites in Koji, which is used in the traditional Japanese cuisine Washoku that is considered to provide a nutritionally balanced diet. It might be possible that Japanese people have so far received these benefits and have also noted the impressive competence of Koji. Together with this report, further studies on Koji are expected to lead to a healthier life for people.
Acknowledgments
The authors sincerely thank K. Kondo, T. Yasui, K. Tanaka, H. Nozawa, and E. Hashizume for their fruitful discussions.
Disclosure
The authors report no conflicts of interest in this work.
References
Sunwoo Y, Chou C, Takeshita J, Murakami M, Tochihara Y. Physiological and subjective responses to low relative humidity. J Physiol Anthropol. 2006;25(1):7–14. | ||
Sunwoo Y, Chou C, Takeshita J, Murakami M, Tochihara Y. Physiological and subjective responses to low relative humidity in young and elderly men. J Physiol Anthropol. 2006;25(3):229–238. | ||
Tupker RA, Bunte EE, Fidler V, Wiechers JW, Coenraads PJ. Irritancy ranking of anionic detergents using one-time occlusive, repeated occlusive and repeated open tests. Contact Dermatitis. 1999;40(6):316–322. | ||
Hashizume H. Skin aging and dry skin. J Dermatol. 2004;31(8):603–609. | ||
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4–S9. | ||
Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288(12):765–770. | ||
Kamata Y, Yamamot o M, Kawakami F, et al. Bleomycin hydrolase is regulated biphasically in a differentiation- and cytokine-dependent manner: relevance to atopic dermatitis. J Biol Chem. 2011;286(10):8204–8012. | ||
Denda M. Functional changes of the epidermis with aging and their treatment. J Soc Cosmet Chem Japan. 1996;30(4):377–387. | ||
Neill US. Skin care in the aging female: myths and truths. J Clin Invest. 2012;122(2):473–477. | ||
Kawada C, Yoshida T, Yoshida H, et al. Ingestion of hyaluronans (molecular weights 800 k and 300 k) improves dry skin conditions: a randomized, double blind, controlled study. J Clin Biochem Nutr. 2015;56(1):66–73. | ||
Uchiyama T, Nakano Y, Ueda O, et al. Oral intake of glucosylceramide improves relatively higher level of transepidermal water loss in mice and healthy human subjects. J Health Sci. 2008;54(5):559–566. | ||
Asserin J, Lati E, Shioya T, Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14(4):291–301. | ||
Inoue H, Sasaki R, Aiso I, Kuwano T. Short-term intake of a Japanese-style healthy lunch menu contributes to prevention and/or improvement in metabolic syndrome among middle-aged men: a non-randomized controlled trial. Lipids Health Dis. 2014;13:57. | ||
Kitamoto K. Cell biology of the Koji mold Aspergillus oryzae. Biosci Biotechnol Biochem. 2015;79(6):863–869. | ||
Koizumi T. [Mystery of fermented foods—there are friendly molds too!]. Nihon Ishinkin Gakkai Zasshi. 2001;42(1):1–5. Japanese [with English abstract]. | ||
Suganuma T, Fujita K, Kitahara K. Some distinguishable properties between acid-stable and neutral types of alpha-amylases from acid-producing koji. J Biosci Bioeng. 2007;104(5):353–362. | ||
Matsuda S, Kudoh Y. Changes of β-glucan contents and, β-glucanase activity in Mugi koji (Barley-koji) making. Food Preserv Sci. 2000; 26(5):257–261. | ||
Miyake Y, Ito C, Itoigawa M, Osawa T. Isolation of the antioxidant pyranonigrin-A from rice mold starters used in the manufacturing process of fermented foods. Biosci Biotechnol Biochem. 2007;71(10):2515–2521. | ||
Su YC, Wang JJ, Lin TT, Pan TM. Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol. 2003;30(1):41–46. | ||
Fujiwara D, Kato M, Koizumi H, Ikado K, Ano Y. Fermentation product of a cereal-derived material and immunomodulator. US 9101566 B2. Aug. 11, 2015. | ||
Ano Y, Ikado K, Shindo K, Koizumi H, Fujiwara D. Identification of 14-dehydroergosterol as a novel anti-inflammatory compound inducing tolerogenic dendritic cells. Sci Rep. 2017;7(1):13903. | ||
Barton DHR, Bruun T. A new sterol from a strain of Aspergillus niger. J Chem Soc. 1951;2728–2733. | ||
Bayer M, Proksch P, Felsner I, et al. Photoprotection against UVAR: effective triterpenoids require a lipid raft stabilizing chemical structure. Exp Dermatol. 2011;20(11):955–958. | ||
Task Force Committee for Evaluation of Anti-Aging Function. Guideline for evaluation of anti-wrinkle products. J Jpn Cosmet Sci Soc. 2007;31:411–431. | ||
Shiratsuchi E, Nakaba M, Yamada M. Elastin hydrolysate derived from fish enhances proliferation of human skin fibroblasts and elastin synthesis in human skin fibroblasts and improves the skin conditions. J Sci Food Agric. 2016;96(5):1672–1677. | ||
Uchiyama T, Kuwazuru S, Ueda O, Maekawa T. Dietary Konjac extract improves TEWL of whole body. Jpn Pharmacol Ther. 2011;39(4):437–445. | ||
Yoshino S, Iwasaki D, Nojima J. Effects of glucosylceramide extracted from pineapple on healthy Japanese males and females with dullness and dry skin-randomized, double-blind, placebo-controlled, parallel-group study. Jpn Pharmacol Ther. 2015;43(11):1593–1600. | ||
Matsushita A, Kameda N, Seike M. Effects of simultaneous intake of soy peptide and collagen peptide on the skin function of healthy adult women. J Home Econ Jpn. 2012;63:35–42. | ||
Dal’Belo SE, Gaspar LR, Maia Campos PM. Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res Technol. 2006;12(4):241–246. | ||
Vázquez B, Avila G, Segura D, Escalante B. Antiinflammatory activity of extracts from Aloe vera gel. J Ethnopharmacol. 1996;55(1):69–75. | ||
Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. | ||
Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44(11):727–732. | ||
Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62(7):681–688. | ||
Zhang M, Caragine T, Wang H, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185(10):1759–1768. | ||
Soda K, Kano Y, Nakamura T, Kasono K, Kawakami M, Konishi F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J Immunol. 2005;175(1):237–245. | ||
Hirata M, Tsuge K, Jayakody LN, et al. Structural determination of glucosylceramides in the distillation remnants of shochu, the Japanese traditional liquor, and its production by Aspergillus kawachii. J Agric Food Chem. 2012;60(46):11473–11482. | ||
Konishi N, Yamada H, Matsue K, et al. Genba reberu deno hifu sokutei hy ka: toraburu zirei taisaku [Measurement and evaluation of skin in the field level: trouble cases and measures]. Science & technology. 2007;228–240. Japanese. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



